Abstract
Introduction
To evaluate the effectiveness of percutaneous image-guided vertebral body stenting (VBS) at restoring vertebral height in acute, stable, traumatic thoracolumbar fractures in a young, non-osteoporotic population.
Materials and Methods
A single-centre retrospective review of all traumatic non-osteoporotic fractures treated with VBS between 2010 and 2017 was performed. Inclusion criteria included patients with recent (< 10 days), symptomatic and stable thoracolumbar compression fractures. Patients with low-energy fractures, osteoporosis and age > 60/50 years (male/female) were excluded. Primary outcomes included: correction of vertebral height, correction of kyphosis angle and Beck Index on reconstructed pre- and post-procedural CBCT images. Secondary outcomes included intra-procedural stent recoil, complications, cement leakage and factors predicting height restoration.
Results
Thirty-nine patients (26 men, 13 women; mean age 33.6 years, range 15–57 years) underwent VBS 5 days post-trauma on average (range 1–10), for stable compression fractures located between T5 and L5. Mean vertebral height gain, vertebral kyphosis angle correction and Beck index improvement were 3.8 mm (95% CI 3.36–4.50; P(> 3 mm) = 99.9%), 4.3° (95% CI 3.50–5.20; P(> 3°) = 99.9%) and 0.07 [95% CI 0.053–0.11], respectively (all statistically significant). Technical success was 92%, with 3 “major” stent recoils resulting in loss of vertebral height correction. No symptomatic complications were observed. No predictive factors for procedural success were identified.
Conclusion
VBS can significantly restore vertebral height in young patients with traumatic vertebral compression fractures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous augmentation techniques are recognized treatments for osteoporotic and malignant compression fractures [1, 2]. They can also be applied for the management of traumatic compression fractures, either as a stand-alone procedure or in combination with a posterior surgical stabilization depending on the type of fracture and the importance of the spinal deformation [3,4,5,6,7,8,9]. The major interest of these minimally invasive procedures is to represent a valuable alternative to conservative treatment, as it allows quick remobilization without the need for a prolonged bracing, for which the efficacy has moreover been questioned in the literature [3, 10, 11]. It can also be applied for fractures which failed conservative treatment, even in the paediatric population [12]. Most of the publications report the use of balloon kyphoplasty to treat traumatic compression fractures, in an attempt to restore as much as possible, the vertebral body height in case of a deformation [6,7,8,9, 13, 14]. Although several studies claim that kyphoplasty is superior to vertebroplasty in correcting vertebral deformity, there might still be some loss of height after balloon deflation due to vertebral elastic recoil, limiting procedural efficacy [15, 16].
Several third-generation percutaneous vertebral augmentation systems (PVAS) have been developed, which aim to maintain vertebral fracture reduction using prosthetic devices [2, 17,18,19]. The vertebral body stenting (VBS) procedure, in particular, is similar to kyphoplasty: two expandable metallic stents mounted on balloons are inflated beneath the fracture and designed to resist vertebral recoil following balloon deflation [20, 21]. According to the manufacturer’s recommendations, VBS is indicated for the management of osteoporotic, malignant and traumatic compression fractures [2, 18, 22]. The technique has shown promising results but has so far been studied almost exclusively in osteoporotic patients [23]. In the trauma population, it can be applied to treat stable thoracic/lumbar vertebral compression fractures according to the AO classification (Types A1.1, A1.2, A1.3 and A3.1), either as a stand-alone procedure or in combination with posterior stabilization for unstable A3.1 fractures [22, 24]. For fractures that do not require surgery but are associated with a significant sagittal deformation, VBS has the theoretical advantage of providing both fracture consolidation and height restoration [2, 18, 22, 25].The purpose of this study was to report our experience of height restoration with VBS in acute traumatic fractures of young non-osteoporotic patients.
Materials and Methods
This was a single-centre retrospective study. All patients gave informed consent for the procedure. Approval of the institutional review board was waived due to the retrospective nature of the study.
