Abstract
Background
Transarterial radioembolization (TARE) has emerged as a newer regional therapy to transarterial chemoembolization (TACE) for treatment of unresectable hepatocellular carcinoma (HCC). The aim of this study is to compare clinical outcomes of both the techniques.
Methods
Online search for studies comparing TARE to TACE from 2005 to present was performed. Primary outcome was overall survival rate for up to 4 years. Secondary outcomes included post-treatment complications and treatment response. Quality of included studies was evaluated by STrengthening the Reporting of OBservational studies in Epidemiology criteria. Relative risk (RR) and 95 % confidence intervals (CI) were calculated from pooled data.
Results
The search strategy yielded 172 studies, five met selection criteria and included 553 patients with unresectable HCC, 284 underwent TACE and 269 underwent TARE. Median ages were 63 and 64 years for TACE and TARE, respectively. Meta-analysis showed no statistically significant difference in survival for up to 4 years between the two groups (HR = 1.06; 95 % CI 0.81–1.46, p = 0.567). TACE required at least one day of hospital stay compared to TARE which was mostly an outpatient procedure. TACE had more post-treatment pain than TARE (RR = 0.51, 95 % CI 0.36–0.72, p < 0.01), but less subjective fatigue (RR = 1.68, 95 % CI 1.08–2.62, p < 0.01). There was no difference between the two groups in the incidence of post-treatment nausea, vomiting, fever, or other complications. In addition, there was no difference in partial or complete response rates between the two groups.
Conclusion
TARE appears to be a safe alternative treatment to TACE with comparable complication profile and survival rates. Larger prospective randomized trials, focusing on patient-reported outcomes and cost–benefit analysis are required to consolidate these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary treatment for hepatocellular carcinoma (HCC) is surgical resection [1].Footnote 1 Unfortunately <30 % of patients with HCC are eligible for surgery [2], mainly because of the multiplicity of the lesions which often occurs on a background of chronic liver disease and low resectability due to late diagnosis and high recurrence following a curative intent surgery [3–8]. Locoregional therapies, such as transarterial chemoembolization (TACE) and radioembolization (TARE) have emerged as tools for palliation, surgical down staging, and bridging therapy prior to transplant.
Although both TACE and TARE are delivered through the hepatic artery, the mechanics are quite different. TACE involves the injection of chemotherapy into liver tumors with a macroembolic effect and arterial occlusion in addition to molecular suppression of tumor growth [9, 10]. TARE involves injection of β-emitting Yttrium-90 (Y90) integrated to either inside the glass matrix or on the surface of the resin microspheres [11, 12]. This represents a novel transarterial brachytherapy that allows concentrated beta radiation administration to tumor tissues while minimizing damages to surrounding liver parenchyma [13, 14]. Based on natural disruptions to the microvasculature surrounding liver tumors, it appears to be tumor selective and can be delivered selectively with sub-segmental, segmental, lobar, or whole-liver approaches [15].
Randomized trials and subsequent meta-analysis have shown a survival advantage for TACE compared to no treatment [16–18]. Hence, the American Association for the Study of Liver Diseases (AASLD) has recommended TACE for intermediate stage or barcelona clinic liver cancer (BCLC)-B stage of unresectable HCC [17–19]. TARE had been advocated as the preferred therapy for HCC with portal vein thrombosis because of lower risk of hepatic parenchymal damage and ischemia [20–22]. Recent institutional reports assessing the efficacy and safety of patients receiving TARE with TACE suggest comparable tumor response and survival rates in patients with unresectable HCC [23, 24].
There are sparse published randomized trails or large-scale prospective studies evaluating the survival advantage conferred by TARE for unresectable HCC. Therefore, in this study, we aimed to systematically review and examine the available evidence comparing clinical outcomes following TARE and TACE for unresectable HCC.
