Abstract
Prognosis of AML in elderly patients is poor due to adverse patient characteristics and comorbidities. In addition, disease-associated parameters reveal differences between older and younger patients with AML. Survival in normal karyotype AML (NK-AML) is influenced by different clinical and molecular markers. The aim of this work was to investigate the frequencies of molecular markers in patients with NK-AML with a focus on NPM1 mutations and FLT3-ITD in different age groups. In the present study, we analyzed the frequencies of mutations of NPM1 and FLT3-ITD in a cohort of 1,321 adult patients and 148 children with AML treated within the AMLCG99, the AML98, and AML04 trials and their distribution in different age groups. Additionally, the frequencies of mutations in CEBPA genes, FLT3-TKD, and MLL-PTD were analyzed in the cohort with NK-AML (n = 729). Our data show that the presence of mutations of NPM1 (from 60% to 40%) and FLT3-ITD (from 50% to 20%) significantly decreased with age in adult AML. Consequently, the proportion of NPM1−/FLT3-ITD− patients increased with age. The decreasing frequency of NPM1 mutations in elderly patients was paralleled by a reduced complete remission (CR) rate in the elderly of 55% compared to 80% in the younger patients. By contrast, the frequencies of other gene mutations, like FLT3-TKD and MLL-PTD, and mutations in CEBPA were not age-dependent. The decreasing frequency of the favorable NPM1 mutations with increasing age may partially explain the worse outcome in the elderly patients. Furthermore, the increasing amount of elderly patients without NPM1 mutations or FLT3-ITD suggests that other molecular and clinical risk factors may influence prognosis in this age group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prognosis in elderly patients with AML is poor. Besides adverse patient characteristics, such as age [1] and comorbidities, this is partly due to an age-dependent increase in the number of patients with an unfavorable complex-aberrant karyotype and unbalanced translocations, whereas the incidence of patients with a normal karyotype AML (NK-AML) moderately increases with age. The favorable prognostic groups with the balanced translocations t(8;21)(q22;q22), inv(16)(p13;q22) or t(15;17)(q22;q11-12) remain almost stable in different age groups [2].
In this work, we focused on patients with a normal karyotype. Long-term survival in NK-AML is influenced by different clinical and molecular markers. Whereas the presence of an NPM1 mutation (NPM1+) [3, 4] is associated with a positive prognostic effect on long-term outcome, the presence of an FLT3-internal tandem duplication (FLT3-ITD+) has a negative impact on survival. Interestingly, a significant interaction between NPM1 and FLT3-ITD has been shown. In adults, the positive prognostic impact on clinical outcome was evident predominantly in patients with NK-AML carrying NPM1 gene mutations when FLT3-ITD was absent. In contrast, the survival in all other groups of combinations between wildtype or mutated NPM1 and FLT3-ITD or wildtype FLT3 was not different so far. In children with NK-AML, the positive prognostic impact of an NPM1 mutation on EFS and OS was seen independent of the FLT3-ITD mutation status [5, 6]. A clinical parameter with negative impact on all outcome parameters [overall survival (OS), event-free survival (EFS), relapse-free survival (RFS), and the rate of complete remission (CR)] is patient age at diagnosis. Certainly, the worse prognosis in elderly patients is due to adverse patient characteristics and comorbidities. Nevertheless, also disease-associated parameters reveal differences between older and younger patients with AML. Therefore, we investigated the frequencies of molecular markers in patients with NK-AML with a focus on NPM1 mutations and FLT3-ITD in different age groups.
Methods
Patients and samples
Analyses were based on 1,321 patients treated in the AMLCG (German AML Cooperative Group) 1999 trial until January 2006 and 148 children with AML who were treated within different AML trials (AML98, AML04). Patients gave their informed consent prior to the study. The studies have been approved by the ethics committees. Inclusion criteria, treatment protocols, and patient outcome in this study have been published [7, 8].
Molecular analyses
Cytomorphology:
Bone marrow cells underwent staining with May–Grünwald–Giemsa, myeloperoxidase, and nonspecific esterase [9] and were then classified according to the French–American–British (FAB) criteria [10, 11].
Cytogenetics and fluorescent in situ hybridization analyses:
Chromosomal and fluorescent in situ hybridization analyses were performed according to standard protocols [12–16].