Institution Algorithm for the Management of Spinal Compression Fractures
Management of compression fractures in our institution is as follows: if the spinal surgeon does not indicate surgery because of spinal canal involvement, two options are offered to the patient: conservative orthopaedic treatment with bracing for 3 months or percutaneous augmentation techniques (vertebroplasty or VBS) as an alternative to immobilization. Inclusion criteria to propose VBS are: stable thoracic/lumbar recent (within 10 days) vertebral compression fractures (Types A1.1, A1.2, A1.3 and A3.1) according to the AO classification [24]), with loss of vertebral height defined by a local kyphotic angle > 15° confirmed on pre-operative CT and MRI.
Study Population
All VBS procedures performed in the department of interventional radiology between April 2010 (date of availability of the VBS device in our department) and December 2017 were retrospectively reviewed. Patients with multi-level fractures were included, where VBS was performed at a single level, and the remaining levels were managed either conservatively or with vertebroplasty alone. In order to minimize bias and study the efficacy solely in traumatic fracture cases, all patients with known osteoporosis, low-energy mechanism (fall from standing, or no fall reported) and age > 50 years (women) or 60 years (men) were excluded.
Vertebral Body Stenting Procedure
All procedures were performed under general anaesthesia (GA) and sterile surgical conditions by two interventional radiologists (each with at least 5 years of consultant experience), using flat-panel digital fluoroscopy with rotational acquisition and Cone-Beam Computed Tomography (CBCT; Allura Xper RD20 CT, Philips, the Netherlands).
Patients were positioned prone in a lumbar lordosing support, and pre-procedural CBCT was performed to plan guide-wire trajectory.
Under fluoroscopic-guidance, two kirschner wires (K-wires) (Vertebral Body Stent access kit, DepuySynthes, Raynham, USA) were advanced through small skin incisions and positioned at the posterior margin of the vertebral body, using a bilateral transpedicular (lumbar) or intercosto-pedicular (thoracic) approach. Access cannulae were advanced over the K-wires and positioned 2 mm anterior to the posterior vertebral wall. Following K-wires removal, a drill and blunt plunger (Vertebral Body Stent access kit, DepuySynthes, Raynham, USA) were used to create an access channel and measure the stent length (based on the landmarks at the tip of the plunger) according to the manufacturer’s protocol. Two VBS systems (Vertebral Body Stenting, DepuySynthes, Raynham, USA) were simultaneously advanced and positioned beneath the collapsed vertebral endplate. These consist of pre-crimped cobalt–chromium expandable metallic stents mounted on balloon catheters. Balloons were simultaneously inflated using a contrast-saline solution to a pressure of up to 30 atmospheres, until complete balloon expansion was achieved. Balloons were then deflated and removed, leaving the stents behind to support the restored vertebral height. Polymethylmethacrylate (PMMA) cement (Osteopal, Heraeus Medical, Wehrheim, Germany) was injected bilaterally into the stent cavities under continuous screening to reinforce the implant, using side-opening needles inserted via the access sleeve (Fig. 1). Injection was ceased when a cement cloud bridged the superior/inferior endplates, or if growing leakage was observed despite the cessation of injection for at least 1 min. Finally, all instruments were removed, and the wound sutured. Post-procedural CBCT was performed in exactly the same prone position, and patients were subsequently transferred to a recovery ward (Fig. 2).
The different steps of a typical VBS procedure. a Lateral projection demonstrates the two introducers just in front of the posterior wall. B Two stents mounted on balloons are inserted below the fractured endplate. C Inflation of the balloons allows to deploy the stents and to restore the vertebral body height. D following removal of the balloons, PMMA bone cement is injected within the stents
Post-operative Management and Follow-Up
All patients were kept overnight for surveillance and were authorized to stand up 6 h after the procedure. There was no bracing following the intervention. Patients were evaluated at a one-month follow-up clinical visit and were authorized to resume their professional activities if the clinical findings were uneventful. Lateral and anteroposterior conventional radiographs were obtained in the upright position one year after the intervention (Fig. 2).
Data Collection and Analysis
Radiological Evaluation
One senior, fellowship trained, interventional radiologist (5 years consultant experience, involved in 50% of the procedures) and one radiology fellow (not involved in any of the procedures) reviewed the cases and performed measurements, blinded to each others’ analyses.
