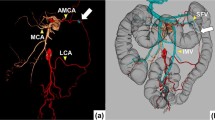
Abstract
Background
Variations of the vasculature at splenic flexure by left colic artery (LCA) and middle colic artery (MCA) remain ambiguous.
Objectives
This study aim to investigate the anatomical variations of the branches from LCA and MCA at splenic flexure area.
Methods
Using ultra-thin CT images (0.5-mm thickness), we traced LCA and MCA till their merging site with paracolic marginal arteries through maximum intensity projection (MIP) reconstruction and computed tomography angiography (3D-CTA).
Results
A total of 229 cases were retrospectively enrolled. LCA ascending branch approached upwards till the distal third of the transverse colon in 37.6%, reached the splenic flexure in 37.6%, and reached the lower descending colon in 23.1%, and absent in 1.7% of the cases. Areas supplied by MCA left branch and aMCA were 33.2%, 44.5% and 22.3% in the proximal, middle and distal third of transverse colon of the cases, respectively. The accessory MCA separately originated from the superior mesenteric artery was found in 17.9% of the cases. Mutual correlation was found that, when the LCA ascending branch supplied the distal transverse colon, MCA left branch tended to feed the proximal transverse colon; when the LCA ascending branch supplied the lower part of descending colon, MCA left branch was more likely to feed the distal third of transverse colon.
Conclusions
Vasculature at splenic flexure by LCA and MCA varied at specific pattern. This study could add more anatomical details for vessel management in surgeries for left-sided colon cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The level of inferior mesenteric artery (IMA) ligation during rectal cancer surgery is still being debated, after Miles and Moynihan proposed low tie and high tie techniques in 1908 [5, 9, 11]. Griffiths and Meyers insisted that after IMA high ligation, the bifurcation of the left colic artery (LCA) should be kept intact to preserve the secondary arterial arch besides Griffiths’ point [2, 6]. Whereas, the splenic vasculature conjured by LCA and middle colic artery (MCA) varies a lot, posing a challenge on the protection of LCA bifurcation and splenic paracolic vessels. For example, if LCA supply the splenic flexure, what is MCA like? Since the left transverse mesocolon is often deprived of vessels, in what condition will MCA or accessory appear in this area? If LCA does not supply the splenic flexure, what is the splenic flexure vasculature like? Does the LCA branches needs to be protected? In addition, due to the variation of the vasculature, vessel division within the radical resection for splenic flexure cancer or distal transverse colon cancer remain unclear. In which scenario that the splenic flexure was supplied by LCA and MCA together, or only by LCA and not by MCA? If LCA supplied distal descending colon or even absent, does it need to be dissected and divided? Most of these anatomical questions were not clearly illustrated before. Anatomic relationships between the terminals of LCA and MCA are hard to be observed by the invasive surgery exploration, or on CT images due to thickness problem (3 mm), ptosis of the transverse colon, and interference from surrounding vessels [10]. In this study, we employed SureStart technique to increase intra-vascular attenuation value [2, 4], and maximum intensity projection (MIP) reconstruction on ultra-thin CT images (0.5 mm) to observe small mesenteric vessels [12]. Through these efforts, the anatomic variations in LCA and MCA at splenic area were illustrated.
Patients and methods
Patients
Patients who had undergone abdominal enhancement CT scan at our hospital between January 2017 and December 2018 were retrospectively enrolled in this study. Patients with previous abdominal surgery were excluded. Ethical approval for this study was obtained and this consent protocol was reviewed and the need for written and informed consent was waived by the Ethical Committee.This study was approved by the Institutional Review Board (IRB) of our hospital.
CT scanning and image processing protocol
The images were acquired by Toshiba Aquilion ONE 320-Row detector CT scanner. The tube potential was 100–120 kVp and the tube current was approximately 280–330 mA (or tube current was automatically controlled). After at least four hours of fasting, the patients were placed in a supine position on the scanner for a plain scan. Subsequently, 60–90 mL of lopromide (370 mg/mL) was injected into the median cubital vein at a rate of 4–5 mL/s. Scan time was set using the SureStart method. When the signal within in the abdominal aorta reached 180 Hounsfield units, arterial phase scan was automatically initiated. 0.75-mm slices were generated by the scanner, and were reconstructed into 0.5-mm images. The images of all the patients were processed through 3D volume rendering performed on Vitrea 6.3 (Vital Images, Minneapolis, Minnesota, USA) and MIP reconstruction performed on RadiAnt DICOM Viewer 5.0.1 (Medixant, Poznan, Poland).