Molecular genetics:
Mutation analyses of NPM1, FLT3-ITD, FLT3-TKD, and MLL-PTD were [12–16] performed according to standard protocols previously described [17]. After screening for the NPM1 mutation by melting curve analyses, the NPM1 mutation was confirmed by nucleotide sequencing [3]. CEBPA mutational screening was performed using a multiplex PCR-based fragment length analysis followed by nucleotide sequencing [18]. In the total cohort of 1,469 patients, 1,244 and 1,450 samples were analyzed for NPM1 mutations and FLT3-ITD, respectively. In all 729 NK-AML patients, FLT3-ITD and NPM1 mutation status were known. FLT3-TKD, CEBPA mutations, and MLL-PTD were analyzed in 619, 665, and 673 patient samples, respectively. In children with NK-AML, molecular analyses of NPM1 and FLT3-ITD were performed in all patients, whereas molecular analyses of CEBPA mutations and MLL-PTD were available for minor proportions of pediatric patients only (≤50%). FLT3-TKD were only accessible in three children.
Statistical analyses:
Patients were divided into eight age groups [group 1, 0–9 years (n all = 64, n NK-AML = 16); group 2, 10–19 years (n all = 92, n NK-AML = 28); group 3, 20–29 years (n all = 49, n NK-AML = 21); group 4, 30–39 years (n all = 105, n NK-AML = 56); group 5, 40–49 years (n all = 198, n NK-AML = 107); group 6, 50–59 years (n all = 258, n NK-AML = 140), group 7, 60–69 years (n all = 449, n NK-AML = 238); group 8, ≥70 years (n all = 254, n NK-AML = 123)]. The frequency and comparison of all molecular markers as well as the four NPM1 and FLT3-ITD combinations were calculated in cross tables (Pearson’s chi-square test) in the different age groups. In addition to the frequency of molecular markers, we analyzed the rate of complete remissions in the different age groups. The Mann–Whitney U test was applied for the analyses of the differences of median age between NPM1+ and NPM1− patients.
Results
Patient characteristics
In the entire patient cohort of 1,469 AML patients consisting of 1,321 adults and 148 children of all karyotypes, median age was 59 years (0–85 years) (Supplementary Figure 1). Median age in the children cohort was 10 years (0.06–19 years). Three patients were ≥18 years and treated according to children AML protocols. Median age in the adult cohort was 61 years (10–85 years). Four patients treated according to the adult protocols were younger than 18 years. In adult patients and children, 57.5% and 35.3%, respectively, had AML with a normal karyotype.
Median age in the 729 patients with NK-AML was 59 years (0–85 years), 10 years (0–18 years) in 41 children, and 60 years (17–85 years) in 688 adults. NPM1 mutations were significantly more frequent in adults (51.0%; 351/688) compared to children (26.8%; 11/41). Distribution of FLT3-ITD was 28.3% (195/688) in adults and 41.5% (17/41) in children (p = 0.072).
Frequency of NPM1 mutations and FLT3-ITD in 1,469 patients with all karyotypes
In 1,469 patients, the NPM1 and FLT3 mutation statuses were available in 1,244 and 1,450 cases.
NPM1 mutations occurred in 40.4% (502/1,244), predominantly in adults (44.3% (486/1,096) adult cohort vs. 10.8% (16/148) in children; p < 0.001).
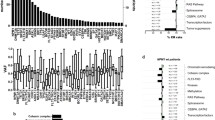
In the eight age groups, we found the lowest frequencies of mutated NPM1 in children between 0 and 9 years (7.9%, 5/63) and 10 and 19 years (13.5%, 23/89). Between 30–59 years, more than 50% (53.1%, 253/476) of patients carried an NPM1 mutation. Interestingly, the frequency of an NPM1 mutation decreased to 37.3% (216/579) in patients ≥60 years (60–69 years, 38.3%, 139/363; ≥70 years, 35.6%, 77/216; Fig. 1a).
Frequencies of NPM1 mutations in different age groups. A in patients with different karyotypes (N = 1,244); B in patients with NK-AML (N = 729). A Frequency of NPM1 mutations in 1,244 patients including children AML and complex and NK-AML. Patients were divided into eight age groups: 0–9 years (n = 63), 10–19 years (n = 89), 20–29 years (n = 37), 30–39 years (n = 86), 40–49 years (n = 167), 50–59 years (n = 223), 60–69 years (n = 363), ≥70 years (n = 216). Frequencies of NPM1 mutations were calculated in cross tables (Pearson chi square): 7.9% (0–9 years), 13.5% (10–19 years), 43.2% (20–29 years), 53.3% (30–39 years), 54.5% (40–49 years), 52.0% (50–59 years), 38.3% (60–69 years), and 35.6% (≥70 years). B Frequencies of NPM1 mutations in 729 patients with NK-AML. Patients were divided into eight age groups: 0–9 years (n = 16), 10–19 years (n = 28), 20–29 years (n = 21), 30–39 years (n = 56), 40–49 years (n = 107), 50–59 years (n = 140), 60–69 years (n = 238), ≥70 years (n = 123). Frequencies of NPM1 mutations were calculated in cross tables (Pearson chi square). NPM1+ Presence of an NPM1 mutation. The analyses were performed in cross tables (Pearson chi square).
