Abstract
It is well established that adjuvant treatment reduces mortality after early breast cancer. However, the optimal timing of adjuvant treatment is not well described. To determine the optimal timing of adjuvant treatment, 402 breast cancer patients who received adjuvant treatment at Ankara Oncology Research and Training Hospital between January 1995 and August 2002 were evaluated retrospectively. Three hundred and fifty-seven (88.8%) patients received adjuvant chemotherapy, 204 (50.7%) of these patients received only adjuvant chemotherapy and 153 (38%) patients received tamoxifen following chemotherapy. Remaining 45 (11.2%) patients received only adjuvant tamoxifen. The median time to start adjuvant treatment after surgery was day 21 (range, days 4 to days 258), and the median follow-up was 50 months (range, 6–105 months). The patients were divided into 5 groups according to starting time of chemotherapy (shorter than 14 days, between days 15–29, between days 30–44, between days 45.−59 and more than 59 days). Overall survival (OS) and disease-free survival (DFS) were not shown significantly different between for 5 groups (P > 0.05). Secondly, patients were divided into two groups as starting adjuvant treatment equal to or shorter than 44 days and longer than 44 days (n = 344, 85.6% and vs. n = 58, 14.4%, respectively). OS was significantly better in patients who started to receive adjuvant treatment within 44 days after surgery compared to patients who received adjuvant treatment after 44 days (92 vs. 83.3%, P = 0.03) for 5 years, but DFS was not significantly different between two groups (83.4 vs. 82.2%, P > 0.05). According to our study, adjuvant treatment of breast cancer should be initiated earlier after surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common type of cancer in women [1]. Proper adjuvant treatment is necessary in indicated patients. Adjuvant treatment of breast cancer includes chemotherapy, hormonal treatment, targeted therapy and radiotherapy based on clinical and pathological characteristics of the tumor [1–5]. Although the adjuvant treatment of breast cancer has been well described, optimal timing of adjuvant treatment is not well known. It has been reported that starting time of the adjuvant treatment after surgery may affect either wound healing or prognosis of breast cancer. Some reports have shown that earlier chemotherapy has effects on drug resistance, micrometastasis and angiogenesis [6]. In the 1980s, the International Breast Cancer Study Group showed a benefit in starting CT earlier by CMF regimen in premenopausal breast cancer patients [7]. Heterogeneity of studies in the literature as a result of differences in prognostic factors of patient populations (age, menopausal status, grade, pathologic subtype, hormone receptor status, HER2, p53 etc.) may affect the role of adjuvant starting time for prognosis of breast cancer [2, 8–13]. Shannon et al. reported that adjuvant chemotherapy timing had no effect on survival in early breast cancer patients with breast sparing surgery [14]. Some benefits have been reported if adjuvant chemotherapy started before the 12th week after surgery [15]. This probable benefit may result from several factors. Micrometastasis has been shown to be doubled after surgery in animal models [16]. This effect may depend on serum transmissible growth factors [16, 17]. On the other hand, angiogenesis has been shown to increase metastasis on animal models after primary tumor had resected by decreasing angiostatin, which is a plasminogen fragment [16, 17]. Theoretically, early adjuvant treatment may inhibit this early rebound effect after surgery on micrometastatic cancer cells. In this study, we investigated optimal timing of adjuvant treatment in patients with early breast cancer.
Materials and methods
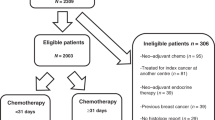
Data were collected retrospectively from 642 breast cancer patients who received chemotherapy and/or hormonal treatment at Ankara Oncology Research and Training Hospital between January 1995 and August 2002. All cases were pathologically documented invasive breast cancer in the same center. Patients receiving neo-adjuvant chemotherapy or neo-adjuvant hormonal treatment, adjuvant chemotherapy with unknown starting time, patients with primary metastatic disease and with follow-up period shorter than 6 months were excluded from the study. Four hundred and two patients were eligible and evaluated. Data on prognostic factors and treatment were collected prospectively including age, tumor size, histological type, tumor grade, hormone receptor status, number of involved lymph nodes, adjuvant irradiation to chest wall and lymph nodes, adjuvant tamoxifen and type of chemotherapy.
Chemotherapy or hormonal treatment starting time after surgery is described as delaying period. All eligible patients were classified by delaying period according to following two methods: first, patients were divided into five groups according to delaying period as shorter than 14 days (Group A, n = 130, 32.3%), between days 15 and days 29 (Group B, n = 148, 36.8%), between days 30 and days 44 (Group C, n = 66, 16.4%), between days 45 and days 59 (Group D, n = 31, 7.7%), more than 59 days (Group E, n = 27, 6.7%). Second, patients were divided into two groups as starting adjuvant treatment equal to or shorter than 44 days and longer than 44 days (n = 344, 85.6% and vs. n = 58, 14.4%, respectively). Disease-free survival (DFS) and overall survival (OS) were calculated.
