Abstract
It has been shown recently that donor and/or recipient cytomegalovirus (CMV) seropositivity is associated with a significant overall survival (OS) decline in acute leukemia patients who underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT). We now analyzed the prognostic impact of the donor/recipient CMV serostatus in 6968 patients with chronic hematological malignancies who underwent allo-HSCT. Donor and/or recipient CMV seropositivity was associated with a significantly reduced 2-year progression-free survival (PFS, 50% vs. 52%, p = 0.03) and OS (62% vs. 65%, p = 0.01). Multivariate Cox regression analyses showed an independent negative prognostic impact of donor and/or recipient CMV seropositivity on PFS (HR, 1.1; 95% CI, 1.0–1.2; p = 0.03), OS (HR, 1.1; 95% CI, 1.0–1.2; p = 0.003), and non-relapse mortality (HR, 1.2; 95% CI, 1.0–1.3; p = 0.02). OS decline was strongest for CMV-seropositive recipients with a CMV-seronegative donor (HR, 1.2; 95% CI, 1.1–1.3), followed by CMV-seropositive patients with a CMV-seropositive donor (HR, 1.1; 95% CI, 1.0–1.2). Conversely, OS did not differ significantly between CMV-seronegative recipients allografted from a CMV-seropositive donor (HR, 1.0; 95% CI, 0.9–1.2) and patients with donor/recipient CMV seronegativity (p = 0.001 for the four groups together). Non-relapse mortality was also significantly (p = 0.01) higher for CMV-seropositive patients with a CMV-seronegative graft (HR, 1.2; 95% CI, 1.1–1.4) than for CMV-seropositive patients with a CMV-seropositive graft (HR, 1.1; 95% CI, 0.9–1.2) or CMV-seronegative recipients with a CMV-seropositive graft (HR, 1.0; 95% CI, 0.8–1.2). Donor and/or recipient CMV seropositivity still results in an OS decline in patients with chronic hematological malignancies who have undergone allo-HSCT. However, this OS decline seems to be lower than that described for acute leukemia patients previously.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A recently published megafile analysis of the European Bone Marrow Transplantation (EBMT) group including 16,628 patients with acute leukemia who underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) between the years 1998 and 2009 showed that donor and/or recipient cytomegalovirus (CMV) seropositivity is still associated with a significant overall survival (OS) decline [1]. This OS decline was significantly stronger for patients with acute lymphoblastic leukemia (ALL, 9% at 2 years) than for those with acute myeloid leukemia (AML, 4% at 2 years) and mainly attributed to an increased non-relapse mortality (NRM).
The incidence of CMV disease has dramatically been reduced by the routine monitoring and preemptive treatment of CMV infection, albeit some recent reports suggest an increasing frequency of (mainly late) CMV disease of up to 20% [2,3,4,5,6]. Despite this, a recently published placebo-controlled study showed that letermovir prophylaxis reduced all-cause mortality at week 24 in patients after allo-HSCT although the incidence of CMV disease was low in both the placebo (1.8%) and in the letermovir arm (1.5%) [6]. Furthermore, it has been suggested recently that CMV replication in plasma detectable by PCR increases the NRM with evidence of a viral load-outcome relationship independently from preemptive treatment [5]. Taken together, these observations led to assume that CMV itself has negative effects besides CMV disease.
Negative consequences of CMV may also include side effects of antivirals and indirect effects. These may increase the risk of both bacterial and fungal infections and increase the NRM of patients with donor and/or recipient CMV seropositivity even in the absence of CMV infection or disease [7,8,9,10].
The situation is further complicated by the fact that CMV might also have favorable effects, i.e., it may reduce the relapse incidence (RI). This eventual “virus-vs.-malignancy” effect has been reported for patients with AML in some studies [11,12,13]. However, the association between CMV infection and RI remains controversial, in particular for patients with malignancies other than AML [14,15,16,17]. To get more insights into the prognostic impact of the donor/recipient CMV serostatus—including its impact on RI and graft-vs.-host disease (GvHD)—we analyzed 6968 patients who were allografted between the years 2005 and 2016.