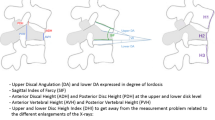
Vertebral height (utilizing five measurements: anterior, central and posterior mid-sagittal height; and left/right lateral mid-coronal height), local kyphotic angle (measured between superior and inferior endplates of the fractured vertebral body) and Beck Index (ratio of anterior to posterior vertebral body height on mid-sagittal images [26]) were measured pre- and post-operatively using multiplanar reconstructed CBCT images (acquired in the same position under GA in each case). Vertebral height restoration (maximum height gain of the most depressed portion of the fracture) was calculated (Fig. 3).
Pre-operative mid-sagittal (A) and mid-coronal (B) CBCT reconstructed images demonstrating vertebral height and vertebral kyphotic angle measurements. Same findings on post-operative mid-sagittal (C) and mid-coronal (D) CBCT. (1) anterior mid-sagittal height, (2) central mid-sagittal height, (3) posterior mid-sagittal height, (4) right lateral mid-coronal, (5) left lateral mid-coronal; dotted lines: local kyphotic angle. In this patient, the most depressed height was on point 1, with a restoration of 6 mm. Local kyphotic angle changed from 16° (pre-operative) to 8° (post-operative)
CBCT and fluoroscopic images were reviewed to assess intra-procedural loss of height restoration after balloon deflation and quoted as “absent”, “minor” (< 2 mm) or “major” (> 2 mm). Implants malpositioned and/or cement leakages were also recorded and classified based on the CIRSE classification for complications [27]. Technical success was defined as a complete insertion and expansion of the stents, with the posterior margin beyond the posterior wall, without a major loss of height following balloon deflation.
The local kyphotic angle was measured on the one-year follow-up radiographs. Radiographs were also reviewed to look for any new adjacent vertebral fracture.
Statistical Analysis
All the analyses were performed using R software: R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. Descriptive statistics were used to present standard measurements for quantitative and qualitative variables. Bayesian inference analysis and Markov Chain Monte Carlo methods were preferred to conventional frequency analysis for significance testing, since these permit calculation of significance level (“p value” equivalent), as well as quantifying a probability of significance. Intercepts with a probability of being positive of more than 97.5% or less than 2.5%, which did not contain zero in the credibility interval, were considered statistically significant. Mixed model linear regression was used to analyse vertebral kyphosis angle correction, including a fixed effect (“Time”: pre-operative and post-operative) and two random effects (“Subjects” and “Reviewers” (senior and junior)) to account for repeated data measurements pre- and post-operatively. Vertebral height correction pre- and post-operatively was estimated using the same method, including the random effect “Subjects” in order to consider the repetition of data for the same subject. Multivariate modelling was used to evaluate the effects of age, sex, intervention delay, fracture type and fracture level on procedural success.
Results
Thirty-nine patients (26 Men, 13 women; mean age 33 years, range 15–57 years) fulfilled the inclusion criteria and were thus included in this study. The mean interval between injury and treatment was 5 days (range 1–10). Spinal fractures were classified as A.1.1 (n = 3), A.1.2 (n = 31), A.1.3 (n = 1) and A.3.1 (n = 4), according to the AO classification. T5 to L5 fractures were treated, but the majority were located at the thoracolumbar junction (70% between T12 and L2; Table 1). In 12 patients, vertebroplasty at a distant level was performed during the same procedure to manage acute compression fractures with no/minimal deformation (i.e. local kyphotic angle < 15°). All cases but four had similar stent sizes on both sides (large = 25 cases; medium = 7 cases; small = 3 cases). There were three cases of different-sized stents and one case of a unilateral stent, all for asymmetric fractures.
Mean vertebral height gain of the most depressed component of the fracture was 3.8 mm (95% CI 3.36–4.50; P(> 3 mm) = 99.9%, statistically significant); mean vertebral kyphosis correction was 4.3° (95% CI 3.50–5.20; P(> 3°) = 99.9%, statistically significant); and mean Beck Index correction was 0.07 [95% CI 0.053–0.11] (Table 2). Stent recoil following balloon removal was observed in 47% of cases (8% “major”; 39% “minor”), with “major” recoil characterized by a loss of vertebral height gain of > 2 mm (Fig. 4). Technical success was therefore 92%, with no equipment failures. Seven (18%) asymptomatic cement leaks were noted on post-procedural CBCT (all grade 1 according to CIRSE classification). There were no other complications. No predictive factors (e.g. age, type of fracture, trauma to procedure time interval) for procedural success were identified, other than a minor contribution for male sex (OR = 19.2 (95% CI 1.03–730; P(OR > 1) = 0.98).