The overall frequency of an FLT3-ITD was 24.3% (353/1,450), detected in 24.9% (327/1,314) of adult patients and 19.1% (26/136) of children. The lowest frequency of an FLT3-ITD (8.3%, 5/60) was found in 60 children between 0 and 9 years, whereas 84 patients between 10 and 19 years showed a frequency of an FLT3-ITD of 27.4% (23/84). The rates of FLT3-ITD positivity in patients <20 years (19.3%; 28/145) and ≥60 years (20.7%; 145/701) were comparably low in contrast to patients between 20 and 59 years in which an FLT3-ITD could be detected in up to 35% (20–29 years, 34.7%, 17/49; 30–39 years, 34.6%, 36/104; 40–49 years, 29.2%, 57/195; 50–59 years, 27.2%, 70/257; Fig. 2a).
Frequencies of FLT3 mutations in different age groups. A in patients with different karyotpyes (N = 1,450); B in patients with NK-AML (N = 729). A Frequency of FLT3-ITD in 1,450 patients including children AML and complex and NK-AML. Patients were divided into eight age groups: 0–9 years (n = 60), 10–19 years (n = 84), 20–29 years (n = 49), 30–39 years (n = 104), 40–49 years (n = 195), 50–59 years (n = 257), 60–69 years (n = 447), ≥70 years (n = 254). Frequencies of FLT-ITD were calculated in cross tables (Pearson chi square): 8.3% (0–9 years), 27.4% (10–19 years), 34.7% (20–29 years), 34.6% (30–39 years), 29.2% (40–49 years), 27.2% (50–59 years), 21.0% (60–69 years), and 20.1% (≥70 years). B Frequencies of FLT3-ITD and FLT3-TKD in 729 patients with NK-AML. Patients were divided into eight age groups: 0–9 years (n = 16), 10–19 years (n = 28), 20–29 years (n = 21), 30–39 years (n = 56), 40–49 years (n = 107), 50–59 years (n = 140), 60–69 years (n = 238), ≥70 years (n = 123). Frequencies of FLT3-ITD and FLT3-TKD were calculated in cross tables (Pearson chi square). FLT3-TKD was not available for pediatric patients. FLT3-ITD+ Presence of an FLT3-ITD (ITD internal tandem duplication) mutation. FLT3-TKD+ Presence of an FLT3-TKD (TKD tyrosine kinase domain) mutation. The analyses were performed in cross tables (Pearson chi square).
Frequency of NPM1 mutations and FLT3-ITD in 729 patients with NK-AML
In 729 patients with available mutation statuses of both NPM1 and FLT3-ITD, we found a significant decrease in the frequency of mutations in NPM1 and FLT3-ITD with higher age.
The lowest frequency of mutated NPM1 was seen in children between 0 and 9 years (12.5%) and between 10 and 19 years (35.7%). In the adult cohort, the frequency of mutated NPM1 was relatively constant in the patient cohorts up to 60 years of age but decreased abruptly from 60.0% in patients aged between 50 and 59 years to 42.4% (153/361) in patients ≥60 years. (Fig. 1b, Table 1). The median age of the NPM1+ versus the NPM1− patients was significantly different in the Mann–Whitney U test [56 years (5–85 years) versus 62 years (0–83 years); p = 0.004].
Similarly to NPM1, an FLT3-ITD was a rare event in children between (0 and 9 years). The frequency of an FLT3-ITD peaked in children between 10 and 19 years and decreased continuously with increasing age from 57.1% in patients between 10 and 19 years of age to 19.5% in elderly patients ≥70 years (<0.001). The median age in FLT3-ITD positive patients was significantly lower compared to patients with wildtype FLT3 (55 vs. 61 years; p < 0.001). The frequency of an MLL-PTD showed a trend to an increase with age, whereas the frequency of the other molecular markers FLT3-TKD and mutated CEBPA in the different age groups did not differ significantly (Fig. 2b, Table 1).