Patients and methods
Study design, data collection, and criteria for patient selection
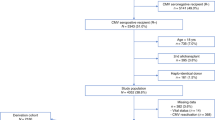
Patients with (B cell) chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), follicular lymphoma, mantle cell lymphoma, myelodysplastic syndrome (MDS), myeloproliferative neoplasm (MPN, diagnosed with either essential thrombocythemia, polycythemia vera, or primary myelofibrosis), multiple myeloma, and Philadelphia chromosome-positive chronic myeloid leukemia (Ph + CML) who underwent allo-HSCT between the years 2005 and 2016 and were documented in the database of the EBMT group were included into this retrospective analysis.
Only patients with a full data set available on both donor and recipient CMV serostatus, donor type (human leukocyte antigen [HLA]-matched related donor vs. another donor type), type of conditioning (myeloablative conditioning [MAC] vs. reduced-intensity conditioning [RIC]), remission status of the underlying malignancy at the time of allo-HSCT (complete remission [CR] vs. another remission status), stem cell source (peripheral blood [PB] vs. bone marrow [BM]), and use of in vivo and in vitro T cell depletion (TCD) were analyzed (n = 6968). Patients who received cord blood as stem cell source were excluded from this analysis, based on the fact that the donor CMV serostatus should be considered to be virtually exclusively CMV-seronegative in cord blood recipients [18, 19].
This study was performed in accordance with the principles of the Declaration of Helsinki and approved by the Infectious Diseases Working Party (IDWP) of the EBMT group.
Endpoints and definitions
The primary endpoint was defined to be progression-free survival (PFS) 2 years after allo-HSCT. Secondary endpoints included OS, NRM, RI, acute and chronic GvHD, and donor/recipient hematopoietic chimerism. PFS was considered to be survival without evidence of relapse or progression of the underlying malignancy. OS was considered to be the time from allo-HSCT to death, regardless of its cause. NRM was defined as death without evidence of relapse or progression. Response, relapse, and progression of the underlying malignancy were defined by standard criteria as used previously [20,21,22,23]. Acute and chronic GvHD were graded according to previously published criteria [24].
Statistical analyses
Patient main characteristics were reported as absolute frequencies (percentages) for categorical variables and medians (ranges) for continuous variables. Differences in the distribution between patient cohorts defined by the donor/recipient CMV serostatus were verified by using the chi-square or the Fisher exact test for categorical variables and the Anova or the Kruskal-Wallis test for continuous variables.
PFS and OS were estimated, together with their respective 95% confidence interval (CI), using the Kaplan-Meier method—testing the differences by the log-rank test. A Cox model was performed in order to estimate the impact of patient and donor CMV serostatus on PFS and OS.
NRM, RI, and the incidence of acute and chronic GvHD were estimated using the cumulative incidence method. Competing events included relapse or death due to relapse (for calculation of NRM), any death not due to relapse (for calculation of RI), and death of any cause (for calculation of the incidence of acute and chronic GvHD).
Differences between groups were verified by the Gray test. A cause-specific Cox model was performed in order to estimate the impact of patient and donor CMV serostatus. All the models have been adjusted for the main confounders taken into account: underlying disease, patient’s age, patient and donor sex, type of donor, conditioning, interval from diagnosis to allo-HSCT, remission status at allo-HSCT, stem cell source, use of TCD, country, and year of allo-HSCT.
Due to a potential distinct effect of the CMV serostatus according to the underlying disease (and other variables), we also investigated a possible interaction between the donor/recipient CMV serostatus and the underlying disease, the donor type and the type of conditioning, respectively. Furthermore, we studied a possible interaction between the donor type and the type of conditioning. Hereby, interaction analyses were done with respect to PFS, OS, NRM, and RI. Additionally, we evaluated the association between the day +100 donor/recipient hematopoietic chimerism and the CMV serostatus.
A p value < 0.05 was considered statistically significant. All the analyses were performed using the statistical software SAS v. 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
CMV-seronegative recipients (n = 2841) were mainly allografted from a CMV-seronegative donor (n = 2026, 71%), while CMV-seropositive donors (n = 2510, 61%) were prevailing among CMV-seropositive recipients (n = 4127) (p < 0.0001), reflecting the current strategy to match donor and recipient according to their CMV serostatus whenever possible [25, 26]. Different patient and transplant characteristics are summarized for the four categories defined by the donor/recipient CMV serostatus in Table 1.
In line with previous observations, the country had a significant impact on both recipient and donor CMV seropositivity (p < 0.0001) [27]. Hereby, Nordic and “other countries” had a higher frequency of recipient and donor seropositivity compared to the Netherlands/Belgium, France, UK, and Germany (Table 1).