Materials and Methods
Literature Search and Study Selection
A comprehensive search of MEDLINE, EMBASE, Google Scholar, SCOPUS, and the Cochrane database was performed for all articles published in the English language evaluating survival outcomes following TACE and TARE for unresectable HCC. Only comparative studies were included as these are the only ones that can be mathematically pooled. The search was conducted using the following MeSH terms: “Hepatocellular carcinoma” and “Chemoembolization” or “Radio embolization” and “HCC” AND “TACE” or “TARE” or “Yttrium-90” or “Y90.” The related articles’ function was used to expand the search from each relevant study identified. All citations and abstracts identified were thoroughly reviewed. Bibliography of retrieved papers was further screened for any additional eligible studies. The latest search was performed in February 1, 2016 and included literature published from the year 2005 to present. The data were pooled and a meta-analysis performed using statistical methods as detailed below. In all reported studies, there was no difference between the compared groups regarding age, sex, histopathology, and preoperative comorbidities.
Outcomes of Interest
The primary end point was survival. We analyzed up to 4 years of overall survival. The secondary endpoints included post-treatment morbidity, nausea and vomiting, pain, fatigue, fever, complications, partial/complete response (RECIST Criteria), and disease-specific mortality.
Inclusion Criteria
In order to be included in the analysis, studies had to
-
(1)
compare the outcome measures mentioned above between unresectable hepatocellular cancer patients who had TARE and those who had TACE;
-
(2)
include, when the same institution reported two studies, the one of better quality (e.g., larger sample size) or the most recent publication.
Exclusion Criteria
Studies were excluded from this analysis if
-
(1)
they were either noncomparative studies or single technique case series;
-
(2)
the outcomes of interest were not reported; and
-
(3)
there was an overlap between authors, institutions, or patient cohorts.
Data Extraction and Quality Assessment
Two reviewers (L.L. and O.P.) independently extracted the following data from each study eligible for the meta-analysis: study characteristics (first author, year of publication, study design) and population characteristics (number of patients included, age, sex, ethnicity, and Child-Pugh class). Up to 4 years of overall survival, overall post-treatment morbidity, nausea and vomiting, pain, fatigue, fever, occurrence of three or more complications, complete pathological response, partial response, stable disease, disease progression, and disease-specific mortality.
Quality assessment of the included studies was conducted using the 22-item checklist “STrengthening the Reporting of OBservational studies in Epidemiology” (STROBE), and this scoring system gives a quality score to studies based on various aspects of study design and reporting. Each item on the checklist is awarded the scores 0–1, except item 1 for title and abstract has 0–2 points, item 12 that evaluates the quality of statistical method has 0–4 points, and item 13 that evaluates the results of participants, item 14 that evaluates the description of the results, and item 16 that evaluates the main results have 0–3 points, making the possible maximum score to be 33 [25, 26].
Statistical Analysis
All statistical analyses were performed using open-source software R version 3.1.4 (Vienna, Austria). Individual-level data were extracted from the studies. For continuous outcomes, data were pooled using the standardized weighted mean difference (WMD). The majority of included studies were cohort studies, and meta-analyses were performed using the Mantel Haenszel method or DerSimonian and Laird [27] model based on whether heterogeneity was statistically significant. Risk ratio (RR) and its 95 % confidence interval were used as the primary measure of treatment effect for dichotomous variables. WMD and 95 % confidence interval were used to evaluate continuous variables. The point estimate was considered statistically significant when the p value was <0.05. Publication bias was evaluated by visual examination of funnel plots for symmetry and by formal statistical calculation using Egger’s test [28]. To evaluate the validity of overall study analysis, subset analysis of higher quality studies (those with a score of >30 on Strobe criteria) was performed.
Interventional Techniques
TARE
Preprocedural angiogram is done to determine eligibility, tumor mapping, hepato-pulmonary shunting, and occlude arterial branches preventing extrahepatic deposition. Following this, an angiogram in which the Yttrium-90 (Y90) was delivered either by the glass matrix (Therasphere) or in resin microspheres (SIRSphere) was done.
TACE
Included studies used chemotherapy drugs including doxorubicin, mitomycin, adriamycin, and cisplatin individually or in combination with contrast media with emulsion.