Distribution of the NPM1/FLT3-ITD genotypes in different age decades in 729 patients with NK-AML
An association between NPM1 mutations and FLT3-ITD has previously been described by different authors [5, 19, 20]. Patients carrying an NPM1 mutation without an FLT3-ITD achieved an improved long-term outcome in comparison to the non-NPM1+/FLT3-ITD− cohort. We investigated the distribution of the different NPM1/FLT3-ITD combinations in different age groups. The majority of children between 0 and 9 years (68.8%) lacked mutations of NPM1 and/or FLT3-ITD. In contrast, only one third of children between 10 and 19 years lacked both mutation markers, one third showed an isolated FLT3-ITD and one third displayed the NPM1+/FLT3-ITD+ genotype. In adults, we found a significant relative increase of NPM1−/FLT3-ITD− patients from 23.8% in the youngest adult patient cohort (n = 20–29 years) to 50.4% in patients aged 70–85 years (p < 0.001). In analogy to the abrupt decrease of the frequency of an NPM1 mutation in patients older than 60 years, we found a strong increase in the NPM1−/FLT3-ITD− genotype at the age of 60 years from 31.4% in patients aged 50–59 years to 49.6% in patients aged 60–69 years. With the exception of children, the percentage of patients with a NPM1+/FLT3-ITD+ genotype decreased continuously with advancing age (p = 0.042). In the eight age groups (0–9, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years), the proportion of the favorable prognostic group of NPM1+/FLT3-ITD− patients was lowest in children with AML, then showed an increase up to 49 years and a decrease afterwards (p = 0.007). Nevertheless, comparing the presence of the favorable prognostic genotype NPM1+/FLT3-ITD− only in adult patients (six age groups: 20–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years), the difference did not reach a statistical significance (chi-square test) (Table 2, Fig. 3).
Distribution of the four NPM1/FLT3-ITD subgroups in different age groups in 729 patients with NK-AML. Patients were divided into eight age groups: 0–9 years (n = 16), 10–19 years (n = 28), 20–29 years (n = 21), 30–39 years (n = 56), 40–49 years (n = 107), 50–59 years (n = 140), 60–69 years (n = 238), ≥70 years (n = 123). Frequencies of the NPM1/FLT3-ITD subgroups were calculated in cross tables (Pearson chi square). NPM1+ Presence of an NPM1 mutation. FLT3-ITD+ Presence of an FLT3-ITD (ITD internal tandem duplication).
The decreased frequency of an NPM1 mutation in older patients is associated with a reduced CR rate
An important parameter to measure early treatment response of AML is the achievement of a complete remission (CR). In the total cohort of 729 patients, 488 (66.9%) achieved a CR. In younger patients, CR rate was significantly higher than in elderly patients (Pearson chi-square, p = 0.001). CR rates differed from more than 80% in young patients to about 55% in the elderly (0–9 years, 81.2%; 10–19 years, 85.7%; 20–29 years, 81.0%; 30–39 years, 75.0%; 40–49 years, 78.5%; 50–59 years, 62.9%; 60–69 years, 63.9%; 70–85 years, 55.3%). In 41 children <18 years, the frequency of an NPM1 mutation was significantly lower (24.4%, 10/41) compared to 688 adults (51.2%, 352/688; p = 0.003).
In contrast to children, in the adult cohorts, the frequencies of mutated NPM1 and CR rate diminished in an almost parallel manner with increasing age (Table 1).
Interestingly, patients carrying an NPM1 mutation did not have significant different CR rates in different age groups (p = 0.131). In contrast, elderly patients with NPM1 wildtype achieved significantly lower CR rates (p = 0.004) (data not shown).
Discussion
The aim of this study was to explore the relation between age and the distribution of mutations of several molecular markers in NK-AML. Our data show in a large cohort of 729 patients with NK-AML that the presence of mutations in NPM1 and FLT3-ITD in adult AML significantly decreased with age, whereas the frequency of other gene mutations, like FLT3-TKD, MLL-PTD, and mutated CEBPA were not age-dependent. This is the largest study of frequencies of NPM1 and FLT3-ITD in NK-AML. These data are in a relative contradiction to previous published reports that showed that an NPM1 mutation was more common in elderly patients [3, 20–22]. Nevertheless, all of these analyses included patients with different karyotypes, the majority of which were not NK-AML. Thus, these data cannot be fully compared to our data restricted to NK-AML unless they are reduced to the actual numbers of patients with NK-AML, which were lower (between 67 and 230 cases) compared to our large study. Including patients regardless of karyotype in our analysis (N = 1469), the lowest frequencies of mutated NPM1 were seen in patients <35 years (22.3%; 50/224). These results are in accordance with Verhaak et al. [20].
