Abstract
Methods for calculating the activity to be administered during yttrium-90 radioembolization (RE) are largely based on empirical toxicity and efficacy analyses, rather than dosimetry. At the same time, it is recognized that treatment planning based on proper dosimetry is of vital importance for the optimization of the results of RE. The heterogeneous and often clustered intrahepatic biodistribution of millions of point-source radioactive particles poses a challenge for dosimetry. Several studies found a relationship between absorbed doses and treatment outcome, with regard to both toxicity and efficacy. This should ultimately lead to improved patient selection and individualized treatment planning. New calculation methods and imaging techniques and a new generation of microspheres for image-guided RE will all contribute to these improvements. The aim of this review is to give insight into the latest and most important developments in RE dosimetry and to suggest future directions on patient selection, individualized treatment planning, and study designs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Yttrium-90 (90Y) radioembolization (RE) is an established treatment modality for chemotherapy resistant, unresectable hepatic malignancies [1]. The radioisotope 90Y (average beta energy 0.93 MeV; half-life 2.67 days; mean/maximum tissue range 2.5/11 mm) is either impregnated onto resin microspheres (SIR-Spheres®, Sirtex Medical Limited, Lane Cove, Australia) or incorporated in glass microspheres (TheraSphere®, BTG International Ltd., Canada). After injection into the hepatic artery, these microspheres distribute according to vascularity and ideally lodge in the arterioles in and around the tumors, delivering high local radiation absorbed doses, while sparing the normal liver parenchyma [2].
Currently, success of the treatment depends largely on the knowledge and experience of the treatment team, who can tailor the amount of injected microspheres or activity. Most 90Y-activity calculation methods are aimed at an ‘average’ patient. Although the safety and efficacy of RE using these methods have been proven to be sufficiently acceptable, the observed toxicity in some patients and the lack of response in other patients post the challenge of optimizing these relatively crude methods. For optimal treatment, it may be necessary to be able to predict the absorbed dose to the tumor and the absorbed dose to the healthy liver and lungs. Accurate prediction of these values can help to optimize patient selection and to truly individualize treatment planning.
Image-based dosimetry may be performed before, during, or after treatment. Substantial progress has been made in each of these areas since adoption of the standard activity calculation methods. The aim of this review is to summarize current status, limitations, developments, and future directions for RE dosimetry.
Pre-treatment Dosimetry
Scout Dose
Before every RE procedure, a preparatory angiography is performed to map the anatomy of the patient, to coil-embolize non-target vessels arising close to the injection site, and to administer a scout dose [3]. Since 90Y emits no direct gamma radiation, it is less suitable for low activity scout dose imaging. Relatively high activity is needed to accurately quantify the 90Y biodistribution. Instead, the surrogate technetium-99m macroaggregated albumin (99mTc-MAA) is used for simulation, and single photon emission computed tomography (SPECT) combined with CT is the preferred modality to determine the intra- and extrahepatic biodistribution of 99mTc-MAA [4].
Its use is three-fold. First, it is used to exclude any extrahepatic deposition of activity in organs other than the lungs. Extrahepatic distribution may also be detectable using catheter-directed CT (e.g. c-arm cone beam CT or hybrid angiography/CT). The benefit of using catheter-directed CT is that the extrahepatic distribution can be corrected immediately and no additional angiography is needed to coil-embolize the culprit vessel. For this reason, a research group from the University of British Columbia is investigating whether it is safe to no longer use 99mTc-MAA and instead coil-embolize non-target vessels during the treatment angiography, right before microsphere injection in a single procedure 90Y-treatment [5]. An advantage of coil-embolizing non-target vessels shortly before treatment is that the chance of recruitment of new non-target collaterals between embolization and microsphere administration decreases [6].
Second, calculation of the lung shunt is performed in every patient in order to exclude the deposition of a critical amount of activity in the lungs, which can lead to radiation pneumonitis [7]. The accuracy of 99mTc-MAA in this aspect is, however, uncertain. There are indications that the lung shunt fraction for radioembolization may be overestimated due to the presence of circulating free pertechnetate [8]. Moreover, the macroaggregated particles have a non-spherical shape and variable sizes. The size distribution is rather broad (90 % of particles have a long axis between 10 and 90 microns) and the size distribution varies between different MAA reagent kit brands, as well as between different lot numbers of kits from the same company [9]. The variability in MAA particle size may affect the accuracy and reproducibility of the lung shunt fraction. As a result, patients may receive an unjustified dose reduction or do not receive treatment at all. To improve pre-treatment dosimetry, there are ongoing efforts to develop surrogate particles other than 99mTc-MAA, which are discussed later on in this article.
And third, intrahepatic 99mTc-MAA biodistribution may be used for dosimetry calculation [10]. If the 99mTc-MAA scout dose confirms a patient’s eligibility for RE (i.e. no extrahepatic deposition of 99mTc-MAA and an acceptable lung shunt fraction), the scout dose may also be used for treatment planning. Some centers deny RE to patients whenever the activity concentration in the tumor is low in comparison with non-tumorous areas, because, supposedly, this means less treatment benefit and an increased risk of liver toxicity [11, 12]. The different methods for activity planning and the role of intrahepatic biodistribution of 99mTc-MAA for activity planning are discussed below.