Results
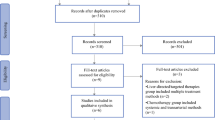
Our search strategy yielded 172 publications. Five studies met inclusion criteria and were included in the final meta-analysis (Fig. 1). Studies were excluded due to reporting incomplete data, noncomparison, treatment neither TARE nor TACE, and combined TACE and TARE. All included studies were retrospective in design. Patient characteristics in included studies are summarized in Table 1. There was no difference between study patients in regard to pretreatment comorbidities, etiology, lesion location, tumor histopathology, associated portal vein thrombosis, source of diagnosis (pathology/radiology), clinical staging including Child-Pugh Class, ECOG score, MELD score, UNOS staging, Okuda class, BCLC Stage, CLIP stage, and Karnofsky score. Treatment characteristics are summarized in Table 2. Survival data are summarized in Table 3.
Studies had a mean score of 29 on STROBE criteria. The following factors affected the scores: no study estimated sample size, four studies did not translate relative risk into absolute risk, three studies did not describe the methods of examining subgroups and interactions, two studies did not perform subgroup or sensitivity analysis, two studies did not describe source of funding, and one study lacked matching criteria (Fig. 2).
Survival
Survival information was extracted from the five studies. This included 284 patients undergoing TACE and 269 patients undergoing TARE. Male-to-female ratio for TACE is 82:18 and for TARE is 77:23. Median age for TACE is 63 with a range of 33–88, whereas median age for TARE is 64 with range of 29–88. Overall survival at 1 year was 42 % for TARE subjects compared to 46 % for TACE. Statistically there was no difference noticed between two modalities (RR = 0.93, 95 % CI 0.81–1.08, p = 0.33). At 2 years, more TARE patients were alive than those that received TACE (27 vs. 18 %), the difference of which was statistically significant (RR = 1.36, 95 % CI 1.05–1.76, p = 0.02). At 3 years, more TARE patients survived (14 vs. 8 %), yet no statistically significant difference was noted (RR = 1.27, 95 % CI 0.88–1.84, p = 0.20). At 4 years, subjects alive from both TACE and TARE were 4 % with no statistically significant difference in survival (RR = 1.64, 95 % CI 0.80–3.34, p = 0.17). At 5 years, only 1 % of subject population was alive from both TACE and TARE treatment modalities. There was minimal heterogeneity among studies (p > 0.05). Disease-specific mortality (RR = 1.58, 95 % CI 0.49–5.10, p = 0.44) did not show difference between studies, but high heterogeneity was noted (π2 = 0.6462, p = 0.0015, I2 = 90 %).
Complications
Postprocedural complications are extracted if they are grade 3 or more according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (Version 3.0) [29]. Pain is less in TARE than TACE (RR = 0.51, 95 % CI 0.36–0.72, p < 0.01) with no heterogeneity noted (π2 = 0.09, p = 0.1648, I2 = 44.5 %). Postprocedural fatigue is more in TARE than TACE (RR = 1.68, 95 % CI 1.08–2.62, p < 0.01) with no heterogeneity (π2 = 0.0573, p = 0.1547, I2 = 50.6 %). Nausea and vomiting (RR = 0.83, 95 % CI 0.60–1.22, p = 0.35), fever (RR = 1.16, 95 % CI 0.07–18.6, p = 0.92), and other complications including diarrhea, anorexia, headache, chest pain, confusion, gastric ulceration, bleeding from puncture site, rash, varicella zoster infection, and hepatic abscess (RR = 1.09, 95 % CI 0.67–1.76, p = 0.74) have shown no statistical significance favoring either of the techniques with no heterogeneity noted (p > 0.05). Analysis of data for overall post-treatment morbidity (RR = 0.84, 95 % CI 0.5–1.26, p = 0.41) and any study subject experiencing more than three complications (RR = 0.83, 95 % CI 0.49–1.39, p = 0.47) showed no difference between two studies.
