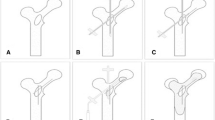
Abstract
Objective
To compare the efficacy of percutaneous long bone cementoplasty (PLBC) with and without embedding a cement-filled catheter in the medullary canal (ECFC) for painful long bone metastases with impending fracture.
Methods
A retrospective study was conducted in 36 consecutive patients undergoing PLBC and ECFC combination (n = 17, group A) or PLBC alone (n = 19, group B). All patients had a high risk of impending fracture in the long bone based on Mirels’ scoring system. Clinical effects were evaluated using both a pre- and a postoperative visual analogue scale (VAS) and Karnofsky performance scale (KPS).
Results
Overall pain relief rate with excellent (VAS 0–2) and good (VAS 2.5–4.5) results during follow-up was significantly higher in group A than in group B (88.2 % vs. 57.9 %, P<0.05). The average VAS and KPS changes in group A were significantly higher than those in group B at 1, 3 and 6 months postoperatively (P<0.05). Also, the rate of fractures of the treated long bone in group A was significantly lower than that in group B (P<0.05).
Conclusions
Combined PLBC and ECFC is a safe and effective procedure for long bone metastases with impending fracture.
Key Points
• Metastases in long bones may cause pain and subsequent pathological fractures.
• Cementoplasty resulted in significant pain relief in patients with long bone metastases.
• Combination of PLBC and ECFC may reduce the incidence of fractures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous long bone cementoplasty (PLBC) has been progressively introduced in clinical practice [1–4]. However, its use for long bone osteolytic metastases is controversial. Several reports have indicated that despite satisfying pain relief after the procedure, there was a risk of fracture due to insufficient mechanical stability [3, 5–7]. To overcome these disadvantages, we adopted an innovative method – PLBC combined with embedding a cement-filled catheter in the long bone medullary canal (ECFC) – to minimize the risk of fracture and to improve mechanical stability [8–10]. We have performed this interventional treatment for painful long bone metastases with impending pathological fracture using a combination of PLBC and ECFC in a population of poor surgical candidates. Herein we analyze and summarize our institutional experience, and compare the efficacies of combined PLBC and ECFC with that of PLBC alone.
Material and methods
Study population
This retrospective study was approved by our institutional review board. We reviewed the clinical and imaging data of consecutive patients who underwent combined PLBC and ECFC (Group A) or PLBC alone (Group B) at our institution between October 2003 and December 2014. Informed consent for the procedure was obtained from all patients.
Inclusion criteria were: nonsurgical candidates due to poor performance status or patient’s refusal for surgery intervention; subjects with osteolytic metastases in the long bone and with high risk of pathological fracture, defined by a Mirels’ score ≥8, and presence of intractable pain unresponsive to conventional therapy, including various analgesics and chemotherapeutic regimens. Osseous metastatic lesions were diagnosed with radiography, computed tomography (CT) or magnetic resonance imaging (MRI). Radiation therapy was not indicated in 21 patients who had already received the maximum radiation dose for local lesions. The procedures were performed pre- or post-radiotherapy for rapid stabilization of the long bones in 15 patients due to either delayed or incomplete action of radiation therapy. Before 2009, only PLBC was performed in these patients. Since 2010, because of a persisting high risk of impending pathological fracture in patients after PLBC, we have adopted a modified Kawai technique, a combination of PLBC and ECFC, to treat these patients [10].
