Abstract
The purpose of this study was to assess the efficacy and safety of percutaneous radiofrequency (RF) ablation therapy combined with cementoplasty under computed tomography and fluoroscopic guidance for painful bone metastases. Seventeen adult patients with 23 painful bone metastases underwent RF ablation therapy combined with cementoplasty during a 2-year period. The mean tumor size was 52 × 40 × 59 mm. Initial pain relief, reduction of analgesics, duration of pain relief, recurrence rate of pain, survival rate, and complications were analyzed. The technical success rate was 100%. Initial pain relief was achieved in 100% of patients (n = 17). The mean VAS scores dropped from 63 to 24 (p < 0.001) (n = 8). Analgesic reduction was achieved in 41% (7 out of 17 patients). The mean duration of pain relief was 7.3 months (median: 6 months). Pain recurred in three patients (17.6%) from 2 weeks to 3 months. Eight patients died and 8 patients are still alive (a patient was lost to follow-up). The one-year survival rate was 40% (observation period: 1–30 months). No major complications occurred, but one patient treated with this combined therapy broke his right femur 2 days later. There was transient local pain in most cases, and a hematoma in the psoas muscle (n = 1) and a hematoma at the puncture site (n = 1) occurred as minor complications. Percutaneous RF ablation therapy combined with cementoplasty for painful bone metastases is effective and safe, in particular, for bulky tumors extending to extraosseous regions. A comparison with cementoplasty or RF ablation alone and their long-term efficacies is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pain relief is one of the priorities for most cancer patients with painful bone metastases. Until now, treatments for bone metastases have been mainly conservative, such as radiation therapy, chemotherapy, or analgesics. However, these are sometimes transient and ineffective, resulting in the taking of a large amount of analgesics such as opiate. Moreover, these patients are increasing because primary tumors are well controlled by recent advanced anticancer treatments.
Recently, there have been some reports about cementoplasty, especially vertebroplasty or radiofrequency (RF) ablation for painful bone metastases showing that it is a safe and effective way to alleviate pain. Pain relief was obtained in 75–94% in vertebroplasty [1–3] and 95–100% in RF ablation [4, 5], respectively. However, these are relatively small lesions. When bone metastases become bulky and extend outside bones, cementoplasty alone is unable to play a sufficient role. We have, therefore, devised this combined therapy. However, there are few reports regarding RF ablation combined with cementoplasty [6–8] for painful bone metastases. RF ablation itself not only has the potential for tumor necrosis but also for pain relief; moreover, cementoplasty gives additional pain relief and stabilization of osteolytic lesions. We hypothesized that this combination therapy would have a synergistic effect for painful bone metastases.
The purpose of this study was to assess the feasibility, efficacy, and safety of RF ablation therapy combined with cementoplasty for painful bone metastasis.
Materials and Methods
Patients
This study was approved by the Institutional Review Board and written informed consent was obtained from patients and their families. The procedure and possible complications were fully explained. Patients’ backgrounds are summarized in Table 1. Between October 2001 and January 2004, 17 patients who had painful bone metastases underwent RF ablation therapy combined with cementoplasty at our institution. Sixteen males and one female who were 54–81 years old (mean age: 64.2 years) had osteolytic metastases. One patient received the second combination therapy 3 months after the initial therapy. The treated numbers were 23 metastases, which were located in the thoracic spine (n = 3), lumber spine (n = 3), sacrum (n = 2), pelvic bone (n = 10), humeral head (n = 2), femur (n = 1), maxillary bone (n = 1), and mandibular bone (n = 1). The tumor size ranged from 22 × 20 × 30 mm to 125 × 67 × 160 mm (mean size: 52.0 × 40.7 × 59.0 mm). Thirteen patients with 16 lesions received radiation therapy (15–90 Gy; mean = 41 Gy, a level not given for its anticancer effect but mainly for pain relief) prior to this combination therapy. All patients were taking various analgesics on a daily basis. The primary sites were hepatocellular carcinoma (n = 6), renal cell carcinoma (n = 6), urinary bladder carcinoma (n = 2), thyroid carcinoma (n = 1), lung carcinoma (n = 1), and colorectal carcinoma (n = 1).