Radiological Response to Treatment
There is no statistical difference in complete radiological response within 3 months of treatment (RR = 2.35, 95 % CI 0.76–7.28, p = 0.14), partial response (RR = 0.85, 95 % CI 0.55–1.31, p = 0.45), disease progression (RR = 1.07, 95 % CI 0.58–1.97, p = 0.84) with no heterogeneity (p > 0.05), whereas stable disease (RR = 0.96, 95 % CI 0.38–2.42, p = 0.92) shows high heterogeneity (π2 = 0.4348, p = 0.0289, I2 = 71.8 %).
Subgroup Analysis
Subset analysis of higher quality studies (those with a score of >30 on STROBE criteria [30, 31]) showed that the results were similar to the overall analysis.
Discussion
TACE was shown to be a promising intervention for unresectable HCC, recently TARE has been introduced for treatment of those patients; it formerly used to be performed predominantly in patients with portal vein thrombosis [32]. In this meta-analysis, TARE and TACE have shown similar survival rates, partial and complete radiological and clinical response to treatment. In terms of toxicity, TARE patients had less pain and more fatigue. Subgroup analysis of studies with relatively higher quality studies indicates similar results, suggesting validity of the overall analysis results.
The results of the current analysis are concordant with what was described in the literature. Other authors as Geschwind et al. and Lewandowski et al. have demonstrated TARE as safe and effective with survival outcomes similar to TACE [33, 34]. Salem et al. have shown increased quality of life among patient treated with TARE compared to TACE [35]. One of the clear indications for TARE includes lesions close to large vasculature in the liver in which TACE may have a higher complication profile.
The role of TARE in the management of unresectable HCC remains undefined. TACE is the standard of care in the management of intermediate stage unresectable disease as defined by the BCLC staging system [36] and reinforced by recommendations of AASLD [19]. This targets unresectable multinodular disease. For TARE, initial results suggest efficacy in advanced-stage disease where guidelines would only offer the multikinase inhibitor, sorafenib. Mazzaferro et al. in a phase II study demonstrated efficacy in both advanced and intermediate stage HCC [37], and in subgroup analysis of the data from the phase III, SHARP Trial in the advanced or BCLC-C group, the overall survival was 11 months which in comparison with sorafenib was reported to be 9.7 months [38]. Head-to-head comparisons with a randomized control trial of sorafenib and TARE are underway, for example, the SARAH and the STOP-HCC.Footnote 2,Footnote 3
The efficacy of TARE in the intermediate group (BCLC B) has been demonstrated in multiple reports including the phase II trial by Mazzaferro with median overall survival as 17 months in compared to that reported with TACE ranging from 16 to 22 months [37].Footnote 4 Comparative studies such as the PREMEIRE trial a randomized phase II trial between TARE and TACE are undergoing enrollment [39].
From a cost–benefit standpoint, TARE is almost three times as expensive as TACE. Nonetheless, the literature has shown that TACE has 60 % subjects receiving multiple treatments compared to 70 % of TARE receiving single treatment [39]. A comprehensive cost–benefit analysis needs to be done to examine those differences; however, it can be suggested that even though the approximate cost of single treatment is high in TARE, this can be comparable due to less need of treatments and therefore may be considered favorable to alleviate some pressure off the already inundated health care system and enhances patient satisfaction and convenience.
Limitations of this study include retrospective design of included studies and inherent selection bias. The long-time interval where selected studies were analyzed where there have been advances in technology and technique may have influenced the results. In addition, results may have been biased depending on different modalities and regimens usage such as different particles in the TARE group and varying chemotherapy in TACE group. Most importantly cost effectiveness leading to health savings was not discussed in any studies.
In conclusion, based on current available data, TARE is comparable to TACE with similar complication profile and survival rates. Larger prospective randomized trials, focusing on patient-reported outcomes and cost–benefit analysis, are required to consolidate these results. An important outcome to examine would be time to progression especially as we see wait times for liver transplant increasing. As results are reported, the role of TARE in HCC treatment algorithm will likely get to be redefined.
Notes
Vilgrain V, Abdel-Rehim M, et al. SARAH Trial Group. Radioembolization with yttrium-90 microspheres versus sorafenib for treatment of advanced hepatocellular carcinoma (SARAH): study protocol for a randomized controlled trial. Trials. 2014 Dec 3;15:474.