Univariate analyses
The estimated PFS, OS, NRM, RI, and incidence of GvHD at different time points after allo-HSCT are shown for the entire population in Table S1. Donor and/or recipient CMV seropositivity (vs. CMV seronegativity of both) was associated with a significantly reduced PFS and OS (Table 2). When analyzing separately the four groups defined by the donor/recipient CMV serostatus, the OS decline was strongest for CMV-seropositive recipients allografted from a CMV-seronegative donor (HR, 1.2; 95% CI, 1.1–1.3), followed by CMV-seropositive patients with a CMV-seropositive donor (HR, 1.1; 95% CI, 1.0–1.2). OS did not differ significantly between CMV-seronegative patients who had a CMV-seropositive donor (HR, 1.0; 95% CI, 0.9–1.2) and those with donor/recipient CMV seronegativity (Table 2, Fig. 1).
The OS decline of CMV-seropositive recipients allografted from a CMV-seronegative donor was accompanied by a significantly increased NRM (HR, 1.2; 95% CI, 1.1–1.4). CMV-seropositive patients who had a CMV-seropositive donor had only a slightly higher NRM (HR, 1.1; 95% CI, 0.9–1.2) than patients with only donor CMV seropositivity (HR, 1.0; 95% CI, 0.8–1.2) or patients with donor/recipient CMV seronegativity (HR, 1.0). The CMV serostatus had no significant impact on RI and GvHD, neither when analyzing donor/recipient-seronegative patients vs. the remaining patients nor when comparing all four groups together (Table 2).
Likewise, the donor/recipient CMV serostatus had no significant impact on the development of donor/recipient hematopoietic chimerism and the median time to engraftment of polymorphonuclear leukocytes (data not shown). Causes of death mainly included relapse/disease progression of the underlying malignancy, GvHD, and infection (Table S2).
Multivariate and interaction analyses
Multivariate Cox regression analyses revealed an independent negative prognostic impact of donor and/or recipient CMV seropositivity regarding PFS (HR, 1.1; 95% CI, 1.0–1.2; p = 0.03), OS (HR, 1.1; 95% CI, 1.0–1.2; p = 0.003), and NRM (HR, 1.2; 95% CI, 1.0–1.3; p = 0.02) (Table 3). In contrast, donor and/or recipient CMV seropositivity had no significant impact on RI (HR, 1.1; 95% CI, 1.0–1.2) and acute or chronic GvHD (HR, 1.0; 95% CI, 0.9–1.1 and HR, 1.0; 95% CI, 0.9–1.0, respectively) in multivariate Cox regression analyses. Furthermore, donor CMV seropositivity had no significant impact on OS when analyzing patients with CMV seropositivity (n = 4127; HR, 1.0; 95% CI, 0.9–1.1; p = 0.6) vs. CMV seronegativity (n = 2841; HR, 1.1; 95% CI, 1.0–1.2; p = 0.3) in separate multivariate analyses.
Interaction analyses did not show a significant association between the donor/recipient CMV serostatus and the underlying disease, the donor type, and the type of conditioning with respect to PFS, OS, NRM, and RI. Furthermore, there was no significant interaction between the donor type and the type of conditioning.
Discussion
Donor and/or recipient CMV seropositivity led to a significant OS decline after allo-HSCT in different recent studies focused to patients with acute leukemia, aplastic anemia or multiple myeloma [1, 20, 28].
We studied a large cohort of 6968 patients with chronic hematological malignancies who underwent allo-HSCT using PB or BM as stem cell source. We found that donor and/or recipient CMV seropositivity was associated with a moderate, but significant 2-year PFS and OS decline of around 2 and 3%, respectively. This PFS and OS decline was confirmed in multivariate Cox regression analyses indicating that donor and/or recipient CMV seropositivity still confers an independent negative prognostic impact in patients allografted in the year 2005 or beyond despite the continuous development of novel strategies to prevent and treat CMV [29, 30]. However, our findings led also to assume that the negative prognostic impact of donor and/or recipient CMV seropositivity is lower in patients with chronic hematological malignancies than in patients with acute leukemia for whom a 2-year OS decline of 9% for ALL and 4% for AML was demonstrated recently [1].
