Abstract
Purpose
The aim of this study was to investigate the association of the thymidylate synthase (TS) and methylenetetrahydrofolate reductase (MTHFR) polymorphisms with the clinical outcomes of gastric cancer patients treated with 5-FU-based adjuvant chemotherapy.
Methods
One-hundred and sixteen patients with gastric cancer were treated with 5-FU-based adjuvant chemotherapy. The TS (a 28-bp tandem repeat polymorphism in the TS enhancer region (TSER) and a 6 bp deletion/insertion polymorphism in the 3′-untranslated region) and MTHFR C677T polymorphisms were determined in blood samples from those patients using PCR and PCR-LDR (ligation detection reaction) method, respectively.
Results
The overall survival (OS) in patients with the TS ins6/ins6 genotype was significantly shorter than those in patients with the del6/del6 (P = 0.017) and ins6/del6 (P = 0.022) genotype. The relapse-free survival (RFS) and OS in patients with the MTHFR C/C genotype were significantly worse than those in patients with the T/T or C/T genotype (P = 0.043 and 0.040, respectively). Cox multivariate analysis also showed that patients with the TS ins6/ins6 genotype have worse OS than patients with the T/T or C/T genotype (HR = 2.437, P = 0.041), and the MTHFR C/C genotype was associated with shorter RFS (HR = 1.723, P = 0.031) and OS (HR = 1.681, P = 0.056). No significant association was found between the TSER polymorphism and the clinical outcomes (P > 0.05).
Conclusion
The polymorphisms of TS 3′-UTR ins6/del6 and MTHFR C677T appear to be potential prognostic factors in gastric cancer patients treated with 5-FU-based adjuvant chemotherapy, which may allow identification of gastric cancer patients who will benefit from 5-FU chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fourth most common cancer and the second most frequent cause of cancer deaths worldwide. Surgery is the primary modality for managing early-stage disease. However, even after radical surgery, the majority of gastric cancer patients develop local or distant recurrence [1]. Meta-analyses of adjuvant chemotherapy clinical trials have confirmed a survival benefit in favor of the systemic medical treatment [2–4]. Despite the development of new agents, 5-fluorouracil (5-FU) remains a cornerstone in the treatment of gastric cancer. However, the response rate is only approximately 25%, even if supplemented by leucovorin (CF), which improves the effect of 5-FU. Despite numerous efforts on identifying suitable predictive markers, there is still a lack of accurate markers to discriminate between the patients who are likely to benefit from 5-FU chemotherapy and those who are not [5–8].
As a pyrimidine analog, 5-FU exerts its anti-tumor effects through anabolism, which is determined by the rate of catabolism. Thus, the genes coding for the key enzymes in 5-FU metabolism may play a pivotal role in the efficacy of 5-FU. Thymidylate synthase (TS) is the target enzyme for 5-FU and catalyzes methylation of dUMP to dTMP, which is an important process of DNA biosynthesis. The expression levels of TS in tumor tissues are considered to influence the sensitivity of several tumors, including gastric cancer, towards 5-FU-based chemotherapy [9, 10]. However, the scarcity of tumor tissue, the potential biases of immunohistochemistry and mRNA quantification, and the genetic heterogeneity of clinical tumor tissue limits its clinical applications. In contrast, it is much easier to obtain DNA isolated from peripheral blood lymphocytes for polymorphisms analysis. Genetic polymorphism is an important mechanism of influencing gene function. A lot of studies indicate that some TS polymorphisms could influence the response to 5-FU. The first found functional polymorphism in the TS promoter is a variable number of tandem repeats with two or three repeats of a 28-base pair sequence in the 5′-untranslated region (UTR) (2R/3R). The 3R allele was associated with enhanced TS expression when compared with the 2R allele [11, 12]. Another important TS polymorphism is a 6 bp deletion or insertion (del6/ins6) in the 3′-UTR. Several studies seem to confirm that the TS 3′-UTR del6 allele is associated with decreased TS mRNA stability and lower intra-tumoral TS expression in comparison with the ins6 allele [13].