RF Ablation
The procedures were performed under general anesthesia with intermittent administration of fentanyl (Fentanest; Sankyo, Tokyo) and continuous infusion of propofol (Diprivan; AstraZeneca, Osaka) and inhalation of nitrous oxide in oxygen using a laryngeal mask. Multidetector row computed tomography (CT) scans (Miyabi; Siemens, Erlangen, Germany) attached with the multistar angiosystem (Multistar; Siemens, Erlangen, Germany) were available at our institution. Hence, RF ablation and inserting the cement needle were performed under CT guidance, and bone cement was injected under C-arm fluoroscopy. First, we placed two dispersive grounding pads on the patients’ thighs bilaterally and markers on the surface around the target lesions. After scanning, a precise route was determined. After sterile preparation, a small skin incision at the puncture site was made, and a 17-gauge Cool-tip needle (Radionics, Burlington, MA) was advanced up to the farthest part of the osteolytic lesion. The Cool-tip needle is a straight electrode that generates up to a 2- or 3-cm-diameter zone of necrosis. This electrode system contains integrated thermocouples for continuous temperature monitoring of the ablated tissue. The energy deposited by the electrode was controlled with a generator (Radionics, Burlington, MA).
The aim of RF ablation is to ablate tumors as widely as possible, but not to ablate beyond the outer margin of the tumors because the tumor is sometimes located close to the spinal cord or major nerves, such as the nerve root or ischiatic nerve. After confirming the location of the electrode, RF ablation was performed. As soft tissue is more easily ablated than liver parenchyma, RF ablation was started at an energy level of 50 W. The deployed energy was manually increased by 10 W every one minute up to 100 W until tissue impedance increased and further current flow was prevented (the roll-off) or up to 5–7 min. When the lesions were large enough for one session, we pulled back the needle 1–2 cm, or punctured a different route, and performed RF ablation using the same protocol. The RF ablation time and final temperature were recorded for each patient. After ablation, the electrodes were insulated and removed.
Cementoplasty
Immediately after the RF ablation procedure, an 8–13-gauge ostyneedle (Hakko, Nagano, Japan) was introduced to the lesion while confirming the needle position under CT guidance. Polymethylmethacrylate (PMMA) (Osteobond Copolymer Bone Cement; Zimmer, Warsaw, IN) was used as the bone cement. We determined the amount of bone cement by measuring the tumor size or by judging by CT the appropriate places to inject. Immediately after mixing the cement, we injected it under fluoroscopic guidance. After injecting the bone cement, the needle was left in for 5 minutes and then withdrawn. After scanning the final images and recovery from anesthesia, the procedures were concluded. When several RF ablations were required, cementoplasty was performed immediately after the last of several RF ablations.
Analysis
We analyzed pain scores at initial pain relief before and after the treatments. Pain was checked every day during the hospital stay, and, occasionally, it was checked at our outpatient office or by phone after discharge. The Wong–Baker FACES Pain Rating Scale for all patients was used and analyzed by Spearman’s correlation coefficient by rank test with tumor size, dosage of radiation therapy, numbers of RF sessions, tumor temperatures, total RF time, and volume of bone cement. The Wong–Baker FACES Pain Rating Scale consisted of six cartoon faces ranging from a happy smiling face to a tearful sad face to indicate no pain to worst pain [9]. The faces were assigned a rating from 0 to 5, with 0 for no pain and 1–5 for increasing intensities of pain. The Visual Analogue Scale (VAS) scores for eight patients were used and analyzed by paired t-test. Reduction of analgesics, activity of daily life, duration of pain relief, rates of pain recurrence, survival rate, and complications were also analyzed.
Results
Results are summarized in Tables 1 and 2. The technical success rate was 100%. The technical success is defined as ablating the target lesion in the tumor and cement injection into the osteolytic lesion as we planned. The RF ablations were performed in 53 sessions for 23 lesions (mean: 3.1 sessions/patient; range: 1–8 sessions). The mean number of sessions of RF ablation was 2.3 sessions/lesion. The mean ablation time was 4.1 min (range: 0.8–7 min). The mean final temperature was 71.8°C (range: 42°–99°C). The mean amount of bone cement (PMMA) was 8.1 ml (range: 1.5–15 ml).
Initial pain relief was achieved in 1 day (n = 8), 2 days (n = 7), and 3 days (n = 2) after the therapy. Pain was relieved in 100% (17 out of 17 patients) within several days. In the Wong–Baker FACES Pain Rating Scale, there were four scales down (n = 2), three scales down (Fig. 1) (n = 4), two scales down (n = 9), and one scale down (n = 2). The more scales that are down, more pain relief patients received in this scale. This means that patients responded more scale down when they felt their pain had decreased more after the treatments. These improved scores had no relationship to tumor size, dosage of radiation therapy, number of RF sessions, tumor temperatures, total RF time, or volume of bone cement. The mean VAS scores in the last eight patients dropped from 63 to 24 mm (p < 0.001). Analgesic reduction was achieved in only 7 out of 17 patients (41%). In activity of daily life, three bedridden patients were able to sit upright in a wheelchair (Fig. 2). This means that the patients went from a lying-down position in a bed to a sitting position. In two patients using wheelchairs, one patient was able to stand upright by himself and another patient was able to walk after the combined therapy. The mean duration of pain relief was 7.3 months (median: 6 months) in all of the patients. The mean period of pain relief was 8.6 months (median: 7 months) in the group without pain recurrence. Pain recurred in three patients (17.6%) from 2 weeks to 3 months. Eight patients died and eight patients are still alive (one patient was lost to follow-up). The 1-year survival rate was 40% (observation period: 1–30 months). No major complications occurred. Most patients complained of transient local pain at the puncture site. There was a hematoma in the psoas muscle (n = 1) and a hematoma of the puncture site (n = 1) as minor complications. One patient broke his right femur 2 days after the treatment due to falling (Fig. 3). He underwent surgery for stabilization with a blade. At that time, there were few viable malignant cells around the lesion.