Percutaneous long bone cementoplasty (PLBC) procedure
The procedure was performed in the angiography room under fluoroscopic guidance. The patient was placed in either a supine or lateral position depending on the lesion site. Under extensive local sterility, anaesthesiology care, cardiovascular monitoring and fluoroscopic guidance, direct access to the lesion was obtained by bone-bevelled needles (11G–13G × 10–15 cm, Cook, Bloomington, IN, USA; Guanlong Co., Jinan, China). Approximately two to three bone-bevelled needles per lesion were placed percutaneously to create a sufficient coverage of the lesion in different orientation. During needle placement, it was possible to confirm the diagnosis by obtaining a core biopsy using a 15-gauge through-cut biopsy needle inserted coaxially through the bevelled needle. Polymethyl methacrylate (PMMA) was mixed to a semiliquid consistency and injected to a maximal possible amount under fluoroscopic guidance. If bone cement appeared at the bone cortex edge or leaked into the adjacent soft-tissue structures, the injection was immediately stopped (Fig. 1). The mean number of inserted needles per lesion was 2.55 ± 0.51 (range 2–3), and the mean injected cement volume was 17.41 ± 8.19 ml (range 8–30).
Metastasis of right distal femur in a 55-year-old male patient with right leg pain and disability. (A) An osteolytic lesion is depicted in the right distal femur on a sagittal CT image. (B) Radiograph obtained after percutaneous long bone cementoplasty (PLBC) shows good distribution of bone cement in the lesion of the femur
PLBC and embedding a cement-filled catheter in the medullary canal (ECFC) procedures
Entry sites were selected at the distal normal cortical bone from the bone lesion or the epiphysis in an adjacent lesion. A 3.2-mm drill was used in the bone cortex drilling and an 11-gauge bevelled needle introduced into the bone medullary canal through the route. Through the needle, a 5-F end-hole catheter (Cordis) combined with a 0.035-in. guide wire (Terumo) was driven with small rotary movements without reaming in the medullary canal. If it was difficult to pass the tumour tissue in the medullary canal, the 0.035-in. wire was replaced by a super-stiff 0.035-in. guide wire (Cordis) through the catheter with the ideal catheter over the guide wire placement in the medullary canal. After withdrawing the 5-F catheter, an 8-F biliary drainage catheter combined with a built-in stiffening cannula (Cook) over the wire was passed down into the medullary canal with the multiple side holes of the catheter corresponding to the level of the bone lesion. On withdrawing the built-in stiffening cannula, bone cement in a slightly more liquid consistency was injected into the catheter, and distributed into the osteolytic lesions through the side holes of the catheter. Then the cement-filled catheter was cut with a 13-gauge circular saw in the puncture site and left in the medullary canal (Fig. 2). The PLBC procedure as described above was performed with a direct access to the lesion through one or two newly inserted bone bevelled needles. The mean number of inserted needles per lesion, including the needle-introduced catheter, was 2.41 ± 0.49 (range 2–3). The mean injected cement volume was 21.06 ± 8.20 ml (range 10–35).
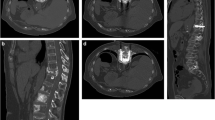
Metastasis in the left proximal femur in a 58-year-old male patient with left leg pain and disability.(A, B) An osteolytic lesion is depicted in the left proximal femur on radiograph and coronal T1WI MR. (C) Radiograph obtained during embedding a cement-filled catheter in the medullary canal (ECFC) shows that an 8-F biliary drainage catheter with multiple side holes was advanced beyond the proximal lesion along the long axis of the femur into the medullary canal. (D) Radiograph obtained after combination of percutaneous long bone cementoplasty (PLBC) and ECFC shows the cement-filled catheter and cement distribution in the osteolytic lesion. (E) The distribution of bone cement is depicted in the left proximal femur on coronal CT image after the procedure
After the procedures, all patients underwent radiographic or CT examination to assess intralesional cement distribution, cement leakage or other possible local complications.
Treatment evaluation
A series of medical assessments for the treatment effect were performed pretherapy at baseline, and at 1 week, 1month and then every 3 months after procedure. Given the clinical relevance of these patients with metastatic disease, a maximum 6-month follow-up was performed. The following clinical data of each patient were recorded: gender, age, histology of the treated lesion, affected bone, metaphyseal or diaphyseal involvement, number of procedures performed, mean lesion size, Mirels’ score, the axial cortical metastasis involvement, radiation treatments preceding or following the procedures, technical success, number of needles inserted, injected cement volume, changes in pain intensity, function improvements following procedures, and secondary fractures and complications.