There were no new vertebral fractures or significant modification of the local kyphotic angle on follow-up plain radiographs after 1 year.
Discussion
Percutaneous vertebral augmentation techniques have emerged as an alternative to conservative management in patients with acute traumatic vertebral compression fractures, without neurological deficit, offering early mobilization and long-term bone consolidation to non-surgical candidates [28, 29]. The first cadaveric experience of a home-made vertebral stenting system was published in 2002 [30], and the first clinical experience with a VBS device was reported in 2011 [31, 32]. Since then, several clinical studies have shown the efficacy of VBS to restore height in vertebral compression fractures, with an average restoration of vertebral kyphosis by 3.2° [33,34,35,36,37]. These publications mainly comprised osteoporotic patients, with an average age over 65 years [33,34,35,36,37]. In contrast, the average age was 33.6 years in our study, significantly younger than the traumatic group in the study of Diel et al. [35].
Our results suggest that VBS is a clinically effective and safe procedure in this patient population. Technical success in our study was 92%, with 3 “major” stent recoils following balloon deflation, resulting in a loss of vertebral height gain. There was no equipment failure. Other studies have reported cannula failure, balloon/stent misplacement and stent mal-deployment in 9–18% of cases, attributed to sclerotic bone and more chronic osteoporotic fractures [34, 36]. Asymptomatic cement leaks were seen on CBCT in 18% of cases, comparable to most other VBS and BK studies [31, 35, 38, 39]. We did not encounter any clinically significant side effects, infection or neural compromise as reported elsewhere [34, 35]. High-quality image guidance is essential for procedural success. All procedures were performed under CBCT guidance, in contrast to fluoroscopic-guidance alone in surgical series’ [30, 32]. CT-guidance permits precise planning of the cannula trajectory and delineation of skin entry-point (particularly for higher thoracic levels); accurate placement of working sleeves and delivery devices in all imaging planes; and confirmation of precise stent position prior to balloon inflation. Continuous fluoroscopic acquisition is mandatory during cement injection, to monitor the cement “cloud” and avoid significant/symptomatic leakage. This also likely contributed by avoiding stent/balloon misplacement, which can lead to disastrous complications if the vertebral cortex is perforated. It also enabled accurate treatment of challenging upper/mid-thoracic fractures (up to T5, which to our knowledge have not been described in other studies).
Immediate restoration of vertebral body height, correction of vertebral kyphotic angle and Beck Index were statistically significant. Our one-year radiographic follow-up did not demonstrate any new fractures or secondary loss of height, which is likely due to the absence of osteoporosis in our study population, keeping in mind that the results should be interpreted with caution due to differing imaging modalities and patient positioning at the late follow-up. Directly comparing vertebral restoration outcomes to other studies is problematic, because restoration potential depends on fracture classification/severity/delay, which are functions of variable patient populations. Moreover, the majority of VBS surgical series also utilized plain radiographs to assess vertebral restoration, where estimation of VBS effect may be significantly confounded by variable ligamentotaxis due to differences in pre- and post-operative radiographic positioning [40]. In this respect, CBCT provides a more accurate measurement attributable to VBS alone, since patients under GA are imaged in precisely the same position pre- and post-operatively, with fixed ligamentotaxis effects.
We believe that our encouraging results are partly due to careful patient selection. All patients were treated urgently (within 10 days of trauma, 48 h of referral, and as soon as practically feasible). Patients with fractures older than 10 days were offered PV rather than VBS according to our institutional protocol, since in our (unpublished) experience, successful percutaneous fracture reduction after this time-point is significantly hindered by dense, reparative bone, coinciding with the onset of the reparative phase of bone healing (1–2 weeks post-injury [41]). Type/level of fracture and age/sex of patient did not influence outcomes on multivariate analysis, but this should be interpreted with caution given the heterogeneous, skewed sample (majority of thoracolumbar type A1.2 fractures).