Statistical analysis
Differences between the five groups were assessed by means of the chi-square test. DFS and OS were calculated using Kaplan–Meier and log-rank tests. Cox regression analysis was used for the prognostic and other treatment variables.
Results
Four hundred and two patients who received adjuvant chemotherapy and/or hormonal treatment were evaluated. Median follow-up was 50 months (range, 6–105 months). Two hundred and four (50.7%) patients received only adjuvant chemotherapy, 45 (11.2%) patients received only adjuvant tamoxifen, and 153 (38.0%) patients received tamoxifen after adjuvant chemotherapy. Two hundred and twenty-three (55.5%) patients received CMF i.v., and 129 (32.1%) patients received anthracycline-based chemotherapy. Axillary lymph node involvement was negative in 141(35.1%) patients, 1–3 positive in 110 (27.3%) patients, 4–9 positive in 83 (20.2%) patients and more than 10 nodes in 60 patients (14.9%). Patients’ characteristics and adjuvant treatment schedules are shown in Table 1. Two hundred and eighty (69.6%) of the 402 patients were without relapse during follow-up. Hundred (24.9%) patients were alive with relapse, and 22 (5.5%) patients died due to disease progression. Metastatic sites were skin (23 patients), bone (45 patients), lung (44 patients), liver (22 patients), brain (6 patients) and supraclavicular or contralateral axillary (17 patients). Six patients who were without metastasis after surgery and adjuvant treatment developed secondary contralateral breast cancer. These six patients are still without relapse after treatment for secondary breast cancer.
Median time to starting time of adjuvant treatment after surgery is day 21 (range, day 4 to day 258). Most of the patients have received first cycle of chemotherapy between second and fourth weeks. Patients’ distribution according to starting time of adjuvant treatment is shown in Fig. 1.
DFS for all patients was 89 and 84% at third and fifth years, respectively. OS for all patients was 97 and 95% at third and fifth years, respectively. The most frequent metastatic sites in decreasing order were bone, lung, skin and liver. Negative receptor status, tumor size and axillary involvement were found to be significantly negatively correlated with prognosis (P < 0.016, P < 0.05, and P < 0.001, respectively) for all patients.
Patient subgroups were similar according to age, menopausal status, receptor status, tumor size, axillary status, surgery type and adjuvant treatments (P > 0.05) according to two methods. OS and DFS were not shown significantly different between 5 groups (P > 0.05) as described in “Materials and method” section. One hundred and twenty-nine patients (32.1%) received anthracycline-based chemotherapy, two hundred and twenty-three (55.5%) patients received CMF regimen, and five patients were given other chemotherapy regimen (P = 0.78).
The patients who received adjuvant treatment within 44 days after surgery had significantly superior OS compared to the patients who received adjuvant treatment 44 days after surgery (92% vs. 83.3% for 5 years, respectively, P = 0.03, Fig. 2) but not significant for DFS. The patients receiving CMF i.v. or anthracycline-based chemotherapy (excluding only adjuvant hormonal treatment patients) with shorter delaying period (shorter or equal to 44 days) had significantly superior OS but not DFS for 5 years (91.4 vs. 83.4% P < 0.05 and 83.4 vs. 82.2% P = 0.03, respectively).
Discussion
Some of the animal studies have shown that starting adjuvant treatment earlier has some benefit on micrometastasis, drug resistance and angiogenesis. Some clinical trials have shown a similar positive effect on survival [18–20], but others have not [14, 21]. In this study, two different methods were used for examining adjuvant treatment starting time. One of the methods used 5 subgroups. However, these 5 subgroups were not significantly different for OS or DFS. On the other hand, to find an optimal starting time of adjuvant treatment, patients were divided into two groups according to 44 days cut-off, which has shown a statistically significant difference in OS between the 2 groups (Fig. 2).
Pronzota et al. have reported that the patients whose adjuvant treatment started before 35 days after surgery had significantly better OS at 4 years with CMF regimen (88 vs. 69%; [19]). International Breast Cancer Study Group (IBCSG) has reported starting adjuvant chemotherapy before 20 days after surgery with CMF regimen had better outcome in OS (60 vs. 34% at 10 years) in node positive, premenopausal, ER negative 226 patients [18]. Moreover, IBCSG has evaluated adjuvant CMF with or without preoperative CMF regimen in node positive premenopausal women. In ER negative 101 patients, they showed better DFS in patients who received perioperative CT than ER + patients (48 vs. 38%) at median 11 years follow-up [7]. On the other hand, Sertoli et al. have shown a benefit with perioperative CEF regimen in ER negative patients, but this effect was not apparent in ER positive patients [22].
In another study, starting adjuvant treatment early at 21 days did not improve either OS or DFS in all patients or in the ER negative group [14]. Endocrine manipulation may act a more positive role than effect of earlier initiation of chemotherapy in ER positive patients. Starting chemotherapy earlier may improve outcomes especially in ER negative patients. However, our report did not show any difference between ER positive and ER negative or node positive and node negative patients. Furthermore, early chemotherapy may help initiating earlier hormonal treatment after chemotherapy, which is especially important in hormone responsive breast cancer patients.