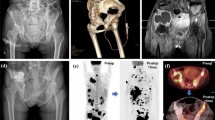
An 81-year-old female with iliac bone metastasis due to thyroid carcinoma (case 5) underwent the combined therapy. (A) CT shows a left iliac bone metastasis (arrow). (B) After RF ablation, bone cement was injected and the pain scores decreased from 4 points to 1 point the next day after treatment. No pain recurrence had occurred after 20 months.
A 61-year-old male with sacral bone metastasis due to renal cell carcinoma (case 14) received the combined therapy. (A) An electrode was introduced into the bulky metastatic tumor at the sacrum. (B) Bone cement was filled inside the tumor (arrow) at the reformatted coronal CT image. After the treatment, pain was relieved and the patient was able to sit upright.
Discussion
Combination therapy with RF ablation and cementoplasty is a new treatment for painful bone metastases. Although RF ablation and cementoplasty procedures are easy to perform, low cost, and less invasive, few cases applying both therapies for painful bone metastases have been reported to date. For both therapies, tumor necrosis or destruction of a sensitive nerve by mechanical or thermal forces plays a role in pain relief. Moreover, destruction of tumor cells that produce several cytokines might be responsible for decreased pain in RF ablation [4]. Stabilization of microfractures and reduction of mechanical forces is also said to be one of the mechanisms of vertebroplasty for metastatic bone tumors [1]. Looking at these direct effects, RF ablation damages malignant tumor cells and cementoplasty provides strength and stabilization to defected bones. In addition, owing to each effect of pain relief, it is expected that this combined therapy could have a synergistic effect of pain relief for larger tumors.
Pain was relieved in all patients and the pain scores improved within several days after the treatment. This result shows this combined therapy’s effect (100%) to be the same as or better than vertebroplasty (75–94%) [1–3] or RF ablation (95–100%) [4, 5] alone for bone metastases in pain relief. However, analgesic reduction was achieved in only 41% (7 out of 17 patients) in our study. Callstrom et al reported analgesic reduction of 80% [4]. The causes of this difference could be due to the fact that our cases had more other painful bone metastases and the tumors’ size was relatively larger than theirs.
The mean duration of pain relief was 7.3 months (median: 6 months). It is hard to evaluate whether this is a long or short period of time for pain relief. However, when it comes to a poor prognosis in our patients, we believe that it is long enough for pain relief. Moreover, pain recurred in only three patients (17.6%) during the observation. One of these patients received this combination therapy again 3 months later because of recurrent pain due to tumor regrowth. After the second procedure, the patient showed more pain scores down than that of the first procedure. This indicates that a repeat therapy is also feasible for recurrent pain due to tumor regrowth. In the group without pain recurrence, the mean period of pain relief was 9.2 months (median: 6 months). There were two cases of patients in our study with no recurrent pain for over 20 months. The primary tumors had already been resected in these two cases. This indicates that it is possible to attain a long period of pain relief after one procedure if the patient’s primary tumors are well controlled.
This combination therapy also contributed to an improvement in the patients’ daily life activity due to pain relief. Three patients became able to sit upright in a wheelchair, having come from a bedridden situation. Furthermore, one patient was able to walk and another patient was able to stand upright by himself from a wheelchair situation. The more the patients’ pain relief, the more their daily life activity improved [10].
Utmost care was taken during the procedure not to ablate tumors close to the spinal cord, nerve roots, or visible nerves because RF ablation has the potential to damage nerve cells. Over 45°C, nerve cells are damaged [11]. Goetz et al. [5] have reported a case of transient bowel and bladder incontinence after ablation of the sacrum. Nakatsuka et al. [8] reported neural damage in four patients in whom the tumor had invaded the posterior cortex of the vertebral body and pedicle. We therefore endeavored to avoid the area closest to a major nerve such as the nerve root or ischiatic nerve. This approach resulted in no major complications such as lower limb or buttock paresis.