The decrease in FLT3-ITD positivity with older age is in accordance with analyses from Schnittger et al. performed in 1,003 patients, 428 of which had NK-AML [23]. Other analyses that did not reveal any age-dependency of FLT3-ITD were performed in cohorts including other cytogenetic groups, smaller patient numbers of NK-AML, or restriction to patients up to 60 years of age [24–26]. In our cohort, more than one third of patients in the elderly cohort (>70 years) lacked a defined molecular marker compared to one fifth of younger patients (≤40 years) (data not shown). Thus, the pathophysiological role of mutated NPM1 and FLT3-ITD in the development of NK-AML in elderly patients is less evident, and other mutations, differences in gene expression patterns, or changes in epigenetic alterations might be relevant for prognosis and have to be further studied. Newly discovered mutations associated with cytogenetically normal AML include the following genes: TET2, IDH1/2, DNMT3A, and RUNX1. Interestingly, median age of patients carrying these mentioned mutations (TET2, IDH1/2, DNMT3A, and RUNX1) has been shown to be higher compared to wildtype. A negative prognostic effect of IDH1/2 mutations (relapse free survival) and TET2 mutations (overall survival) on survival has been demonstrated only in patients with the favorable NPM1+/FLT3-ITD− genotype, but not in patients with wildtype NPM1/wildtype FLT3 [27, 28]. DNMT3A mutations have been described recently and shown to be associated with a negative effect on overall survival in patients with NK-AML. The effect of DNMT3A mutations in different NPM1/FLT3 subgroups still warrants further investigation [29]. Interestingly, RUNX1 mutations have been found to display a negative prognostic effect on overall survival and event-free survival in the intermediate cytogenetic risk group without molecular mutations [30, 31]. Thus, the higher occurrence of RUNX1 mutations in elderly patients with NK-AML who have a lower frequency of NPM1 and FLT3-ITD might in part explain the dismal prognosis in this patient cohort.
In our results, an FLT3-TKD mutation occured with equal frequencies in all adult age groups, whereas the frequency of an FLT3-ITD showed a strong dependence on age. Additionally, in the literature, an FLT3-TKD occurs in all genetic AML subgroups. An overrepresentation of FLT3-TKD has been shown for AML with normal karyotype or complex aberrant karyotype. FLT3-TKD is significantly associated with an NPM1 mutation, whereas an FLT3-TKD hardly occurs together with an FLT3-ITD. A positive prognostic impact on EFS has been described for patients with an FLT3-TKD in the combination with the NPM1 mutation [32]. In our cohort, the frequency of an NPM1+/FLT3-TKD+ genotype in adult patients ≤60 years was 6.4% (19/298), whereas only 3.1% (10/320) of patients ≥ 60 years showed this genotype (p = 0.056). The effect of a NPM1+/FLT3-TKD+ genotype is probably similar in all age groups, but the lower frequency of this prognostic favorable subgroup may explain at least in part the worse EFS in the elderly subgroup.
Furthermore, we could show that the decreased frequency of an NPM1 mutation with increasing age was associated with an almost parallel decrease of achievement of a CR in adult NK-AML patients. The achievement of a CR is associated with a longer overall survival [33]. Thus, we postulate that the inferior prognosis in elderly patients is partly due to a lower frequency of an NPM1 mutation resulting in a reduced CR rate. The independence of the prognostic impact of NPM1 mutations and age on overall survival has been proven in a multivariate cox regression model (data not shown).
In earlier investigations, we and others have shown that the presence of an NPM1 mutation is significantly associated with the achievement of a complete remission as a marker of early response to therapy [34]. We found a higher rate of adequate blast cell reduction in the bone marrow 1 week after the end of the first induction cycle (day 16 blast cell clearance) in NPM1-mutation-positive AML compared to AML with wildtype NPM1 [34], thus postulating a higher in vivo chemosensitivity of NPM1-mutated blasts. As the presence of an NPM1 mutation and CR rate decrease equally with higher age, we propose that the reduced frequency of the NPM1 mutation leads to a lower CR rate and consequently contributes to a dismal prognosis in elderly patients.
The analysis of the four NPM1/FLT3-ITD subgroups confirmed the results of the single markers. In adult AML, we found a significant higher number of NPM1−/FLT3-ITD− patients with older age, whereas the double positive fraction significantly decreased. The NPM1+/FLT3-ITD− patients showed an increase to up to almost 40% in patients between 40 and 49 years and a decrease in older patients. Comparing adult patients ≤40 years to patients >70 years, there was no statistical difference in the occurrence of the different NPM1/FLT3-ITD subgroups (data not shown). Thus, the simplest explanation for the inferior survival of the older patient—which would have been the decrease of the favorable prognostic NPM1+/FLT3-ITD− group—cannot be substantiated by our results. This is clinically relevant since the NPM1+/FLT3-ITD− genotype has been shown to be associated with a better prognosis in the elderly cohort [1].