Methylenetetrahydrofolate reductase (MTHFR) plays a key role in folate metabolism. The substrate for MTHFR, 5,10-methylenetetrahydrofolate, is essential to the DNA synthesis by acting as a cofactor in the conversion of dUMP to dTMP by TS. A common polymorphism in the MTHFR has been identified (C677T). The T/T variant occurs frequently in most populations and correlates with reduced enzyme activity and increased thermolability compared with the C/C wild-type homozygotes [14]. Because the activity of 5-FU is dependent on a competitive interaction with folate metabolism, the MTHFR 677 T allele may also influence the effect of 5-FU-based chemotherapy by causing the accumulation of 5, 10- methylenetetrahydrofolate. Some in vitro studies have identified the MTHFR polymorphism as an important predictor for 5-FU treatment [15], but the clinical data are controversial [16–19].
Most studies on the TS and MTHFR polymorphisms were focused on advanced or metastatic diseases. However, the results of those studies could not be transferred to adjuvant chemotherapy without reservation. The objective of the present study was to analyze whether the TS and MTHFR polymorphisms could influence the prognosis of gastric cancer patients receiving 5-FU-based adjuvant treatment.
Materials and methods
Patients
From May 2001 to November 2006, one hundred and sixteen patients with histologically confirmed gastric cancer were enrolled in this study at the Fourth Affiliated Hospital of Suzhou University. All those patients received radical surgery and classified as stage of IB-IV (M0). Of the patients, 12 curatively resected patients with stage IV (M0) patients were included based on following consideration: complete resection of the tumor with D (2–3) resection, defined as resection performed with curative intent and resulting in negative resection margins. After surgery, these patients were treated with at least four cycles of 5-FU-based adjuvant treatment (83 with 5-FU/CF/oxaliplatin, 14 with 5-FU/CF/taxanes, 19 with 5-FU/CF/oxaliplatin/other regimens (taxanes or hydroxycamptothecin)). Follow-up of those patients was performed at 3-month intervals after chemotherapy at outpatient clinics or by routine phone calls. The relapse of gastric cancer had to be proven by cytology biopsy or surgery. This study was approved by the ethics and research committee of our hospital.
DNA extraction and genotyping
Blood samples were collected in EDTA containing tubes. Genomic DNA was isolated from peripheral blood lymphocytes using Axygene genomic DNA purification Kit (Axygen Biotechnology, China).
The primers and probes were listed in Table 1. Genotyping of MTHFR was performed using PCR-LDR (polymerase chain reaction-ligation detection reaction) method as described previously [20]. The PCRs for TS genotyping were carried out in a total volume of 20 μl including 20 ng genomic DNA, 1 × PCR buffer, 2.5 m mol/l MgCl2, 0.2 m mol/l dNTPs, 0.25 μ mol/l each primer, 1 U hot-start Taq DNA polymerase (QIAgen) and 0.5% DMSO (only for TSER). Cycling parameters were as follows: 95°C for 15 min; 35 cycles of 94°C for 30 s, 60°C for 40 s, and 72°C for 30 s; and a final extension step at 72°C for 10 min. PCR products were analyzed by 2% (TSER) or 3% (TS 3′-UTR) agarose gel electrophoresis and ethidium bromide staining following by visualization with ultraviolet illumination using a gel imaging analyzing system.
In addition, the representative PCR products were subjected to direct DNA sequencing to confirm the accuracy of the genotyping results.