We did not observe a significant impact of the donor CMV serostatus among CMV-seropositive vs. CMV-seronegative patients in multivariate analyses. Furthermore, interaction analyses did not show a significant association between the donor/recipient CMV serostatus and different parameters such as the donor type and the type of conditioning.
Contrasting our findings, a recently published EBMT megafile analysis studying the impact of the donor CMV serostatus in 49542 patients after allo-HSCT reported a negative impact of serostatus discrepancy in case of unrelated donor: reduced OS for CMV-seronegative recipients with a CMV-seropositive donor (vs. a CMV-seronegative donor) and improved OS for patients with both donor and recipient CMV seropositivity (vs. only recipient CMV seropositivity) if they received MAC [26]. Smaller patient number in our study might be the reason for the differences between this and the present EBMT megafile analysis. Additionally, donor CMV seronegativity vs. seropositivity has been associated with several negative effects in CMV-seropositive recipients such as a delayed CMV-specific immune reconstitution, a higher peak virus load or a prolonged duration of CMV infections [31,32,33]. Therefore, we still recommend to a match donor and recipient according to their CMV serostatus, albeit other criteria for donor search might be more important, particular if the recipient is CMV-seronegative.
Donor and/or recipient CMV seropositivity had no significant impact on RI. Since recipient CMV seropositivity is one of the most important risk factors for CMV infection and disease, this observation argues against a clinically relevant “virus-vs.-malignancy” effect in patients with chronic hematological malignancies which has been suggested for patients with AML [11,12,13].
We did not find a significant impact of the donor/recipient CMV serostatus on the incidence of acute or chronic GvHD, neither in univariate nor in multivariate analysis. The association between the CMV serostatus and GvHD remains incompletely understood. Some previous studies found an increased incidence of acute GvHD in the case of recipient CMV seropositivity [7, 34,35,36,37]. This association is commonly explained by the suggestion that CMV and GvHD trigger each other, besides an additional risk of acute GvHD due to the frequently tapered immunosuppressives after detection of CMV [1, 37]. However, other studies failed to describe any association between GvHD and the CMV serostatus or even reported a decreased acute GvHD incidence for CMV-seropositive recipients [1, 38, 39]. This association has at least partly been attributed to confounding factors such as an increased early death rate of CMV-seropositive recipients.
Our multivariate analyses further showed that a diagnosis of MDS, MPN, or multiple myeloma (vs. CLL/SLL) in addition to recipient’s male sex, increasing recipient’s age, a donor other than an HLA-matched related donor, MAC, a remission status other than CR prior to allo-HSCT, use of alemtuzumab-based TCD, and allografting in the Netherlands/Belgium, France, and “other country” (vs. the UK) are all associated with an inferior OS. The year of transplant had also a significant impact on OS if using it as a continuous variable with a more favorable outcome of patients allografted in recent years.
Our data show that donor and/or recipient CMV seropositivity in patients with chronic hematological malignancies who underwent allo-HSCT is still associated with a significant OS decline. However, this OS decline seems to be lower than that described for patients with acute leukemia. Despite the fact that the negative impact of donor CMV seronegativity in the case of recipient CMV seropositivity was less evident in the present megafile analysis than previously described, we still recommend to use a CMV-seropositive donor for a CMV-seropositive recipient whenever possible.