Potential limitations of VBS include elastic vertebral recoil following balloon deflation, resulting in loss of height restoration prior to cement injection. Although VBS significantly reduces recoil compared with BK ex vivo, a single in vivo study did not demonstrate superiority over BK, possibly due to technical difficulties [42,43,44]. There are currently no studies comparing VBS with alternative PVAS for traumatic fractures in young patients, despite the availability of several alternative devices (including Vertelift, Osseofix, Spine Jack, and Kiva implants) which have demonstrated promising initial results in osteoporotic patients [2]. The use of PMMA bone cement (which is mandatory with VBS) in a young population can also be a source a controversy, as the safety and/or adverse effects of PMMA on a long-term basis are not yet fully understood [45, 46]. In this series, the benefits of the intervention were considered to significantly outweigh any delayed adverse risk of PMMA utilization and such decisions were clearly explained to the patients or guardians when collecting informed consent. Finally, although restoration of anatomy and sagittal balance to prevent kyphotic deformity and maintain biomechanics is an accepted indication, there is currently no data evaluating long-term effects of PVAS in traumatic non-osteoporotic fractures in young patients, and its precise role in management remains unclear. Unfortunately, one limitation is the inability to prove that the use of VBS is cost-effective in this subpopulation, even though early mobilization should theoretically help to resume working activities sooner than with conservative treatment [47].
Study limitations include a small sample size and selective inclusion criteria, limiting applicability to a wider patient cohort. The retrospective design limits availability of clinical data, follow-up and confounding factors (e.g. analgesic therapy). There was no comparison group, and follow-up was at a relatively short interval. The comparison between the immediate CBCT and the one-year conventional radiographs is also limited as it involved different imaging modalities and different patient positions. Finally, bone mineral density was not measured prior to the intervention in every case, but our selection criteria make underlying osteoporosis/osteopenia unlikely.
In conclusion, percutaneous image-guided VBS is a safe, effective treatment for traumatic vertebral compression fractures in young non-osteoporotic patients, facilitating vertebral height restoration. Further, larger studies with longer follow-up periods are required to confirm efficacy, and evaluate the role of VBS versus alternative PVAS in the management of this specific patient cohort.
References
Filippiadis DK, Marcia S, Masala S, et al. Percutaneous vertebroplasty and kyphoplasty: current status, new developments and old controversies. Cardiovasc Intervent Radiol. 2017;40(12):1815–23. https://doi.org/10.1007/s00270-017-1779-x.
Filippiadis DK, Marcia S, Ryan A, et al. New implant-based technologies in the spine. Cardiovasc Intervent Radiol. 2018;41(10):1463–73. https://doi.org/10.1007/s00270-018-1987-z.
Amoretti N. Early percutaneous vertebroplasty helps motorsport professionals to resume competition soon after vertebral fracture. Eur Radiol. 2018;28(7):2870–1. https://doi.org/10.1007/s00330-017-5250-9.
Huwart L, Foti P, Andreani O, et al. Vertebral split fractures: technical feasibility of percutaneous vertebroplasty. Eur J Radiol. 2014;83(1):173–8.
Amoretti N, Huwart L. Combination of percutaneous osteosynthesis and vertebroplasty of thoracolumbar split fractures under CT and fluoroscopy guidance: a new technique. Cardiovasc Intervent Radiol. 2014;37(5):1363–8. https://doi.org/10.1007/s00270-014-0849-6.
de Falco R, Scarano E, Di Celmo D, et al. Balloon kyphoplasty in traumatic fractures of the thoracolumbar junction. Preliminary experience in 12 cases. J Neurosurg Sci. 2005;49(4):147–53.
Hartmann F, Gercek E, Leiner L, et al. Kyphoplasty as an alternative treatment of traumatic thoracolumbar burst fractures Magerl type A3. Injury. 2012;43(4):409–15. https://doi.org/10.1016/j.injury.2010.03.025.
Tsai PJ, Hsieh MK, Fan KF, et al. Is additional balloon Kyphoplasty safe and effective for acute thoracolumbar burst fracture? BMC Musculoskelet Disord. 2017;18(1):393. https://doi.org/10.1186/s12891-017-1753-4.
Verlaan JJ, Somers I, Dhert WJ, et al. Clinical and radiological results 6 years after treatment of traumatic thoracolumbar burst fractures with pedicle screw instrumentation and balloon assisted endplate reduction. Spine J. 2015;15(6):1172–8. https://doi.org/10.1016/j.spinee.2013.11.044.