A Phase III Clinical Trial of Intra-arterial TheraSphere® in the Treatment of Patients With Unresectable Hepatocellular Carcinoma (HCC). https://clinicaltrials.gov/ct2/show/NCT01556490?term=stop+hcc&rank=1.
An Investigator Initiated Multicenter Prospective Randomized Study of Chemoembolization Versus Radioembolization for the Treatment of Hepatocellular Carcinoma. https://clinicaltrials.gov/ct2/show/NCT00956930?term=premiere+radioembolization&rank=1.
References
El-Serag K. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60(5):1767–75.
Mittal S, Mittal S, El-Serag HB, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2–6.
El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167(18):1983–9.
Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2007;4(7):424–32.
Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7(8):448–58.
Lau WY. Future perspectives for hepatocellular carcinoma. HBP. 2003;5(3):206–13.
Llovet JM, Llovet JM, Fuster J, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10(2 Suppl 1):S115–20.
Bilimoria MM, Lauwers GY, Doherty DA, Nagorney DM, Belghiti J, Do KA, Regimbeau JM, Ellis LM, Curley SA, Ikai I, Yamaoka Y, Vauthey JN, International Cooperative Study Group on Hepatocellular Carcinoma. Underlying liver disease, not tumor factors, predicts long-term survival after resection of hepatocellular carcinoma. Arch Surg. 2001;136(5):528–35.
Leelawat K, Laisupasin P, Kiatdilokrut A, Pongtongpool T, Narong S, Samkhumphim N, Ket-Horm S. The effect of doxorubicin on the changes of serum vascular endothelial growth factor (VEGF) in patients with hepatocellular carcinoma after transcatheter arterial chemoembolization (TACE). J Med Assoc Thai. 2008;91(10):1539–43.
Virmani S, Rhee TK, Ryu RK, Sato KT, Lewandowski RJ, Mulcahy MF, Kulik LM, Szolc-Kowalska B, Woloschak GE, Yang GY, Salem R, Larson AC, Omary RA. Comparison of hypoxia-inducible factor-1alpha expression before and after transcatheter arterial embolization in rabbit VX2 liver tumors. J Vasc Interv Radiol. 2008;19(10):1483–9.
Sato K, Sato K, Lewandowski RJ, Lewandowski RJ, Bui JT, Bui JT, Omary R, Hunter RD, Kulik L, Kulik L, Mulcahy M, Mulcahy M, Liu D, Chrisman H, Resnick S, Nemcek AA Jr, Vogelzang R, Vogelzang R, Salem R. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol. 2006;29(4):522–9.
Murthy R, Kamat P, Nuñez R, Nuñez R, Salem R, Salem R. Radioembolization of yttrium-90 microspheres for hepatic malignancy. Semin Intervent Radiol. 2008;25(1):48–57.
Pöpperl G, Helmberger T, Münzing W, Schmid R, Jacobs TF, Tatsch K. Selective internal radiation therapy with SIR-Spheres in patients with nonresectable liver tumors. Cancer Biother Radiopharm. 2005;20(2):200–8.
Lim L, Gibbs P, Yip D, Shapiro JD, Dowling R, Smith D, Little A, Bailey W, Liechtenstein M. Prospective study of treatment with selective internal radiation therapy spheres in patients with unresectable primary or secondary hepatic malignancies. Intern Med J. 2005;35(4):222–7.
Ibrahim SM, Lewandowski RJ, Ryu RK, Sato KT, Gates VL, Mulcahy MF, Mulcahy MF, Kulik L, Larson AC, Larson AC, Omary RA, Salem R. Radiographic response to yttrium-90 radioembolization in anterior versus posterior liver segments. Cardiovasc Intervent Radiol. 2008;31(6):1124–32.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37(2):429–42.
Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J, Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–9.
Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–71.
Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2. doi:10.1002/hep.24199.
Jelic S, Sotiropoulos GC, ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v59–64. doi:10.1093/annonc/mdq166.
Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. 2010;116(5):1305–14.
Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, Sato KT, Benson A 3rd, Nemcek AA Jr, Gates VL, Abecassis M, Omary RA, Salem R. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47(1):71–81.
Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, Galle PR, Dufour JF, Greten TF, Raymond E, Roskams T, De Baere T, Ducreux M, Mazzaferro V, Bernardi M, Bruix J, Colombo M, Zhu A. European Association for the study of the liver, European Organisation for research and treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43.
Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, Ettorre GM, Salvatori R, Giampalma E, Geatti O, Wilhelm K, Hoffmann RT, Izzo F, Iñarrairaegui M, Maini CL, Urigo C, Cappelli A, Vit A, Ahmadzadehfar H, Jakobs TF, Lastoria S. European Network on Radioembolization with Yttrium-90 Resin Microspheres (ENRY). Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54(3):868–78.
Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. STROBE initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–24.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. STROBE initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–80.
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–81.
Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, Gansen DN, de Groen PC, Lazaridis KN, Narayanan Menon KV, Larusso NF, Alberts SR, Gores GJ, Fleming CJ, Slettedahl SW, Harmsen WS, Therneau TM, Wiseman GA, Andrews JC, Roberts LR. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36(3):714–23.
Salem R, Lewandowski RJ, Lewandowski RJ, Lewandowski RJ, Kulik L, Wang E, Wang E, Riaz A, Ryu RK, Sato KT, Gupta R, Gupta R, Nikolaidis P, Miller FH, Yaghmai V, Yaghmai V, Yaghmai V, Ibrahim SM, Senthilnathan S, Baker T, Gates VL, Gates VL, Atassi B, Newman S, Memon K, Chen R, Vogelzang RL, Nemcek AA, Resnick SA, Resnick SA, Chrisman HB, Carr J, Carr J, Omary RA, Abecassis M, Benson AB, Mulcahy MF. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(2):497–507.
Memon K, Kulik L, Lewandowski RJ, Mulcahy MF, Benson AB, Ganger D, Riaz A, Gupta R, Vouche M, Gates VL, Miller FH, Omary RA, Salem R. Radioembolization for hepatocellular carcinoma with portal vein thrombosis: impact of liver function on systemic treatment options at disease progression. J Hepatol. 2013;58(1):73–80.
Geschwind JF, Salem R, Carr BI, Soulen MC, Thurston KG, Goin KA, Van Buskirk M, Roberts CA, Goin JE. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S194–205.
Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, Omary R, Abecassis M, Salem R. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9(8):1920–8.
Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R, Baker T, Abecassis MM, Atassi R, Riaz A, Cella D, Burns JL, Ganger D, Benson AB 3rd, Mulcahy MF, Kulik L, Lewandowski R. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol. 2013;11(10):1358–65.
Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262(1):43–58.
Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, Maccauro M, Marchianò A, Bongini M, Lanocita R, Civelli E, Bombardieri E, Camerini T, Spreafico C. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57(5):1826–37.
Bruix J, Raoul J, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57(4):821–9.
Lance C, McLennan G, Obuchowski N, Cheah G, Levitin A, Sands M, Spain J, Srinivas S, Shrikanthan S, Aucejo FN, Kim R, Menon KV. Comparative analysis of the safety and efficacy of transcatheter arterial chemoembolization and yttrium-90 Radioembolization in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22(12):1697–705.
Akinwande O, Kim D, Edwards J, Brown R, Philips P, Scoggins C, Martin RC 2nd. Is radioembolization ((90)Y) better than doxorubicin drug eluting beads (DEBDOX) for hepatocellular carcinoma with portal vein thrombosis? a retrospective analysis. Surg Oncol. 2015;24(3):270–5.
Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, Staley CA 3rd, Kim HS. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21(2):224–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00270-017-1693-2.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lobo, L., Yakoub, D., Picado, O. et al. Unresectable Hepatocellular Carcinoma: Radioembolization Versus Chemoembolization: A Systematic Review and Meta-analysis. Cardiovasc Intervent Radiol 39, 1580–1588 (2016). https://doi.org/10.1007/s00270-016-1426-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-016-1426-y