Pain relief was measured by a visual analogue scale (VAS) score ranging from 0 to 10 (0 indicating no pain and 10 indicating the worst pain) and categorized into four types: excellent (0–2), good (2.5–4.5), fair and poor. Changes in quality of life were measured on a 100-point Karnofsky performance scale (KPS).
Statistical analysis
Quantitative variables were presented as mean ± SD. Qualitative variables were reported as numbers and percentages. Comparisons of the variables between the two groups were performed by applying the Mann-Whitney test, x 2 test or Fisher’s exact test, as appropriate. All statistical analyses were performed using SPSS version 13.0 (SPSS, Chicago, IL, USA). The patients were assessed from the time of the procedure until the appearance of a pathological fracture in the treated sites during follow-up, pathological fracture and patient death on a Kaplan-Meier survival curve.
Results
Patients
A total of 36 patients (21 males and 15 females) with a mean age of 62.69 ± 7.07 years (range 52–73) were included in this study. Primary tumour types were lung (n = 14), liver (n = 8), breast (n = 6), kidney (n = 4) and stomach (n = 4). Seventeen and 19 patients were classified into group A (PLBC with ECFC) and group B (PLBC alone), respectively. Baseline characteristics of the patients are summarized in Table 1. There was no statistical significance between the two groups.
Clinical evaluation
All procedures were technically successful and well tolerated in all patients. There were no intra- or postoperative complications from procedures performed. Asymptomatic cement leakages occurred six cases in group A, with leaking into the puncture path (n=3), periosteum (n=2) and muscle space (n=1), and seven cases in group B, with leaking into the puncture path (n=4), periosteum (n=2) and veins (n=1). There was no intra-articular cement leakage. The clinical outcomes of the two groups are shown in Table 2.
In group A, excellent pain relief was obtained in 8/17 patients (47.06 %), good pain relief in 6/17 patients (35.29 %) and no improvement in 3/17 patients (17.65 %) at 1week post-procedure. Clinical follow-up data (mean 5 ± 1.5 months; 95 % CI 4–6; range 1.5–6) were available for 17 patients. Among the three patients with no improvement at 1week post-procedure, one patient achieved good pain relief at 1 month after the procedure. Four patients died from progression of cancer within 6 months after the initial procedure. The overall clinical assessment during follow-up exhibited excellent pain relief (n=8) and good pain relief (n=7) in 15 patients with a pain relief rate of 88.2 %. The initial average VAS was7.53 ± 1.23, and the KPS scale was 61.76 ± 5.29. At 1 month, 3 months and 6 months post-procedure, both the VAS and the KPS differed significantly from the values at baseline (P < 0.01).
In group B, excellent pain relief was obtained in 7/19 patients (36.84 %), good pain relief in 9/19 patients (47.37 %) and no improvement in 3/19 patients (15.79 %) at 1week after the procedure. Clinical follow-ups were available for 19 patients (mean 5 ± 1.8 months; 95 % CI 4–6; range 1–6). Five of the 16 patients with excellent and good pain relief at initial follow-up experienced worsening of pain due to pathological fractures of the treated long bone during daily activity (two on getting up, three on walking) at the 1.5-, 2-, 3- and 6-month follow-ups (Fig. 3) and had to undergo further surgery. Pain relief was never obtained in the four patients who had no improvement. Five patients died from progression of cancer within 6 months of the initial procedure. The overall clinical assessment during follow-ups exhibited excellent pain relief (n=6) and good pain relief (n=5) in 11 patients with a pain relief rate of 57.9 %. The initial average VAS was 7.52 ± 1.02, and the KPS scale was 61.58 ± 6.02; both the VAS and the KPS differed significantly from the initial baseline values at each follow-up time point (P < 0.01). VAS and KPS results of the two groups are summarized in Table 3.