Hitchon PW, Abode-Iyamah K, Dahdaleh NS, et al. Nonoperative management in neurologically intact thoracolumbar burst fractures: clinical and radiographic outcomes. Spine (Phila Pa 1976). 2016;41(6):483–9. https://doi.org/10.1097/brs.0000000000001253.
Shamji MF, Roffey DM, Young DK, et al. A pilot evaluation of the role of bracing in stable thoracolumbar burst fractures without neurological deficit. J Spinal Disord Tech. 2014;27(7):370–5. https://doi.org/10.1097/BSD.0b013e31826eacae.
Thomas AM, Fahim DK. Stand-alone balloon kyphoplasty for the treatment of a traumatic burst fracture in a pediatric patient: case report. World Neurosurg. 2019. https://doi.org/10.1016/j.wneu.2019.01.184.
Belkoff SM, Mathis JM, Fenton DC, et al. An ex vivo biomechanical evaluation of an inflatable bone tamp used in the treatment of compression fracture. Spine. 2001;26(2):151–6.
Wang H, Sribastav SS, Ye F, et al. Comparison of percutaneous vertebroplasty and balloon kyphoplasty for the treatment of single level vertebral compression fractures: a meta-analysis of the literature. Pain Phys. 2015;18(3):209–22.
Saliou G, Rutgers DR, Kocheida EM, et al. Balloon-related complications and technical failures in kyphoplasty for vertebral fractures. AJNR Am J Neuroradiol. 2010;31(1):175–9.
Verlaan JJ, van de Kraats EB, Oner FC, et al. The reduction of endplate fractures during balloon vertebroplasty: a detailed radiological analysis of the treatment of burst fractures using pedicle screws, balloon vertebroplasty, and calcium phosphate cement. Spine (Phila Pa 1976). 2005;30(16):1840–5.
Vanni D, Pantalone A, Bigossi F, et al. New perspective for third generation percutaneous vertebral augmentation procedures: preliminary results at 12 months. J Craniovertebral Junction Spine. 2012;3(2):47–51.
Marcia S, Saba L, Marras M, et al. Percutaneous stabilization of lumbar spine: a literature review and new options in treating spine pain. Br J Radiol. 2016;89(1065):20150436.
Muto M, Marcia S, Guarnieri G, et al. Assisted techniques for vertebral cementoplasty: why should we do it? Eur J Radiol. 2015;84(5):783–8. https://doi.org/10.1016/j.ejrad.2014.04.002.
Vanni D, Pantalone A, Bigossi F, et al. New perspective for third generation percutaneous vertebral augmentation procedures: preliminary results at 12 months. J Craniovertebral Junction Spine. 2012;3(2):47–51.
Marcia S, Saba L, Marras M, et al. Percutaneous stabilization of lumbar spine: a literature review and new options in treating spine pain. Br J Radiol. 2016;89(1065):20150436.
http://synthes.vo.llnwd.net/o16/LLNWMB8/INT%20Mobile/Synthes%20International/Product%20Support%20Material/legacy_Synthes_PDF/DSEM-SPN-0614-0144-1_LR.pdf. Available online on 30 May 2019.
Martín-López JE, Pavón-Gómez MJ, Romero-Tabares A, et al. Stentoplasty effectiveness and safety for the treatment of osteoporotic vertebral fractures: a systematic review. Orthop Traumatol Surg Res. 2015;101(5):627–32. https://doi.org/10.1016/j.otsr.2015.06.002.
Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201.
Bousson V, Hamze B, Odri G, et al. Percutaneous vertebral augmentation techniques in osteoporotic and traumatic fractures. Semin Intervent Radiol. 2018;35(4):309–23. https://doi.org/10.1055/s-0038-1673639.
Beck E. Radiographic measuring methods in vertebral fractures. Hefte zur Unfallheilkunde. 1971;108:36–7.
Filippiadis DK, Binkert C, Pellerin O, et al. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40(8):1141–6. https://doi.org/10.1007/s00270-017-1703-4.
Maestretti G, Sutter P, Monnard E, et al. A prospective study of percutaneous balloon kyphoplasty with calcium phosphate cement in traumatic vertebral fractures: 10-year results. Eur Spine J. 2014;23(6):1354–60.