Statistical analysis
Data analysis was performed using SPSS 13.0 for Windows. The genotypes for each polymorphism were analyzed firstly as a three-group categoric variable (referent model), and if it was necessary some SNPs were also grouped further according to the dominant and recessive model. The relationships between the genotype frequencies and clinical characteristics were assessed by χ2 or Fisher’s exact probability tests. Relapse-free survival (RFS) was defined as the time interval between the date of surgery and the date of confirmed relapse or the date of last follow-up. Overall survival (OS) was defined as the time between surgery and either death or the time of the last follow-up. The end date of the follow-up was 24 February 2008 and the median follow-up period was 26.1 months (range 5.2–75.1 months). The mortality rate and relapse rate in those gastric cancer patients were 51.7% (60/116) and 60.3% (70/116), respectively. Survival curves were generated by the Kaplan-Meier method and verified by the log-rank test. Cox proportional hazards regression analysis was used to estimate Hazard Ratios (HRs) and their 95% confidence intervals (CIs), representing the overall relative risk of relapse and death associated with polymorphism, and to adjust for potential confounding variables. All of the values were two-sided and statistical significance was defined as P < 0.05.
Results
TS and MTHFR genotypes
A total of 116 patients were analyzed. Their demographic and disease characteristics were shown in Table 2. The allelic discrimination data from PCR and PCR-LDR assay were confirmed by direct sequencing of representative PCR products. In those cases, the genotype determined by PCR or PCR-LDR assay was identical to that determined by DNA sequencing (Supplementary Fig. 1). Table 3 listed the genotypes distribution of TS and MTHFR polymorphisms. No significant associations were found between those polymorphisms and age, gender, pathologic stage, or grading (data not shown).
Association between polymorphisms and clinical outcomes
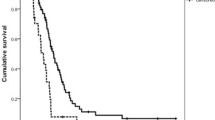
According to the Kaplan-Meier survival analysis, median RFS was 11.5 months for the ins6/ins6 genotype, 20.8 months for the ins6/del6 genotype, and 36.9 months for the del6/del6 genotype (log-rank χ2 = 3.483, P = 0.175, Fig. 1a). Patients with the ins6/ins6 genotype had a shorter OS of 20.7 months when compared with 29.8 and 41.0 months in those with the ins6/del6 (log-rank χ2 = 5.219, P = 0.022) and del6/del6 genotype(log-rank χ2 = 6.676, P = 0.017) (Fig. 1d).
Kaplan-Meier curves considering the influence of the thymidylate synthase (TS) and methylenetetrahydrofolate reductase (MTHFR) genotypes on relapse-free survival and overall survival of gastric cancer patients treated with 5-FU-based adjuvant chemotherapy. a–c Kaplan-Meier estimates of relapse-free survival by the TS 5′-untranslated region (5′-UTR), TS 3′-UTR and MTHFR C677T genotypes, respectively; d–f Kaplan-Meier estimates of overall survival by the TS 5′-UTR, TS 3′-UTR and MTHFR C677T genotypes, respectively
Patients with the 3R/3R genotype had a shorter RFS of 19.1 months when compared with 31.8 and 29.8 months in those with the 2R/3R and 2R/2R genotype; however, the difference was not statistically significant (log-rank χ2 = 1.541, P = 0.463, Fig. 1b). No significant association was seen between the OS of patients with the 3R/3R genotype and that of patients with the 2R/3R and 2R/2R genotype (log-rank χ2 = 3.795, P = 0.150, Fig. 1e).
The Kaplan-Meier survival analysis showed that patients with the MTHFR C/C genotype had shorter RFS (19.9 vs. 31.8 months, log-rank χ2 = 4.103, P = 0.043) (Fig. 1c) and OS (24.5 vs. 52.0 months, P = 0.040) than patients with the other two genotypes (Fig. 1f).
After adjusted for age, gender, pathologic stage and grading, multivariate analysis showed that the ins6/ins6 genotype appeared to be an independent risk factor for OS when compared with the other two genotypes (adjusted HR = 2.437, P = 0.041). The MTHFR C/C genotype was a potential risk factor for RFS (adjusted HR = 1.723, P = 0.031) for gastric cancer patients, and a trend for worse OS in patients with the C/C genotype versus those with the T/T and C/T genotypes also was noted (adjusted HR = 1.681, P = 0.056) (Table 4).