Büchner et al. [1] investigated the frequency of NPM1 mutations and FLT3-ITD in a larger patient cohort of de novo AML patients from the AMLCG 92 and 99 trials including all cytogenetic risk groups. In contrast to their analyses, our analyses of NPM1/FLT3 subgroups focused on patients with NK-AML allowing inclusion of secondary AML and therapy-related AML. Furthermore, our adult patients were treated only within the AMLCG 99 trial, except for three patients treated within children AML protocols.
In accordance with the observation of Büchner et al., we found a higher proportion of older patients lacking mutations of NPM1 and FLT3-ITD compared to the younger patient cohort (49.9% versus 29.6% in our analysis; 40.2% versus 26.3% in the Büchner publication).
Whereas Büchner et al. found an almost equal frequency of the favorable NPM1+/FLT3-ITD− genotype in patients <60 years compared to ≥60 years (36.5% versus 33.2%), in our cohort of NK-AML, the frequency of the favorable NPM1+/FLT3-ITD− genotype was significantly lower in adult patients <60 years compared to those ≥60 years (35.0% versus 26.0%).
The distribution of the NPM1/FLT3-ITD subgroups seemed to be comparable in patients <20 years compared to ≥70 years: The majority of children lacked mutations of NPM1 and FLT3, and the frequency of the favorable prognostic NPM1+/FLT3-ITD− group in children was even lower compared to the elderly patients (9% versus 29%). The fact, that the highest CR rates were achieved by children and the lowest CR rates were achieved by elderly patients suggests that children and adult NK-AML might have to be considered as different entities with different disease biologies.
In conclusion, the decreasing frequency of the NPM1 mutation in elderly patients with NK-AML contributes to the lower CR rate and might explain part of the drug resistance to chemotherapeutic agents. These observations shed light on the disease biology in older patients with AML and might in part explain the dismal prognosis.
References
Büchner T, Berdel WE, Haferlach C, Haferlach T, Schnittger S, Müller-Tidow C, Braess J, Spiekermann K, Kienast J, Staib P, Gruneisen A, Kern W, Reichle A, Maschmeyer G, Aul C, Lengfelder E, Sauerland MC, Heinecke A, Wörmann B, Hiddemann W (2009) Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol 27(1):61–69. doi:10.1200/JCO.2007.15.4245
Bacher U, Kern W, Schnittger S, Hiddemann W, Haferlach T, Schoch C (2005) Population-based age-specific incidences of cytogenetic subgroups of acute myeloid leukemia. Haematologica 90(11):1502–1510
Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, La Starza R, Diverio D, Colombo E, Santucci A, Bigerna B, Pacini R, Pucciarini A, Liso A, Vignetti M, Fazi P, Meani N, Pettirossi V, Saglio G, Mandelli F, Lo-Coco F, Pelicci PG, Martelli MF (2005) Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 352(3):254–266. doi:10.1056/NEJMoa041974
Falini B, Nicoletti I, Martelli MF, Mecucci C (2007) Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood 109(3):874–885. doi:10.1182/blood-2006-07-012252
Hollink IH, Zwaan CM, Zimmermann M, Arentsen-Peters TC, Pieters R, Cloos J, Kaspers GJ, de Graaf SS, Harbott J, Creutzig U, Reinhardt D, van den Heuvel-Eibrink MM, Thiede C (2009) Favorable prognostic impact of NPM1 gene mutations in childhood acute myeloid leukemia, with emphasis on cytogenetically normal AML. Leukemia 23(2):262–270. doi:10.1038/leu.2008.313
Thiede C, Creutzig E, Reinhardt D, Ehninger G, Creutzig U (2007) Different types of NPM1 mutations in children and adults: evidence for an effect of patient age on the prevalence of the TCTG-tandem duplication in NPM1-exon 12. Leukemia 21(2):366–367. doi:10.1038/sj.leu.2404519
Büchner T, Berdel WE, Schoch C, Haferlach T, Serve HL, Kienast J, Schnittger S, Kern W, Tchinda J, Reichle A, Lengfelder E, Staib P, Ludwig WD, Aul C, Eimermacher H, Balleisen L, Sauerland MC, Heinecke A, Wörmann B, Hiddemann W (2006) Double induction containing either two courses or one course of high-dose cytarabine plus mitoxantrone and postremission therapy by either autologous stem-cell transplantation or by prolonged maintenance for acute myeloid leukemia. J Clin Oncol 24(16):2480–2489. doi:10.1200/JCO.2005.04.5013