The same patient as in Fig. 1. (A, B) A pathological fracture is depicted at the treated site in the right distal femur on the frontal and lateral radiograph at the 1-month follow-up
Comparison of group A and group B
The overall excellent and good pain relief rate during follow-ups was significantly higher in group A than in group B (88.2 vs. 57.9 %, P = 0.047, Fisher’s exact test). The average VAS score at 1, 3 and 6 months in group A were significantly lower than those in group B (P<0.05) (Fig. 4), while the average KPS score at 1, 3 and 6 months in group A were obviously higher than those in group B (P<0.05) (Fig. 5). The mean cement filling volume was significantly higher in group A (21.06 ± 8.20 ml; range 10–35 ml) in 18 treated sites with PLBC and ECFC than that in 20 treated sites with PLBC in group B (17.41 ± 8.19 ml; range 8–30) (P<0.05). No patients experienced pathological fractures of the treated long bone in group A during follow-ups, while five patients in group B had pathological fractures of the treated long bones during follow-ups (P<0.023, Fisher’s exact test). In addition, there were no significant differences in the average VAS score and KPS score preoperatively and at 1 week postoperatively between the two groups. There were no significant differences in patient survival between the two groups (P>0.05, log-rank test) (Fig. 6).
Discussion
Osteolytic metastases in the long bones with intractable pain are usually seen in cancer patients at advanced stage with a very limited life expectancy. Subsequent pathological fracture of these lesions would expose patients to excruciating pain, hospitalization and emergency surgery in compromised circumstances [11, 12]. For these patients, non-surgical management has been shown to be ineffective in providing complete pain relief or a return to function of the affected limb. According to Mirels’ recommendation, a prophylactic surgical approach is strongly indicated for a lesion with an overall score of ≥8 [13]. Other authors state that the predictive fracture criterion is axial cortical involvement>30 mm [14]. However, surgical treatment of impending fracture of the long bones only provides palliative care in most patients, and is usually associated with considerable postoperative morbidity and mortality.
A retrospective study reported 3 % deep vein thrombosis within 3 months after surgery, and a death rate of 1 % during surgery, 9 % during the hospital stay and 13.9 % within 3 months after surgery [15]. Although percutaneous cementoplasty has been used as an alternative for stabilization of impending pathological fractures of the long bones, several groups have reported that despite satisfactory pain relief after the procedure, pathological fractures were the most common delayed adverse event following PLBC [3, 5–7]. The main reason is that injecting cement directly into osteolytic lesions cannot provide adequate mechanical stability for long bones, which is considered resistant to compressive forces, but less so to torsional forces [16–18], and long bones treated by PLBC are more subject to torsional forces. For such fractures within the treated area, if we consider the minimal or absent local tumour progression, one may speculate that PMMA stress fracture occurred [2]. Several techniques for these patients were reported that included the use of screws, nails and metallic mesh in combination with PMMA injection, or of a photodynamic bone stabilization system [19–23]. A technique such as photodynamic balloon nails appears promising; however, it requires special material and equipment, which are not commercially available in most countries outside Europe. Our study is the first one advocating the use of the ECFC technique combined with PLBC, and showed better clinical outcome compared with that of PLBC alone.
A combination of PLBC and ECFC, developed from percutaneous cementoplasty, can provide internal fixation with more bone cement filling in the treated long bone. To perform such a procedure, a catheter with multiple side holes was first introduced into the medullary canal of the long bone, bone cement in a slightly more liquid consistency was then injected into the catheter, and distributed into the osteolytic lesions by the side holes of the catheter. The procedure surpasses the limitations of cement injection in the long bones by implanting an ‘internal fixation’ with a cement-filled catheter. An additional PLBC procedure was performed through direct access to the lesion. In our opinion, bone cement injection of PLBC was useful to surround and stabilize the cement-filled catheter and to fill more bone cement into the lesions.