Discussion
In this study, we investigated whether the determination of two common polymorphisms of the TS gene and the MTHFR C677T polymorphism in gastric cancer patients receiving 5-FU-based adjuvant chemotherapy, could be used to predict the relapse and survival of those patients. Our analysis suggests that the polymorphisms of the TS 3′UTR and MTHFR C677T can result in differences of RFS and/or OS among gastric cancer patients treated with 5-FU-based adjuvant chemotherapy, highlighting its potential utility for the rational choosing 5-FU in the treatment of gastric cancer.
It is well known that some genetic or biologic features of cancer cell happen during the course of cancer progression. It is possible that some variations between early and advanced (metastatic) cancer may account for their different response to a certain drug. Most of the current studies on the TS and MTHFR polymorphisms focused on advanced or metastatic diseases; and the results of these studies may not be able to exactly reflect the potential influence of the polymorphisms of TS and MTHFR on adjuvant chemotherapy.
Recently, the TS polymorphisms have been suggested to influence 5-FU sensitivity in vitro and in vivo. Several studies on colorectal cancer found that the 3R/3R genotype was associated with worse clinical outcomes in patients treated with 5-FU-based chemotherapy compared with the 2R/2R or 2R/3R genotype [21, 22], but Jakobsen et al. [17] got opposite conclusion. Different results also were reported in gastric cancer. Ishida et al. [5] observed that a longer survival was associated with the 2R/2R or 2R/3R genotype in gastric cancer patients who had received the oral fluoropyrimidines therapy, compared with the 3R/3R genotype, although it did not reach significance. Ott et al. [7] reported that the 3R/3R is a risk factor for tumor-related survival in neoadjuvant treated locally advanced gastric cancer. However, several other studies did not observe any significant difference in the outcome of patients according to TSER genotypes [6, 19, 23, 24]. Uncertainty about the effect of the TS 3′UTR polymorphism in patients treated with FU also exists. Dotor et al. [25] found a better effect of the TS 3′UTR del6/del6 on survival of colorectal cancer patients treated with 5-FU-based adjuvant chemotherapy. Stoehlmacher et al. [26] also showed that the TS del6/del6 genotype was associated with better clinical outcomes of colorectal cancer patients receiving 5-FU-based chemotherapy. In advanced gastric cancer patients treated with FU, Lu et al. [27] also found higher response rate in patients with the TS 3′UTR del6 allele compared to the patients with the ins6/ins6 genotype, but several other studies showed that the TS 3′UTR polymorphism could not predict the efficacy of 5-FU treatment [19, 23, 24]. Possible explanations for divergent findings may include genotyping in normal or tumor tissues, variable doses and schedules of FU-based therapy, variable tumor stage or different kind of cancers, limited patient’s numbers in some studies and different ethnic populations among those studies.
In this study, the OS in patients with the TS ins6/ins6 genotype was significantly shorter than those in patients with the del6/del6 and ins6/del6 genotype, but no significant association was found between the clinical outcomes and the TSER polymorphism. A limitation of the present study was failed to analyze another important polymorphism (a G > C substitution at the 12th nucleotide in the second repeat of the 3R alleles, named 3RG/3RC) in the TSER, which has been identified recently and has opened new perspectives for studying TS 5′-UTR genotypes. TS 5′-UTR genotypes were classified into high expression type (2R/3G, 3C/3G, and 3G/3G) and low expression type (2R/2R, 2R/3C, and 3C/3C). Although Dotor et al. [25] found that the analysis of the double polymorphism in TS 5′-UTR did not add prognostic information, several studies analyzed the TS polymorphisms based on 5′-UTR/3′-UTR combined genotypes and found that those polymorphisms may all have functional influence on the effect of 5-FU, even though no significant association was found between the 5-FU sensitivity and the sole TS polymorphisms [18, 28, 29]. However, in the present study, we failed to analyze the novel polymorphism that functionally transfers a TS 3R genotype into a TS 2R genotype in their analysis, which may influence the effect of the TS 5′UTR polymorphisms on the prognosis of gastric cancer patients treated with 5-FU-based adjuvant chemotherapy.