References
Schmidt-Hieber M, Labopin M, Beelen D, Volin L, Ehninger G, Finke J, Socié G, Schwerdtfeger R, Kröger N, Ganser A, Niederwieser D, Polge E, Blau IW, Mohty M (2013) CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood 122(19):3359–3364. https://doi.org/10.1182/blood-2013-05-499830
Jang JE, Hyun SY, Kim YD, Yoon SH, Hwang DY, Kim SJ, Kim Y, Kim JS, Cheong JW, Min YH (2012) Risk factors for progression from cytomegalovirus viremia to cytomegalovirus disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 18(6):881–886. https://doi.org/10.1016/j.bbmt.2011.10.037
Marty FM, Winston DJ, Rowley SD, Vance E, Papanicolaou GA, Mullane KM, Brundage TM, Robertson AT, Godkin S, Momméja-Marin H, Boeckh M (2013) CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med 369(13):1227–1236. https://doi.org/10.1056/NEJMoa1303688
Chemaly RF, Ullmann AJ, Stoelben S, Richard MP, Bornhäuser M, Groth C, Einsele H, Silverman M, Mullane KM, Brown J, Nowak H, Kölling K, Stobernack HP, Lischka P, Zimmermann H, Rübsamen-Schaeff H, Champlin RE, Ehninger G (2014) Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med 370(19):1781–1789. https://doi.org/10.1056/NEJMoa1309533
Green ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, Sorror ML, Goyal S, Özkök S, Yi J, Sahoo F, Kimball LE, Jerome KR, Marks MA, Boeckh M (2016) Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol 3(3):27–e127. https://doi.org/10.1016/S2352-3026(15)00289-6
Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, Haider S, Ullmann AJ, Katayama Y, Brown J, Mullane KM, Boeckh M, Blumberg EA, Einsele H, Snydman DR, Kanda Y, DiNubile MJ, Teal VL, Wan H, Murata Y, Kartsonis NA, Leavitt RY, Badshah C (2017) Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 377(25):2433–2444. https://doi.org/10.1056/NEJMoa1706640
Broers AE, van der Holt R, van Esser JW, Gratama JW, Henzen-Logmans S, Kuenen-Boumeester V, Löwenberg B, Cornelissen JJ (2000) Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood 95(7):2240–2245
Kröger N, Zabelina T, Krüger W, Renges H, Stute N, Schrum J, Kabisch H, Schafhausen P, Jaburg N, Löliger C, Schäfer P, Hinke A, Zander AR (2001) Patient cytomegalovirus seropositivity with or without reactivation is the most important prognostic factor for survival and treatment-related mortality in stem cell transplantation from unrelated donors using pretransplant in vivo T-cell depletion with anti-thymocyte globulin. Br J Haematol 113(4):1060–1071
Nichols WG, Corey L, Gooley T, Davis C, Boeckh M (2002) High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis 185(3):273–282. https://doi.org/10.1086/338624
Yong MK, Ananda-Rajah M, Cameron PU, Morrissey CO, Spencer A, Ritchie D, Cheng AC, Lewin SR, Slavin M (2017) Cytomegalovirus reactivation is associated with increased risk of late-onset invasive fungal disease after allogeneic hematopoietic stem cell transplantation: a multicenter study in the current era of viral load monitoring. Biol Blood Marrow Transplant 23(11):1961–1967. https://doi.org/10.1016/j.bbmt.2017.07.025
Lönnqvist B, Ringdèn O, Ljungman P, Wahren B, Gahrton G (1986) Reduced risk of recurrent leukaemia in bone marrow transplant recipients after cytomegalovirus infection. Br J Haematol 63(4):671–679
Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, Christoph S, Gromke T, Kordelas L, Ottinger HD, Ross RS, Horn PA, Schnittger S, Beelen DW (2011) Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 118(5):1402–1412. https://doi.org/10.1182/blood-2010-08-304121
Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM, Riddell SR, Boeckh M (2013) CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood 122(7):1316–1324. https://doi.org/10.1182/blood-2013-02-487074
George B, Pati N, Gilroy N, Ratnamohan M, Huang G, Kerridge I, Hertzberg M, Gottlieb D, Bradstock K (2010) Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl Infect Dis 12(4):322–329. https://doi.org/10.1111/j.1399-3062.2010.00504.x
Ljungman P (2013) CMV: a warrior against leukemia? Blood 122(7):1101–1102. https://doi.org/10.1182/blood-2013-06-508515
Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, Green JS, Saad A, Antin JH, Savani BN, Lazarus HM, Seftel M, Saber W, Marks D, Aljurf M, Norkin M, Wingard JR, Lindemans CA, Boeckh M, Riches ML, Auletta JJ (2016) Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood 127(20):2427–2438. https://doi.org/10.1182/blood-2015-11-679639
Koldehoff M, Ross SR, Dührsen U, Beelen DW, Elmaagacli AH (2017) Early CMV-replication after allogeneic stem cell transplantation is associated with a reduced relapse risk in lymphoma. Leuk Lymphoma 58(4):822–833. https://doi.org/10.1080/10428194.2016.1217524
Albano MS, Taylor P, Pass RF, Scaradavou A, Ciubotariu R, Carrier C, Dobrila L, Rubinstein P, Stevens CE (2006) Umbilical cord blood transplantation and cytomegalovirus: posttransplantation infection and donor screening. Blood 108(13):4275–4282. https://doi.org/10.1182/blood-2006-04-020313
Mikulska M, Raiola AM, Bruzzi P, Varaldo R, Annunziata S, Lamparelli T, Frassoni F, Tedone E, Galano B, Bacigalupo A, Viscoli C (2012) CMV infection after transplant from cord blood compared to other alternative donors: the importance of donor-negative CMV serostatus. Biol Blood Marrow Transplant 18(1):92–99. https://doi.org/10.1016/j.bbmt.2011.05.015
Auner HW, Szydlo R, van Biezen A, Iacobelli S, Gahrton G, Milpied N, Volin L, Janssen J, Nguyen Quoc S, Michallet M, Schoemans H, El Cheikh J, Petersen E, Guilhot F, Schönland S, Ahlberg L, Morris C, Garderet L, de Witte T, Kröger N (2013) Reduced intensity-conditioned allogeneic stem cell transplantation for multiple myeloma relapsing or progressing after autologous transplantation: a study by the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 48(11):1395–1400. https://doi.org/10.1038/bmt.2013.73
Stern M, de Wreede LC, Brand R, van Biezen A, Dreger P, Mohty M, de Witte TM, Kröger N, Ruutu T (2014) Sensitivity of hematological malignancies to graft-versus-host effects: an EBMT megafile analysis. Leukemia 28(11):2235–2240. https://doi.org/10.1038/leu.2014.145
Robinson SP, Boumendil A, Finel H, Schouten H, Ehninger G, Maertens J, Crawley C, Rambaldi A, Russell N, Anders W, Blaise D, Yakoub-Agha I, Ganser A, Castagna L, Volin L, Cahn JY, Montoto S, Dreger P (2016) Reduced intensity allogeneic stem cell transplantation for follicular lymphoma relapsing after an autologous transplant achieves durable long term disease control: an analysis from the Lymphoma Working Party of the EBMT. Ann Oncol 27:1088–1094. https://doi.org/10.1093/annonc/mdw124
Martino R, Henseler A, van Lint M, Schaap N, Finke J, Beelen D, Vigouroux S, Alessandrino EP, Mufti GJ, Veelken JH, Bruno B, Yakoub-Agha I, Volin L, Maertens J, Or R, Leblond V, Rovira M, Kalhs P, Alvarez AF, Vitek A, Sierra J, Wagner E, Robin M, de Witte T, Kröger N (2017) Long-term follow-up of a retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic transplantation from matched related donors in myelodysplastic syndromes. Bone Marrow Transplant 52(8):1107–1112. https://doi.org/10.1038/bmt.2017.19
Sullivan KM (1999) Graft-versus-host-disease. In: Thomas E, Blume K, Forman SJ (eds) Hematopoietic Cell Transplantation, 2nd edn. Blackwell Science, Boston, MA, pp 515–526
Ljungman P (2014) The role of cytomegalovirus serostatus on outcome of hematopoietic stem cell transplantation. Curr Opin Hematol 21(6):466–469. https://doi.org/10.1097/MOH.0000000000000085
Ljungman P, Brand R, Hoek J, de La CR, Cordonnier C, Einsele H, Styczynski J, Ward KN, Cesaro S (2014) Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis 59(4):473–481. https://doi.org/10.1093/cid/ciu364
Ljungman P, Brandan R (2007) Factors influencing cytomegalovirus seropositivity in stem cell transplant patients and donors. Haematologica 92(8):1139–1142
Bacigalupo A, Socié G, Hamladji RM, Aljurf M, Maschan A, Kyrcz-Krzemien S, Cybicka A, Sengelov H, Unal A, Beelen D, Locasciulli A, Dufour C, Passweg JR, Oneto R, Signori A, Marsh JCW (2015) Current outcome of HLA identical sibling versus unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica 100(5):696–702. https://doi.org/10.3324/haematol.2014.115345
Nishihori T, Shaheen M, El-Asmar J, Aljurf M, Kharfan-Dabaja MA (2015) Therapeutic strategies for cytomegalovirus in allogeneic hematopoietic cell transplantation. Immunotherapy 7(10):1059–1071. https://doi.org/10.2217/imt.15.70