The effect of the combination of this therapy with radiation therapy is little known. As radiation therapy is the first choice and a standard treatment now, over 70% of cases were introduced to our institution when pain recurred after radiation therapy or irradiation had proved ineffective. However, we believe that combination with radiation therapy is more beneficial for patients because whole tumor is not ablated or the whole tumor injected with cement.
There are several limitations of this new technique. First, in the high-dose irradiation case (90 Gy), the tumor (sacral metastasis by colorectal carcinoma) temperatures did not rise over 50°C because the generator showed roll-off instantly. Clinically, this patient showed pain relief and was able to sit after treatment. However, pain recurred 2 weeks later. This indicates that we might not be able to ablate the tumor well because tumor impedence could be much higher than that in a low-dose-irradiation case. Second, Schaefer et al. reported that they used this combined therapy of RF ablation and cementoplasty for a pathological fracture of the knee joint [6]. They stressed that it was useful for pain relief and stabilization of the fracture. However, one of our patients broke his right femur after treatment with this technique due to falling 2 days after the procedure. The reason why this occurred was that the whole-body weight was loaded onto this femur, and the femur was unable to support it. Although the situation between the distal femur and the shaft of the femur might be different, this therapy for the shaft of long bones, especially weight-bearing bones, could be ineffective for the prevention of pathological fracture. Third, this treatment is palliative and has little contribution to a longer prognosis. Indeed, the 1-year survival rate was only 40% in our study.
In conclusion, the combination therapy of percutaneous RF ablation and cementoplasty is feasible, effective, and safe. This treatment is a very promising technique for painful bone metastases and can improve a patient’s quality of life. As a next step, further studies of a comparison with cementoplasty or RF ablation alone and their long-term efficacy are needed.
References
A Cotton F Dewatre B Cortet et al. (1996) ArticleTitlePercutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at the clinical follow-up Radiology 200 525–530 Occurrence Handle10.1148/radiology.200.2.8685351
H Deramond C Depriester P Galibert et al. (1998) ArticleTitlePercutaneous vertebroplasty with polymethylmethacrylate: technique, indications, and results Radiol Clin North Am 36 533–546 Occurrence Handle1:STN:280:DyaK1c3lvFemuw%3D%3D Occurrence Handle10.1016/S0033-8389(05)70042-7 Occurrence Handle9597071
JB Martin B Jean K Sugiu et al. (1999) ArticleTitleVertebroplasty: clinical experience and follow-up results Bone 25 11S–15S Occurrence Handle1:STN:280:DyaK1MzosFCnuw%3D%3D Occurrence Handle10.1016/S8756-3282(99)00126-X Occurrence Handle10458267
MR Callstrom JW Charboneau MP Goetz et al. (2002) ArticleTitlePainful metastases involving bone: feasibility of percutaneous CT- and US-guided radio-frequency ablation Radiology 224 87–97 Occurrence Handle10.1148/radiol.2241011613 Occurrence Handle12091666
MP Goetz MR Callstrom JW Charboneau et al. (2004) ArticleTitlePercutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study J Clin Oncol 22 300–306 Occurrence Handle10.1200/JCO.2004.03.097 Occurrence Handle14722039
Schaefer O, Lohrmann C, Herling M, et al. (2002) Combined radiofrequency thermal ablation and percutaneous cementoplasty treatment of a pathologic fracture J Vasc Intervent Radiol 13:1047–1050
Schaefer O, Lohrmann C, Markmiller M, et al. (2003) Combined treatment of a spinal metastasis with radiofrequency heat ablation and vertebroplasty Am J Radiol 180:1075–1077
Nakatsuka A, Yamakado K, Maeda M, et al. (2004) Radiofrequency ablation combined with bone cement injection for the treatment of bone malignancies J Vasc Intervent Radiol 15:707–712
D Wong C Baker (1988) ArticleTitlePain in children: comparison of assessment scales Pediatr Nurs 14 9–17 Occurrence Handle1:STN:280:DyaL1c7ktlyitQ%3D%3D Occurrence Handle3344163
Hierholzer J, Anselmetti G, Fuchs H, et al. (2003) Percutanous osteoplasty as a treatment for painful malignant bone lesions of the pelvis and femur J Vasc Intervent Radiol 14:773–777
Dupuy DE, Hong R, Oliver B, et al. (2000) Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal Am J Radiol 175:1263–1266
Acknowledgments
The authors thank Hiroto Fujimura for his assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toyota, N., Naito, A., Kakizawa, H. et al. Radiofrequency Ablation Therapy Combined with Cementoplasty for Painful Bone Metastases: Initial Experience. Cardiovasc Intervent Radiol 28, 578–583 (2005). https://doi.org/10.1007/s00270-004-0208-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-004-0208-0