Definition and classification
LCA was defined as the first branch originated from IMA and supplying the left-sided colon. Several branches might be given out on its approaching track. A branch was defined as a vessel bifurcated from LCA or MCA, at least 3 cm away from the marginal artery. The ascending branch of LCA was defined as the branch that approached most upwards, or as LCA itself when LCA do not bifurcate. The MCA was defined as the first branch of the superior mesenteric artery (SMA) and supplying the transverse colon. The marginal artery was defined as the vessel runs in the mesentery 2–3 cm interior to the large intestine, and serve as a vascular arcade that connects the branches from SMA and IMA. The MCA right branch was defined as the branch running toward the most right before reaching the marginal artery, and the MCA left branch was defined as the branch other than MCA right branch running toward the middle or left of transverse colon. The accessory MCA (aMCA) was defined as the other artery separately originated from SMA besides the existing MCA, and run along the inferior border of the pancreas supplying the splenic flexure.
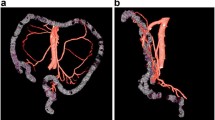
The supplying areas of LCA ascending branch and MCA left branch were classified by the region of their anastomosis site with paracolic marginal artery. Therefore, the descending colon was divided into splenic flexure region and distal descending colon (shown in Fig. 1). The transverse colon was classified into three areas: proximal third, middle third, and distal third (shown in Fig. 2). The splenic flexure supplying artery was defined as the vessel reached the regions of distal 3rd of transverse colon and splenic flexure.
LCA subtypes classified by the terminal reach of LCA ascending branch, regardless of other LCA branches. Type A (37.6%, 86/229): LCA ascending branch reached the distal third of the transverse colon; Type B (37.6%, 86/229): LCA ascending branch reached splenic flexure; Type C (23.1%, 53/229): LCA reached the descending colon; Type D (1.7%, 4/229): LCA absent
Classifications of MCA left branches by their terminal reaches. A MCA approached the proximal third of transverse colon (33.2%, 76/229); B MCA left branch reached the middle third of transverse colon (44.5%, 102/229); C MCA left branch reached the distal third of transverse colon (4.4%,10/229); D Accessory MCA separately originated from SMA and reached the distal third of transverse colon (17.9%, 41/229)
Statistical analysis
IBM SPSS Statistics 22.0 (IBM Corporation, Somers, New York, USA) was used for statistical calculations. Percentages were performed using χ2 analysis. Chi-square and logistic regression were used for binary variables. For continuous data, the Mann–Whitney test was used. Correlation between the presence of accessory MCA, for each item of the analysis grid, was evaluated by both univariate and multivariate analyses. Correlations were tested using the nonparametric Spearman's rank test. P value of less than 0.05 was considered to be statistically significant.
Results
Variations of the terminal reach of LCA and its bifurcation
A total of 229 patients (144 males and 85 females) were retrospectively enrolled, with a median age of 51.9 years old (range, 15–80 years). 60.7% of them (139/229) were diagnosed as gastrointestinal neoplasms, 16.6% (38/229) as inflammatory bowel disease, and 22.7% (52/229) as other gastrointestinal abnormalities. The LCA ascending branch anastomosed with the marginal artery at the distal third of the transverse colon in 37.6% (type A, 86/229), at the splenic flexure area in 37.6% (type B, n = 86/229), 23.1% at the lower descending colon of total cases (type C, n = 53/229). LCA was absent in 1.7% (type D, n = 4/229) (Table 1) (shown in Fig. 1). LCA bifurcated in 73.8% (169/229) of total patients, and 45.0% (76/169) in type A, 42.6% (72/169) in type B and 12.4% (21/169) in type C. The number of LCA branches was correlated with LCA subtype, highest in type A and lowest in type C/D (ρ = − 0.418, P < 0.0001) (Table 2).
Variations of the MCA left branch and accessory MCA
Two MCA branches were observed in 73.8% (169/229), 3 branches in 4.4% (10/229), and no bifurcation in 21.8% (50/229) of total cases (Table 1). The number of MCA branches was correlated with LCA subtype, lowest in type A LCA. (ρ = 0.1491, P < 0.0241). Areas supplied by MCA left branch and aMCA were 44.5% (102/229) in the middle third of the transverse colon, 33.2% (76/229) in the proximal third and 22.3% (51/229) in the distal third of the transverse colon of total cases (Table 1) (shown in Fig. 2). Accessory MCA (aMCA) was found in 17.9% (41/229) of total cases (Table 1) (shown in Fig. 2), and in 1 case, it originated from celiac flow. Multivariate analysis showed that this anatomic structure was more common in type C/D of LCA and MCA without bifurcation (Table 3). In the MCAs without bifurcations, 78.0% (39/50) of MCA left branch supplied the proximal third, 22.0% (11/50) supplied the middle third, and none for distal third of the transverse colon, but 46.0% (23/50) of aMCA supplied the distal third of the transverse colon as supplementary.