Creutzig U, Büchner T, Sauerland MC, Zimmermann M, Reinhardt D, Döhner H, Schlenk RF (2008) Significance of age in acute myeloid leukemia patients younger than 30 years: a common analysis of the pediatric trials AML-BFM 93/98 and the adult trials AMLCG 92/99 and AMLSG HD93/98A. Cancer 112(3):562–571. doi:10.1002/cncr.23220
Bain BJ (2005) Leukaemia diagnosis, 4th edn. Wiley-Blackwell, Oxford
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C (1976) Proposals for the classification of the acute leukaemias. French–American–British (FAB) co-operative group. Br J Haematol 33(4):451–458
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C (1985) Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French–American–British Cooperative Group. Ann Intern Med 103(4):620–625
Büchner T, Hiddemann W, Wormann B, Löffler H, Gassmann W, Haferlach T, Fonatsch C, Haase D, Schoch C, Hossfeld D, Lengfelder E, Aul C, Heyll A, Maschmeyer G, Ludwig WD, Sauerland MC, Heinecke A (1999) Double induction strategy for acute myeloid leukemia: the effect of high-dose cytarabine with mitoxantrone instead of standard-dose cytarabine with daunorubicin and 6-thioguanine: a randomized trial by the German AML Cooperative Group. Blood 93(12):4116–4124
Heim S, Mitelman F (2009) Cancer cytogenetics, 3rd edn. Wiley-Blackwell, Hoboken
Kjeldsberg CR (2000) Practical diagnosis of hematologic disorders, 3rd edn. ASCP, Chicago
Standing Committee on Human Cytogenetic Nomenclature, Mitelman F (1995) ISCN 1995: an international system for human cytogenetic nomenclature (1995): recommendations of the International Standing Committee on Human Cytogenetic Nomenclature, Memphis, TN, USA, October 9–13, 1994. Karger, Basel, New York
Swerdlow SH, Jaffe ES, International Agency for Research on Cancer, World Health Organization (2008) WHO classification of tumours of haematopoietic and lymphoid tissues. World Health Organization classification of tumours. International Agency for Research on Cancer, Lyon
Dufour A, Schneider F, Metzeler KH, Hoster E, Schneider S, Zellmeier E, Benthaus T, Sauerland MC, Berdel WE, Büchner T, Wörmann B, Braess J, Hiddemann W, Bohlander SK, Spiekermann K (2010) Acute myeloid leukemia with biallelic CEBPA gene mutations and normal karyotype represents a distinct genetic entity associated with a favorable clinical outcome. J Clin Oncol 28(4):570–577. doi:10.1200/JCO.2008.21.6010
Benthaus T, Schneider F, Mellert G, Zellmeier E, Schneider S, Kakadia PM, Hiddemann W, Bohlander SK, Feuring-Buske M, Braess J, Spiekermann K, Dufour A (2008) Rapid and sensitive screening for CEBPA mutations in acute myeloid leukaemia. Br J Haematol 143(2):230–239. doi:10.1111/j.1365-2141.2008.07328.x
Döhner K, Schlenk RF, Habdank M, Scholl C, Rucker FG, Corbacioglu A, Bullinger L, Fröhling S, Döhner H (2005) Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood 106(12):3740–3746. doi:10.1182/blood-2005-05-2164
Verhaak RG, Goudswaard CS, van Putten W, Bijl MA, Sanders MA, Hugens W, Uitterlinden AG, Erpelinck CA, Delwel R, Lowenberg B, Valk PJ (2005) Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood 106(12):3747–3754. doi:10.1182/blood-2005-05-2168
Chou WC, Tang JL, Lin LI, Yao M, Tsay W, Chen CY, Wu SJ, Huang CF, Chiou RJ, Tseng MH, Lin DT, Lin KH, Chen YC, Tien HF (2006) Nucleophosmin mutations in de novo acute myeloid leukemia: the age-dependent incidences and the stability during disease evolution. Cancer Res 66(6):3310–3316. doi:10.1158/0008-5472.CAN-05-4316
Suzuki T, Kiyoi H, Ozeki K, Tomita A, Yamaji S, Suzuki R, Kodera Y, Miyawaki S, Asou N, Kuriyama K, Yagasaki F, Shimazaki C, Akiyama H, Nishimura M, Motoji T, Shinagawa K, Takeshita A, Ueda R, Kinoshita T, Emi N, Naoe T (2005) Clinical characteristics and prognostic implications of NPM1 mutations in acute myeloid leukemia. Blood 106(8):2854–2861. doi:10.1182/blood-2005-04-1733
Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, Löffler H, Sauerland CM, Serve H, Büchner T, Haferlach T, Hiddemann W (2002) Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 100(1):59–66
Fröhling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, Döhner H, Döhner K (2002) Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood 100(13):4372–4380. doi:10.1182/blood-2002-05-1440