Compared with conventional surgery, this procedure has several advantages. First, the placement of an 8-F catheter in the medullary canal raises less intramedullary pressure than the metal hardware used in surgical intramedullary fixation, with most injected cement remaining inside the catheter or distributing into the osteolytic lesions through the side holes of the catheter, which would reduce the risk of fat pulmonary embolism commonly seen in conventional surgery. Second, the procedure is minimally invasive and only requires local anaesthesia, which would decrease the risk of infection and blood loss in the hypervascular metastases, and avoid damage to the muscles around limbs with less injury and disability after the procedure. Third, the embedded cement-filled catheter in the bone medullary canal interferes minimally with limb movements, and reduces the risk of thromboembolic complications. Lastly, additional cementoplasties can be performed during the procedure for patients with other bone metastases during one session.
As shown in our data, compared with PLBC alone, a combination of PLBC and ECFC can provide enough mechanical stability as well as durability of the long bone and reduce the risk of pathological fracture with significant quality-of-life improvement after the procedure. The results of our study showed significant differences in the VAS and KPS scores in group A compared with those in group B during follow-up. The clinical effect seemed to be predominantly attributable to the internal fixation of an embedded cement-filled catheter and injection of more bone cement into the long bone, thereby providing mechanical stability of resistance torque and making the cement eliminate more of the malignant tissue. A combination of PLBC and ECFC may be a good alternative for patients who are not candidates for standard surgical stabilization even if the mechanical stability obtained with this technique is probably less than that with surgical prostheses or osteosynthesis. Indeed, the level of stability obtained in our study seems sufficient in patients with advanced cancer disease, as we did not experience occurrence of pathological fracture in the treated long bone in group A during 6 months of follow-up, while five patients had pathological fractures in group B. Meanwhile, we also observed no differences in complications and survival between the groups. In some ways, death without fracture is the goal of this palliative treatment.
This study has several limitations. First, the number of cases is too small to make broad generalizations. However, to our knowledge, this is the first study providing direct comparison of two techniques for palliative treatment of intractable painful osteolytic long bone metastases with impending pathological fracture. According to the results of our study, a combination of PLBC and ECFC has a positive impact on impending pathological fracture of the long bone. Second, the follow-up was relatively short as the patients in this study were at the end-stage of their lives with advanced cancers. Death due to rapid progression of the primary cancers might have masked both benefits and risks of the procedure. The treatment of bone metastases is for palliation only in the vast majority of patients, and a good mechanical stability of the affected limbs in these patients is vital for achieving pain control, faster mobilization and overall improvement in their quality of life. Additionally, there were no biomechanical studies about a cement-filled catheter and measurement of the intramedullary canal pressure during the cement-filled catheter being introduced in the canal of the long bone.
In conclusion, percutaneous stabilization for impending pathological fracture of the long bone with a combination of PLBC and ECFC is an effective and safe procedure. It is a minimally invasive technique that can be performed under conscious sedation, with satisfying clinical outcome.