The third polymorphism with putative influence on 5-FU-based chemotherapy is the MTHFR C677T. The reduced activity of the thermolabile variant of the MTHFR enzyme in T allele carriers increases availability of 5,10-methylenetetrahydrofolate, which is a cofactor for fluorouracil inhibition of TS. In previous studies, the MTHFR C677T polymorphism showed significant [16, 17] or no clear prognostic effect [18, 19, 24, 30] in patients treated with FU-based palliative chemotherapy. Lu et al. [31] reported that the MTHFR T/T genotype was associated with higher response rate compared to the C/C or C/T genotype in advanced gastric cancer treated with 5-FU-based chemotherapy in Chinese population, but several studies on other populations showed that the T allele was not associated with improved outcomes [18, 19, 24, 30]. In the present study, we also found the favorable effect of the T allele on survival of gastric cancer patients receiving 5-FU-based adjuvant chemotherapy in Chinese population. Basing on the functional effect of the MTHFR 677 T allele, perhaps there is another possible explanation for divergent results in vivo in addition to the aforementioned reasons about the TS polymorphisms. Cellular availability of cofactor 5,10-methylenetetrahydrofolate for fluorouracil TS inhibition may be influenced not only by the MTHFR C677T polymorphism, but also other factors, like folate level in the diet. It is possible that patients with low folate diet are more susceptible to the effects of the MTHFR C677T than patients with sufficient folate intake. MTHFR C677T polymorphism may show different pharmacogenetic effects on the 5-FU sensitivity in studied populations with different diet features.
Conclusion
Our data show that the polymorphisms of TS 3′-UTR and MTHFR C677T appear to be potential prognostic factors in gastric cancer patients treated with 5-FU-based adjuvant chemotherapy.Further prospective clinical studies on the prognostic role of the TS and MTHFR polymorphisms are needed before the polymorphisms can be used in clinical practice to select patients who are most likely to benefit from 5-FU-based chemotherapy.
References
Macdonald JS (2004) Treatment of localized gastric cancer. Semin Oncol 31:566–573
Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, Cascinu S, Barni S, Labianca R, Torri V (2000) Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente). Ann Oncol 11:837–843
Hejna M, Wohrer S, Schmidinger M, Raderer M (2006) Postoperative chemotherapy for gastric cancer. Oncologist 11:136–145
Carrato A, Gallego-Plazas J, Guillen-Ponce C (2005) Adjuvant therapy of resected gastric cancer is necessary. Semin Oncol 32:S105–S108
Ishida Y, Kawakami K, Tanaka Y, Kanehira E, Omura K, Watanabe G (2002) Association of thymidylate synthase gene polymorphism with its mRNA and protein expression and with prognosis in gastric cancer. Anticancer Res 22:2805–2809
Goekkurt E, Hoehn S, Wolschke C, Wittmer C, Stueber C, Hossfeld DK, Stoehlmacher J (2006) Polymorphisms of glutathione S-transferases (GST) and thymidylate synthase (TS)–novel predictors for response and survival in gastric cancer patients. Br J Cancer 94:281–286
Ott K, Vogelsang H, Marton N, Becker K, Lordick F, Kobl M, Schuhmacher C, Novotny A, Mueller J, Fink U, Ulm K, Siewert JR, Hofler H, Keller G (2006) The thymidylate synthase tandem repeat promoter polymorphism: a predictor for tumor-related survival in neoadjuvant treated locally advanced gastric cancer. Int J Cancer 119:2885–2894
Park DJ, Lenz HJ (2006) Determinants of chemosensitivity in gastric cancer. Curr Opin Pharmacol 6:337–344