In conclusion, our study showed that starting adjuvant systemic treatment within 44 days improves OS in patients with early breast cancer. A larger number of studies, especially those with subgroup analyses, are needed. Adjuvant treatment of breast cancer is very important for both DFS and OS. Some factors such as wound healing and performance status may be reason to delay adjuvant treatment. Our study puts forward an optimal starting time for adjuvant therapy and describes the possibility of poor outcomes if adjuvant therapy is delayed.
References
Haskel CM. Breast cancer: In: Haskell CM, editor. Cancer treatment, 5th edn. Philedelphia PA: W.B. Saunders Company; 2001. pp. 505–595.
Box BA, Russell CA. Breast cancer: manuel of clinical oncology. In: Casciato DA, editor. Fifth Edition. Philadelphia: Lippincott Williams and Wilkins; 2004. pp. 233–253.
Green MC, Hortobagyi GN. Adjuvant chemotherapy for breast cancer. Langenbecks Arch Surg. 2002;387:109–16.
Burdette-Radoux S, Muss HB. Optimizing the use of anthracyclines in the adjuvant treatment of early-stage breast cancer. Clin Breast Cancer. 2003;4:264–72.
Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–42.
McCulloch P, Choy A, Martin L. Association between tumour angiogenesis and tumour cell shedding into effluent venous blood during breast cancer surgery. Lancet. 1995;18:1334–5.
The Ludwig Breast Cancer Study Group. Combination adjuvant chemotherapy for node-positive breast cancer. Inadequacy of a single perioperative cycle. N Engl J Med. 1988;5:677–83.
Nixon AJ, Neuberg D, Hayes DF, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II Breast Cancer. J Clin Oncol. 1994;12:888–94.
Buzdar AU. Role of anastrozole in adjuvant therapy for postmenopausal patients. Semin Oncol. 2003;30:21–9.
Pestalozzi BC, Luporsi-Gely E, Jost LM, Bergh J. ESMO guidelines task force. ESMO minimum clinical recommendations for diagnosis, adjuvant treatment and follow-up of primary breast cancer. Ann Oncol. 2005;16(Suppl 1):7–9.
Martin C, Cutuli B, Velten M. Predictive model of axillary lymph node involvement in women with small invasive breast carcinoma: axillary metastases in breast carcinoma. Cancer. 2002;94:314–22.
Pinto AE, Andre S, Pereira T, et al. C-erbB-2 oncoprotein overexpression identifies a subgroup of estrogen receptor positive (ER +) breast cancer patients with poor prognosis. Ann Oncol. 2001;12:525–33.
Silvestrini R, Benini E, Daidone MG, Veneroni S, Boracchi P, Cappelletti V, Di Fronzo G. Veronesi U: p53 as an independent prognostic marker in lymph node-negative breast cancer patients. J Natl Cancer Inst. 1993;85:965–70.
Shannon C, Ashley S, Smith IE. Does timing of adjuvant chemotherapy for early breast cancer influence survival? J Clin Oncol. 2003;21:3792–7.
Crown J. Adjuvant systemic chemotherapy of patients with node-positive breast cancer. In: Perry MC, editor. Educational book of American Society of Clinical Oncology. Alexandria, VA: American Society of Clinical Oncology;2004. pp. 28–35.
Fisher B, Gunduz N, Coyle J, et al. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res. 1989;49:1996–2001.
Fisher B, Saffer E, Rudock C, et al. Effect of local or systemic treatment prior to primary tumor removal on the production and response to a serum growth-stimulating factor in mice. Cancer Res. 1989;49:2002–4.
Colleoni M, Bonetti M, Coates AS, et al. Early start of adjuvant chemotherapy may improve treatment outcome for premenopausal breast cancer patients with tumors not expressing estrogen receptors. The international breast cancer study group. J Clin Oncol. 2000;18:584–90.
Pronzato P, Campora E, Amoroso D, et al. Impact of administration-related factors on outcome of adjuvant chemotherapy for primary breast cancer. Am J Clin Oncol. 1989;12:481–5.
Altundag MK, Celik I, Ozisik Y. Is there a range of time for initiation of adjuvant chemotherapy in patients with malignancy? Ann Oncol. 2000;11:1209.
van der Hage JA, van de Velde CJ, et al. Improved survival after one course of perioperative chemotherapy in early breast cancer patients. Long-term results from the European organization for research and treatment of cancer (EORTC) Trial 10854. Eur J Cancer. 2001;37:2184–93.
Sertoli MR, Bruzzi P, Pronzato P, et al. Randomized cooperative study of perioperative chemotherapy in breast cancer. J Clin Oncol. 1995;13:2712–21.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alkis, N., Durnali, A.G., Arslan, U.Y. et al. Optimal timing of adjuvant treatment in patients with early breast cancer. Med Oncol 28, 1255–1259 (2011). https://doi.org/10.1007/s12032-010-9566-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9566-4