Anatomical relationships between LCA ascending branch and MCA left branch/aMCA at splenic flexure
A close anatomical relationship was found between the LCA ascending branch and MCA left branch/aMCA. When the LCA ascending branch supplied the distal transverse colon (type A), MCA left branch tended to feed the proximal transverse colon. When the LCA ascending branch supplied splenic flexure, MCA left branch tended to feed the middle third of the transverse colon. When the LCA ascending branch supplied the lower part of descending colon (type C/D), MCA left branch/aMCA was more likely to feed the distal third of the transverse colon (ρ = 0.6093, P < 0.001) (Table 2) (shown in Fig. 3).
Correlation between the terminal reaches of LCA ascending and MCA left branch. A MCA left branch reached the proximal third of transverse colon while LCA ascending branch reached the distal third of transverse colon (24.5%, 56/229); B MCA left branch reached the middle third of transverse colon while LCA ascending branch fed the region of splenic flexure (26.6%, 61/229); C LCA ascending branch reached the middle part of descending colon and MCA left branch supplied the distal third of transverse colon (16.2%, 37/229); D LCA ascending branch reached the middle part of descending colon and MCA left branch supplied the proximal third of transverse colon (2.2%, 5/229)
Discussion
In the current study, we reported the anatomical variations in the LCA ascending branch and MCA left branch within the splenic mesenteric vasculature. It was believed that the terminals of these arteries are hard to trace, but we successfully displayed their anatomical relationships through MIP reconstruction with ultra-thin images. Our findings provided a basis for surgeons to protect the splenic mesenteric vasculature, to ensure the blood supply of anastomosis of rectal cancer surgery.
We found that the splenic flexure was more often supplied by LCA ascending branch than by MCA left branch/aMCA (69.0 vs 22.3%). This is of significance that, for splenic flexure cancer, LCA and MCA might not always be the drainage vessel that need to be divided at the root level. Notably, a close correlation of the supplying areas was found that, when the LCA ascending branch supplied the distal transverse colon (type A), MCA left branch tended to feed the proximal transverse colon; when the LCA ascending branch supplied the lower part of the descending colon (type C/D), MCA left branch/aMCA is prone to feed the distal third of the transverse colon. Thus, the vasculature at splenic flexure could be anticipated by the track of LCA ascending branch, which can be usually observed within laparoscopic splenic flexure cancer resection using a caudal-cranial approach. This relationship was previously hypothesized [12] yet verified for the first time in this study. It is also notable that in 5 cases (2.2%), the splenic flexure was neither approached by LCA ascending branch nor by MCA left branch. Special caution should be given to this type of patients within the rectal cancer surgery, especially with elder age and smoking habit, because the poorly structured marginal artery at splenic flexure might jeopardize the blood compensation from MCA, after IMA high ligation.
In addition, Areas supplied by MCA left branch/AMCA was found in 22.3% of the cases (51/229). This finding may contradict the notion that the left side of transverse mesocolon is an “avascular area” that can be used as the access for postcolic Billroth II or Roux-en-Y anastomosis. 17.9% in all cases) were the aMCA separately arising from SMA. This percentage was similar to the study by Hamabe, Atsushi et al. (14.3%) [3], whereas lower than that by H. Miyake et al. (36.4%) [7] since they also included vessels originated from celiac flow. The presence of aMCA was strongly correlated with LCA branching pattern in this study, which was also found by H. Miyake et al. [7]. Surgical dissection for this type of splenic flexure or descending colon cancer might be more challenging, since the regional lymph nodes drains into SMA instead of MCA [1, 8].
The main limitation of this study was that the anatomy was not confirmed intra-operatively. However, previous studies have suggested that MIP reconstruction with ultra-thin images can achieve satisfactory accuracy [4, 8]. In this study, the thickness of the image was 0.5 mm, which can significantly improve the accuracy of fine vessel delineation.
Conclusions
This study presents the variations in splenic vasculature caused by the branches of LCA and MCA. This information is helpful to increase the accuracy and safety of left-sided and rectal cancer surgery.