Hua D, Huang ZH, Mao Y, Deng JZ (2007) Thymidylate synthase and thymidine phosphorylase gene expression as predictive parameters for the efficacy of 5-fluorouracil-based adjuvant chemotherapy for gastric cancer. World J Gastroenterol 13:5030–5034
Wei J, Zou Z, Qian X, Ding Y, Xie L, Sanchez JJ, Zhao Y, Feng J, Ling Y, Liu Y, Yu L, Rosell R, Liu B (2008) ERCC1 mRNA levels and survival of advanced gastric cancer patients treated with a modified FOLFOX regimen. Br J Cancer 98:1398–1402
Kawakami K, Omura K, Kanehira E, Watanabe Y (1999) Polymorphic tandem repeats in the thymidylate synthase gene is associated with its protein expression in human gastrointestinal cancers. Anticancer Res 19:3249–3252
Kawakami K, Salonga D, Park JM, Danenberg KD, Uetake H, Brabender J, Omura K, Watanabe G, Danenberg PV (2001) Different lengths of a polymorphic repeat sequence in the thymidylate synthase gene affect translational efficiency but not its gene expression. Clin Cancer Res 7:4096–4101
Mandola MV, Stoehlmacher J, Zhang W, Groshen S, Yu MC, Iqbal S, Lenz HJ, Ladner RD (2004) A 6 bp polymorphism in the thymidylate synthase gene causes message instability and is associated with decreased intratumoral TS mRNA levels. Pharmacogenetics 14:319–327
Ueland PM, Hustad S, Schneede J, Refsum H, Vollset SE (2001) Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci 22:195–201
Sohn KJ, Croxford R, Yates Z, Lucock M, Kim YI (2004) Effect of the methylenetetrahydrofolate reductase C677T polymorphism on chemosensitivity of colon and breast cancer cells to 5-fluorouracil and methotrexate. J Natl Cancer Inst 96:134–144
Cohen V, Panet-Raymond V, Sabbaghian N, Morin I, Batist G, Rozen R (2003) Methylenetetrahydrofolate reductase polymorphism in advanced colorectal cancer: a novel genomic predictor of clinical response to fluoropyrimidine-based chemotherapy. Clin Cancer Res 9:1611–1615
Jakobsen A, Nielsen JN, Gyldenkerne N, Lindeberg J (2005) Thymidylate synthase and methylenetetrahydrofolate reductase gene polymorphism in normal tissue as predictors of fluorouracil sensitivity. J Clin Oncol 23:1365–1369
Ruzzo A, Graziano F, Kawakami K, Watanabe G, Santini D, Catalano V, Bisonni R, Canestrari E, Ficarelli R, Menichetti ET, Mari D, Testa E, Silva R, Vincenzi B, Giordani P, Cascinu S, Giustini L, Tonini G, Magnani M (2006) Pharmacogenetic profiling and clinical outcome of patients with advanced gastric cancer treated with palliative chemotherapy. J Clin Oncol 24:1883–1891
Pare L, Altes A, Ramon y Cajal T, Del Rio E, Alonso C, Sedano L, Barnadas A, Baiget M (2007) Influence of thymidylate synthase and methylenetetrahydrofolate reductase gene polymorphisms on the disease-free survival of breast cancer patients receiving adjuvant 5-fluorouracil/methotrexate-based therapy. Anticancer Drugs 18:821–825
Huang ZH, Hua D, Li LH, Zhu JD (2008) Prognostic role of p53 codon 72 polymorphism in gastric cancer patients treated with fluorouracil-based adjuvant chemotherapy. J Cancer Res Clin Oncol (in press)
Iacopetta B, Grieu F, Joseph D, Elsaleh H (2001) A polymorphism in the enhancer region of the thymidylate synthase promoter influences the survival of colorectal cancer patients treated with 5-fluorouracil. Br J Cancer 85:827–830
Martinez-Balibrea E, Manzano JL, Martinez-Cardus A, Moran T, Cirauqui B, Catot S, Taron M, Abad A (2007) Combined analysis of genetic polymorphisms in thymidylate synthase, uridine diphosphate glucoronosyltransferase and X-ray cross complementing factor 1 genes as a prognostic factor in advanced colorectal cancer patients treated with 5-fluorouracil plus oxaliplatin or irinotecan. Oncol Rep 17:637–645