Piazzolla A, De Giorgi S, Solarino G, et al. Vertebral body reconstruction system B-Twin(R) versus corset following non-osteoporotic Magerl A.12 thoracic and lumbar fracture. Functional and radiological outcome at 12 month follow-up in a prospective randomized series of 50 patients. Orthop Traumatol Surg Res OTSR. 2011;97(8):846–51.
Furderer S, Anders M, Schwindling B, et al. Vertebral body stenting. A method for repositioning and augmenting vertebral compression fractures. Der Orthopade. 2002;31(4):356–61.
Muto M, Greco B, Setola F, et al. Vertebral body stenting system for the treatment of osteoporotic vertebral compression fracture: follow-up at 12 months in 20 cases. Neuroradiol J. 2011;24(4):610–9.
Klezl Z, Majeed H, Bommireddy R, et al. Early results after vertebral body stenting for fractures of the anterior column of the thoracolumbar spine. Injury. 2011;42(10):1038–42.
Werner CM, Osterhoff G, Schlickeiser J, et al. Vertebral body stenting versus kyphoplasty for the treatment of osteoporotic vertebral compression fractures: a randomized trial. J Bone Joint Surg Am. 2013;95(7):577–84.
Matejka J, Zeman J, Belatka J, et al. Vertebral body augmentation using a vertebral body stent. Acta chirurgiae orthopaedicae et traumatologiae Cechoslovaca. 2011;78(5):442–6.
Diel P, Roder C, Perler G, et al. Radiographic and safety details of vertebral body stenting: results from a multicenter chart review. BMC Musculoskelet Disord. 2013;14:233.
Thaler M, Lechner R, Nogler M, et al. Surgical procedure and initial radiographic results of a new augmentation technique for vertebral compression fractures. Eur Spine J. 2013;22(7):1608–16.
Hartmann F, Griese M, Dietz SO, et al. Two-year results of vertebral body stenting for the treatment of traumatic incomplete burst fractures. Minim Invasive Ther Allied Technol. 2015;24(3):161–6. https://doi.org/10.3109/13645706.2014.962546.
Hulme PA, Krebs J, Ferguson SJ, et al. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine. 2006;31(17):1983–2001.
Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009;373(9668):1016–24.
Cawley DT, Sexton P, Murphy T, et al. Optimal patient positioning for ligamentotaxis during balloon kyphoplasty of the thoracolumbar and lumbar spine. J Clin Neurosci. 2011;18(6):834–6. https://doi.org/10.1016/j.jocn.2010.10.009.
Kostenuik P, Mirza FM. Fracture healing physiology and the quest for therapies for delayed healing and nonunion. J Orthop Res. 2017;35(2):213–23. https://doi.org/10.1002/jor.23460.
Rotter R, Martin H, Fuerderer S, et al. Vertebral body stenting: a new method for vertebral augmentation versus kyphoplasty. Eur Spine J. 2010;19(6):916–23.
Disch AC, Schmoelz W. Cement augmentation in a thoracolumbar fracture model: reduction and stability after balloon kyphoplasty versus vertebral body stenting. Spine. 2014;39(19):E1147–53.
Werner CM, Osterhoff G, Schlickeiser J, et al. Vertebral body stenting versus kyphoplasty for the treatment of osteoporotic vertebral compression fractures: a randomized trial. J Bone Joint Surg Am. 2013;95(7):577–84.
Lewis G. Properties of acrylic bone cement: state of the art review. J Biomed Mater Res. 1997;38(2):155–82.
Lieberman IH, Togawa D, Kayanja MM. Vertebroplasty and kyphoplasty: filler materials. Spine J. 2005;5(6 Suppl):305S–16S.
Martelli N, Devaux C, van den Brink H, Pineau J, Prognon P, Borget I. A systematic review of the level of evidence in economic evaluations of medical devices: the example of vertebroplasty and kyphoplasty. PLoS ONE. 2015;10(12):e0144892. https://doi.org/10.1371/journal.pone.0144892.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garnon, J., Doré, B., Auloge, P. et al. Efficacy of the Vertebral Body Stenting System for the Restoration of Vertebral Height in Acute Traumatic Compression Fractures in a Non-osteoporotic Population. Cardiovasc Intervent Radiol 42, 1579–1587 (2019). https://doi.org/10.1007/s00270-019-02265-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02265-y