Gale RE, Hills R, Pizzey AR, Kottaridis PD, Swirsky D, Gilkes AF, Nugent E, Mills KI, Wheatley K, Solomon E, Burnett AK, Linch DC, Grimwade D (2005) Relationship between FLT3 mutation status, biologic characteristics, and response to targeted therapy in acute promyelocytic leukemia. Blood 106(12):3768–3776. doi:10.1182/blood-2005-04-1746
Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, Wermke M, Bornhauser M, Ritter M, Neubauer A, Ehninger G, Illmer T (2002) Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 99(12):4326–4335
Paschka P, Schlenk RF, Gaidzik VI, Habdank M, Krönke J, Bullinger L, Spath D, Kayser S, Zucknick M, Gotze K, Horst HA, Germing U, Döhner H, Döhner K (2010) IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol 28(22):3636–3643. doi:10.1200/JCO.2010.28.3762
Metzeler KH, Maharry K, Radmacher MD, Mrozek K, Margeson D, Becker H, Curfman J, Holland KB, Schwind S, Whitman SP, Wu YZ, Blum W, Powell BL, Carter TH, Wetzler M, Moore JO, Kolitz JE, Baer MR, Carroll AJ, Larson RA, Caligiuri MA, Marcucci G, Bloomfield CD (2011) TET2 mutations improve the new European LeukemiaNet risk classification of acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol 29(10):1373–1381. doi:10.1200/JCO.2010.32.7742
Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, Kandoth C, Payton JE, Baty J, Welch J, Harris CC, Lichti CF, Townsend RR, Fulton RS, Dooling DJ, Koboldt DC, Schmidt H, Zhang Q, Osborne JR, Lin L, O'Laughlin M, McMichael JF, Delehaunty KD, McGrath SD, Fulton LA, Magrini VJ, Vickery TL, Hundal J, Cook LL, Conyers JJ, Swift GW, Reed JP, Alldredge PA, Wylie T, Walker J, Kalicki J, Watson MA, Heath S, Shannon WD, Varghese N, Nagarajan R, Westervelt P, Tomasson MH, Link DC, Graubert TA, DiPersio JF, Mardis ER, Wilson RK (2010) DNMT3A mutations in acute myeloid leukemia. N Engl J Med 363(25):2424–2433. doi:10.1056/NEJMoa1005143
Schnittger S, Dicker F, Kern W, Wendland N, Sundermann J, Alpermann T, Haferlach C, Haferlach T (2011) RUNX1 mutations are frequent in de novo AML with noncomplex karyotype and confer an unfavorable prognosis. Blood 117(8):2348–2357. doi:10.1182/blood-2009-11-255976
Gaidzik VI, Bullinger L, Schlenk RF, Zimmermann AS, Rock J, Paschka P, Corbacioglu A, Krauter J, Schlegelberger B, Ganser A, Spath D, Kundgen A, Schmidt-Wolf IG, Gotze K, Nachbaur D, Pfreundschuh M, Horst HA, Döhner H, Döhner K (2011) RUNX1 mutations in acute myeloid leukemia: results from a comprehensive genetic and clinical analysis from the AML Study Group. J Clin Oncol 29(10):1364–1372. doi:10.1200/JCO.2010.30.7926
Bacher U, Haferlach C, Kern W, Haferlach T, Schnittger S (2008) Prognostic relevance of FLT3-TKD mutations in AML: the combination matters—an analysis of 3082 patients. Blood 111(5):2527–2537. doi:10.1182/blood-2007-05-091215
Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, Rees JK, Stevens RF, Walker H (1999) A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties. Br J Haematol 107(1):69–79
Schneider F, Hoster E, Unterhalt M, Schneider S, Dufour A, Benthaus T, Mellert G, Zellmeier E, Bohlander SK, Feuring-Buske M, Buske C, Braess J, Fritsch S, Heinecke A, Sauerland MC, Berdel WE, Büchner T, Wörmann BJ, Hiddemann W, Spiekermann K (2009) NPM1 but not FLT3-ITD mutations predict early blast cell clearance and CR rate in patients with normal karyotype AML (NK-AML) or high-risk myelodysplastic syndrome (MDS). Blood 113(21):5250–5253. doi:10.1182/blood-2008-09-172668
Acknowledgments
The authors would like to thank Gudrun Mellert and Evelyn Zellmeier (Laboratory for Leukemia Diagnostics, University Hospital Munich) for their excellent technical support.
Conflict of interest
The author indicates no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Overview of patient cohorts (DOC 41 kb)
Rights and permissions
About this article
Cite this article
Schneider, F., Hoster, E., Schneider, S. et al. Age-dependent frequencies of NPM1 mutations and FLT3-ITD in patients with normal karyotype AML (NK-AML). Ann Hematol 91, 9–18 (2012). https://doi.org/10.1007/s00277-011-1280-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-011-1280-6