Lecomte T, Ferraz JM, Zinzindohoue F, Loriot MA, Tregouet DA, Landi B, Berger A, Cugnenc PH, Jian R, Beaune P, Laurent-Puig P (2004) Thymidylate synthase gene polymorphism predicts toxicity in colorectal cancer patients receiving 5-fluorouracil-based chemotherapy. Clin Cancer Res 10:5880–5888
Ruzzo A, Graziano F, Loupakis F, Rulli E, Canestrari E, Santini D, Catalano V, Ficarelli R, Maltese P, Bisonni R, Masi G, Schiavon G, Giordani P, Giustini L, Falcone A, Tonini G, Silva R, Mattioli R, Floriani I, Magnani M (2007) Pharmacogenetic profiling in patients with advanced colorectal cancer treated with first-line FOLFOX-4 chemotherapy. J Clin Oncol 25:1247–1254
Dotor E, Cuatrecases M, Martinez-Iniesta M, Navarro M, Vilardell F, Guino E, Pareja L, Figueras A, Mollevi DG, Serrano T, de Oca J, Peinado MA, Moreno V, Germa JR, Capella G, Villanueva A (2006) Tumor thymidylate synthase 1494del6 genotype as a prognostic factor in colorectal cancer patients receiving fluorouracil-based adjuvant treatment. J Clin Oncol 24:1603–1611
Stoehlmacher J, Park DJ, Zhang W, Yang D, Groshen S, Zahedy S, Lenz HJ (2004) A multivariate analysis of genomic polymorphisms: prediction of clinical outcome to 5-FU/oxaliplatin combination chemotherapy in refractory colorectal cancer. Br J Cancer 91:344–354
Lu JW, Gao CM, Wu JZ, Cao HX, Tajima K, Feng JF (2006) Polymorphism in the 3′-untranslated region of the thymidylate synthase gene and sensitivity of stomach cancer to fluoropyrimidine-based chemotherapy. J Hum Genet 51:155–160
Kawakami K, Graziano F, Watanabe G, Ruzzo A, Santini D, Catalano V, Bisonni R, Arduini F, Bearzi I, Cascinu S, Muretto P, Perrone G, Rabitti C, Giustini L, Tonini G, Pizzagalli F, Magnani M (2005) Prognostic role of thymidylate synthase polymorphisms in gastric cancer patients treated with surgery and adjuvant chemotherapy. Clin Cancer Res 11:3778–3783
Marcuello E, Altes A, del Rio E, Cesar A, Menoyo A, Baiget M (2004) Single nucleotide polymorphism in the 5′ tandem repeat sequences of thymidylate synthase gene predicts for response to fluorouracil-based chemotherapy in advanced colorectal cancer patients. Int J Cancer 112:733–737
Marcuello E, Altes A, Menoyo A, Rio ED, Baiget M (2006) Methylenetetrahydrofolate reductase gene polymorphisms: genomic predictors of clinical response to fluoropyrimidine-based chemotherapy? Cancer Chemother Pharmacol 57:835–840
Lu JW, Gao CM, Wu JZ, Sun XF, Wang L, Feng JF (2004) Relationship of methylenetetrahydrofolate reductase C677T polymorphism and chemosensitivity to 5-fluorouracil in gastric carcinoma. Ai Zheng 23:958–962
Acknowledgments
This work was supported in part by a grant from the Scientific and Technologic Bureau of Wuxi (CLZ00612).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2008_815_MOESM1_ESM.pdf
Supplementary Fig. 1 Genotyping results of the TS and MTHFR polymorphisms. A: Electrophoresis results of the TSER and TS 3′-UTR PCR products; B: Sequencing-verified results for the representative genotypes of TSER and TS 3′-UTR polymorphisms; C: Electrophoresis results of PCR-LDR products for MTHFR; D: Sequencing-verified result for the representative PCR products of MTHFR (PDF 220 kb)
Rights and permissions
About this article
Cite this article
Huang, ZH., Hua, D. & Li, LH. The polymorphisms of TS and MTHFR predict survival of gastric cancer patients treated with fluorouracil-based adjuvant chemotherapy in Chinese population. Cancer Chemother Pharmacol 63, 911–918 (2009). https://doi.org/10.1007/s00280-008-0815-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0815-6