References
Anselmetti GC (2010) Osteoplasty: percutaneous bone cement injection beyond the spine. Semin Interv Radiol 27:199–208
Cazzato RL, Buy X, Eker O et al (2014) Percutaneous long bone cementoplasty of the limbs : experience with fifty-one non-surgical patients. Eur Radiol 24:3059–3068
Anselmetti GC, Manca A, Ortega C et al (2008) Treatment of extraspinal painful bone metastases with percutaneous cementoplasty: a prospective study of 50 patients. Cardiovasc Intervent Radiol 31:1165–1173
Chang SW, Murphy KP (2005) Percutaneous CT-guided cementoplasty for stabilization of a femoral neck lesion. J Vasc Interv Radiol 6:889–890
Basile A, Giuliano G, Scuderi V et al (2008) Cementoplasty in the management of painful extraspinal bone metastases: our experience. Radiol Med 113:1018–1028
Dayer R, Peter R. Percutaneous cementoplasty complicating the treatment of a pathologic subtrochanteric fracture: a case report. Injury.7:801–804
Cazzato RL, Buy X, Eker O, Fabre T, Palussiere J (2014) Percutaneous long bone cementoplasty of the limbs: experience with fifty-one non-surgical patients. Eur Radiol 24:3059–3068
Sun G, Jin P, Li M et al (2011) Percutaneous cementoplasty for painful osteolytic humeral metastases: initial experience with an innovative technique. Skelet Radiol 40:1345–1348
Sun G, Jin P, Liu XW et al (2014) Cementoplasty for managing painful bone metastases outside the spine. Eur Radiol 24:731–737
Kawai N, Sato M, Iwamoto T et al (2007) Percutaneous cementoplasty with use of a cement-filled catheter for a pathologic fracture of the humerus. J Vasc Interv Radiol 18:805–809
Wedin R, Bauer HC (2005) Surgical treatment of skeletal metastatic lesions of the proximal femur. Endoprosthesis or reconstruction nail? J Bone Joint Surg 87B:1653–1657
Jawad MU, Scully SP (2010) In brief: classifications in brief: Mirels' classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res 468:2825–2827
Mirels H (1989) Metastatic disease in long bones. A proposed scoring sys-tem for diagnosing impending pathologic fractures. Clin Orthop Relat Res 249:256–264
Van der Linden YM, Dijkstra PD, Kroon HM et al (2004) Comparative analysis of risk factors for pathological fracture with femoral metastases. J Bone Joint Surg (Br) 86:566–573
Ristevski B, Jenkinson RJ, Stephen DJ et al (2009) Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg 52(4):302–308
Gangi A, Buy X (2010) Percutaneous bone tumor management. Semin Intervent Radiol 27:124–136
Heini PF, Franz T, Fankhauser C et al (2004) Femoroplasty- augmentation of mechanical properties in the osteoporotic proximal femur: a biomechanical investigation of PMMA reinforcement in cadaver bones. Clin Biomech 19:506–512
Sutter EG, Mears SC, Belkoff SM (2010) A biomechanical evaluation of femoroplasty under simulated fall conditions. J Orthop Trauma 24:95–99
Kelekis A, Filippiadis D, Anselmetti G, Brountzos E (2015) Percutaneous augmented peripheral osteoplasty in long bones of oncologic patients for pain reduction and prevention of impeding pathologic fracture: the rebar concept. Cardiovasc Intervent Radiol (Epub ahead of print)
Kim JH, Kang HG, Kim JR, Lin PP (2011) Minimally invasive surgery of humeral metastasis using flexible nails and cement in high-risk patients withadvanced cancer. Surg Oncol 20:e32–e37
Anselmetti GC, Manca A, Chiara G, Tutton S (2011) Painful pathologic fracture of the humerus:percutaneous osteoplasty with bone marrow nails under hybridcomputed tomography and fluoroscopic guidance. J Vasc Interv Radiol 22:1031–1034
Deschamps F, Farouil G, Hakime A, Teriitehau C et al (2012) Percutaneous stabilization of impending pathological fracture of the proximal femur. Cardiovasc Intervent Radiol 35:1428–1432
Vegt P, Muir JM, Block JE (2014) The photodynamic bone stabilization system: a minimally invasive, percutaneous intramedullary polymericosteosynthesis for simple and complex long bone fractures. Med Devices (Auckl) 7:453–461
Acknowledgments
The scientific guarantor of this publication is Gang Sun. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study was supported by Grant Nos. 51073173 from the National Nature Science Foundation of China (NNSFC) and 2013AA032203 from the National High Technology Research and Development Program of China (863 programme). No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: retrospective, observational, multicentre study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xun-wei Liu and Peng Jin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Xw., Jin, P., Liu, K. et al. Comparison of percutaneous long bone cementoplasty with or without embedding a cement-filled catheter for painful long bone metastases with impending fracture. Eur Radiol 27, 120–127 (2017). https://doi.org/10.1007/s00330-016-4347-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4347-x