References
Garcia-Granero A, Sanchez-Guillen L, Carreno O, Sancho Muriel J, Alvarez Sarrado E, Fletcher Sanfeliu D, FlorLorente B, Frasson M, Martinez Soriano F, Garcia-Granero E (2017) Importance of the Moskowitz artery in the laparoscopic medial approach to splenic flexure mobilization: a cadaveric study. Tech Coloproctol 21:567–572. https://doi.org/10.1007/s10151-017-1663-3
Griffiths JD (1961) Extramural and intramural blood-supply of colon. Br Med J 1:323–326. https://doi.org/10.1136/bmj.1.5222.323
Hamabe A, Park S, Morita S, Tanida T, Tomimaru Y, Imamura H, Dono K (2018) Analysis of the vascular interrelationships among the first jejunal vein, the superior mesenteric artery, and the middle colic artery. Ann Surg Oncol 25:1661–1667. https://doi.org/10.1245/s10434-018-6456-z
Kwon O, Kang ST, Kim SH, Kim YH, Shin YG (2015) Maximum intensity projection using bidirectional compositing with block skipping. J Xray Sci Technol 23:33–44. https://doi.org/10.3233/XST-140468
Lange MM, Buunen M, van de Velde CJ, Lange JF (2008) Level of arterial ligation in rectal cancer surgery: low tie preferred over high tie. A review. Dis Colon Rectum 51:1139–1145. https://doi.org/10.1007/s10350-008-9328-y
Meyers MA (1976) Griffiths’ point: critical anastomosis at the splenic flexure. Significance in ischemia of the colon. AJR Am J Roentgenol 126:77–94. https://doi.org/10.2214/ajr.126.1.77
Miyake H, Murono K, Kawai K, Hata K, Tanaka T, Nishikawa T, Otani K, Sasaki K, Kaneko M, Emoto S, Nozawa H (2018) Evaluation of the vascular anatomy of the left-sided colon focused on the accessory middle colic artery: a single-centre study of 734 patients. Colorectal Dis 20:1041–1046. https://doi.org/10.1111/codi.14287
Murono K, Miyake H, Hojo D, Nozawa H, Kawai K, Hata K, Tanaka T, Nishikawa T, Shuno Y, Sasaki K, Kaneko M, Emoto S, Ishii H, Sonoda H, Ishihara S (2020) Vascular anatomy of the splenic flexure, focusing on the accessory middle colic artery and vein. Colorectal Dis 22:392–398. https://doi.org/10.1111/codi.14886
Pezim ME, Nicholls RJ (1984) Survival after high or low ligation of the inferior mesenteric artery during curative surgery for rectal cancer. Ann Surg 200:729–733. https://doi.org/10.1097/00000658-198412000-00010
Stenzel F, Rief M, Zimmermann E, Greupner J, Richter F, Dewey M (2014) Contrast agent bolus tracking with a fixed threshold or a manual fast start for coronary CT angiography. Eur Radiol 24:1229–1238. https://doi.org/10.1007/s00330-014-3148-3
Surtees P, Ritchie JK, Phillips RK (1990) High versus low ligation of the inferior mesenteric artery in rectal cancer. Br J Surg 77:618–621. https://doi.org/10.1002/bjs.1800770607
Tanaka T, Matsuda T, Hasegawa H, Yamashita K, Nakamura T, Suzuki S, Kakeji Y (2019) Arterial anatomy of the splenic flexure using preoperative three-dimensional computed tomography. Int J Colorectal Dis 34:1047–1051. https://doi.org/10.1007/s00384-019-03289-z
Funding
This study was funded in part by National Natural Science Foundation of China (81770656; 81970452) and Sun Yat-Sen University Clinical Research 5010 Program (2019022).
Author information
Authors and Affiliations
Contributions
JXZ, XFJ and JBF contributed to study concept and design, acquisition, analysis, interpretation of data and drafting of the manuscript. JWC, DCK, WTC, HYZ and DYZ contributed to data collections and interpretation of data and critical revision of the manuscript for important intellectual content. XCM and JK supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Ethical approval for this study was obtained from the Ethical Committee of The Sixth Affiliated Hospital, Sun Yat-Sen University and the reference number is 2021ZSLYEC-154. This consent protocol was reviewed and the need for written and informed consent was waived by the Ethical Committee of The Sixth Affiliated Hospital, Sun Yat-Sen University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zou, J., Jiang, X., Feng, J. et al. Anatomical variations of the branches from left colic artery and middle colic artery at splenic flexure. Surg Radiol Anat 44, 467–473 (2022). https://doi.org/10.1007/s00276-022-02898-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-022-02898-8