Maffini E, Giaccone L, Festuccia M, Brunello L, Busca A, Bruno B (2016) Treatment of CMV infection after allogeneic hematopoietic stem cell transplantation. Expert Rev Hematol 9(6):585–596. https://doi.org/10.1080/17474086.2016.1174571
Gor D, Sabin C, Prentice HG, Vyas N, Man S, Griffiths PD, Emery VC (1998) Longitudinal fluctuations in cytomegalovirus load in bone marrow transplant patients: relationship between peak virus load, donor/recipient serostatus, acute GVHD and CMV disease. Bone Marrow Transplant 21(6):597–605. https://doi.org/10.1038/sj.bmt.1701139
Ganepola S, Gentilini C, Hilbers U, Lange T, Rieger K, Hofmann J, Maier M, Liebert UG, Niederwieser D, Engelmann E, Heilbronn R, Thiel E, Uharek L (2007) Patients at high risk for CMV infection and disease show delayed CD8+ T-cell immune recovery after allogeneic stem cell transplantation. Bone Marrow Transplant 39:293–299
van der Heiden PLJ, van Egmond HM, Veld SAJ, van de Meent M, Eefting M, de Wreede LC, Halkes CJM, Falkenburg JHF, Marijt WAF, Jedema I (2018) CMV seronegative donors: effect on clinical severity of CMV infection and reconstitution of CMV-specific immunity. Transpl Immunol 49:54–58. https://doi.org/10.1016/j.trim.2018.04.003
Verduyn Lunel FM, Raymakers R, van Dijk A, van der Wagen L, Minnema MC, Kuball J (2016) Cytomegalovirus status and the outcome of T cell-replete reduced-intensity allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 22(10):1883–1887. https://doi.org/10.1016/j.bbmt.2016.07.009
Ringdén O, Paulin T, Lönnqvist B, Nilsson B (1985) An analysis of factors predisposing to chronic graft-versus-host disease. Exp Hematol 13(10):1062–1067
Miller W, Flynn P, McCullough J, Balfour HH, Goldman A, Haake R, McGlave P, Ramsay N, Kersey J (1986) Cytomegalovirus infection after bone marrow transplantation: an association with acute graft-v-host disease. Blood 67(4):1162–1167
Boeckh M, Nichols WG (2004) The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 103(6):2003–2008. https://doi.org/10.1182/blood-2003-10-3616
Yakoub-Agha I, Mesnil F, Kuentz M, Boiron JM, Ifrah N, Milpied N, Chehata S, Esperou H, Vernant JP, Michallet M, Buzyn A, Gratecos N, Cahn JY, Bourhis JH, Chir Z, Raffoux C, Socié G, Golmard JL, Jouet JP (2006) Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol 24(36):5695–5702. https://doi.org/10.1200/JCO.2006.08.0952
Hahn T, McCarthy PL, Zhang M-J, Wang D, Arora M, Frangoul H, Gale RP, Hale GA, Horan J, Isola L, Maziarz RT, van Rood JJ, Gupta V, Halter J, Reddy V, Tiberghien P, Litzow M, Anasetti C, Pavletic S, Ringdén O (2008) Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol 26(35):5728–5734. https://doi.org/10.1200/JCO.2008.17.6545
Author information
Authors and Affiliations
Contributions
M.S.H., G.T., P.L., M.Mik., and J.S. designed the research. M.S.H., G.T., P.L., M.Mik., N.K., D.B., G.S., L.V., N.B., N.F., I.Y.A., E.F., J.M, P.C., J.P., J.C., N.R., C.C., J.H.B., T.M., P.R., J.Y.C., M.Mic., S.M., N.K., B.G., and J.S. provided important clinical data and/or performed statistical analyses. M.S.H. wrote the first draft of the manuscript. All authors approved the final version of the manuscript. A complete list of contributors appears in the online data supplement.
The authors thank all allogeneic transplantation centers of the European Bone Marrow Transplantation group for reporting the data to this registry.
Corresponding author
Ethics declarations
Conflict of interest
I.Y.A received honorarium from Biotest and MSD Sharp & Dohme GmbH that commercialize anti-CMV drugs. The other authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
According to EBMT policy, patients give informed consent for data reporting to the EBMT registry.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 44.8kb)
Rights and permissions
About this article
Cite this article
Schmidt-Hieber, M., Tridello, G., Ljungman, P. et al. The prognostic impact of the cytomegalovirus serostatus in patients with chronic hematological malignancies after allogeneic hematopoietic stem cell transplantation: a report from the Infectious Diseases Working Party of EBMT. Ann Hematol 98, 1755–1763 (2019). https://doi.org/10.1007/s00277-019-03669-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03669-z