Activity Planning: BSA Method (90Y Resin Microspheres)
In early clinical studies, the used activity ranged between 2 and 3 GBq depending on the metastatic tumor load (the so-called ‘empirical’ method since it has no theoretical background), but this method was soon abandoned after clinically observed toxicity [13]. The ‘body surface area (BSA)’ method was subsequently developed to overcome this problem, because the BSA generally correlates with liver volume and thus the administered activity could be semi-empirically adjusted for liver volume without the need for liver volumetry on cross-sectional imaging [14]. This is currently the preferred method for calculating the prescribed activity in patients with multiple liver metastases, who are treated with resin microspheres [15].
The standard BSA formula for resin microspheres is based on BSA, and the fraction of the total liver volume involved by tumor [15]:
In the case of a significant shunt to the lungs, the prescribed activity is empirically reduced (10–15 % shunt fraction; 20 % activity reduction; 15–20 % shunt fraction: 40 % activity reduction; >20 % shunt fraction: no treatment).
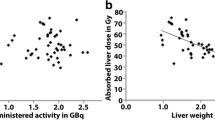
A major drawback of the BSA method is that while BSA may be related to liver weight in the healthy general population, this is not necessarily the case in patients with liver tumors. In addition, the BSA method assumes a favorable tumor-to-normal liver ratio, which may not be true in every patient. As a result, treatment planning according to the BSA-based method (Eq. 1) leads to considerable inter-patient variation in liver and tumor absorbed doses. Large patients with small livers will receive relatively high doses on the liver and vice versa (Fig. 1) [16].
The BSA-based activity calculation method does not take the patient-specific target volume into account, leading to a wide variation in absorbed target doses. Following the BSA method, patient A with a large liver (2928 mL, 65 % tumor involvement, BSA 1.73) and patient B with a small liver (957 mL, 29 % tumor involvement, BSA 2.03), would receive similar amounts of 90Y-activity (2.18 and 2.12 GBq, respectively), resulting in mean target absorbed doses of 37 and 111 Gy, respectively
Activity Planning: MIRD Method (90Y Glass Microspheres)
The majority of treatment centers use either the BSA or the MIRD method depending on the microspheres used. Activity calculation for glass microspheres (MIRD method) is based on an estimation of the mean absorbed dose in the target liver volume [17]. It uses a simplified calculation method derived from the MIRD equations for dose calculation [18]. An absorbed dose of 50 Gy per GBq per kilogram tissue is used with assumptions of homogeneous intrahepatic microsphere distribution and absorption of all the administered activity and energy in the liver using the following formula:
where M Target is the mass of the target volume. The desired dose may range from 80 to 120 Gy depending on the clinical judgment of the treating physician. The lung dose should not exceed 30 Gy (or 50 Gy for repeated treatment), which, with the above-mentioned assumptions and an assumed lung mass of 1 kg, is equivalent to an absolute lung shunt of approximately 600 MBq of 90Y-microspheres [17, 19].
According to the MIRD method for glass microspheres, the treating physician prescribes an amount of activity based on a desired liver absorbed dose, which depends on the patient’s clinical status. Although this seems to be scientifically sound, the intrahepatic biodistribution varies per patient and thus the dose to tumor and healthy liver can vary between patients as well. This method (Eq. 2) is particularly useful for so-called radiation segmentectomy in patients with disease limited to ≤2 liver segments [20]. These patients are treated with supra-therapeutic absorbed doses to a limited target volume that will ablate both the tumor and the normal liver parenchyma within that target volume, while sparing the untreated part of the liver.
Activity Planning: Anatomic Partition Models (90Y Resin Microspheres)
A huge step forward with regard to dosimetry was the development of a MIRD-derived method for 90Y-resin microspheres, called the ‘partition’ method [21]. This method involves selecting safe absorbed doses to the normal liver and lung and implanting the maximum activity that does not exceed these limits. The tumor and normal liver compartments are in general delineated on anatomical imaging modalities, and the anticipated activity in these compartments is calculated on scout dose SPECT imaging using 99mTc-MAA. The ratio between the activity concentrations in the tumor and normal liver compartments (R T/N) is calculated as
where A is the activity in GBq and M is the mass of the compartment in kg. Subsequently, the prescribed activity may be calculated as
where D Normal liver is the maximum desired absorbed dose to the normal liver in Gy, R T/N follows from Eq. (3), and LSF is the lung shunt fraction. Importantly, it adjusts for the difference between absorbed dose to the tumor and the normal liver (T/N). However, the partition model is still not routinely used today [15, 22].
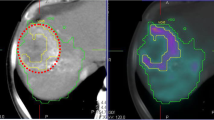
Many patients presented for radioembolization have multiple tumors distributed over the whole liver, which hinders accurate segmentation of all tumors and direct registration with 99mTc-MAA distribution. A method that has recently been introduced to overcome this problem involves a second SPECT scan after intravenous injection of 99mTc-MAA sulfur colloid (99mTc-SC; 185 MBq) directly following conventional 99mTc-MAA SPECT imaging (37 MBq) (Fig. 2) [23]. Sulfur colloid is used as a biomarker for normal hepatic reticulo-endothelial tissue, and its distribution can be visualized with a second SPECT shortly after the 99mTc-MAA SPECT. The absorbed dose to functional liver tissue and tumor tissue can then be automatically estimated. This method offers an automatic physiology-based functional imaging tool for hepatic RE treatment and may lead to improved individualized treatment planning.
A routine 99mTc macroaggregated albumin SPECT simulating treatment microsphere distribution (A) is followed by a second SPECT after IV injection of 99mTc sulfur colloid to show functional liver parenchyma (B). These data may be used for functional voxel-based segmentation and pre-treatment dosimetry
Multiple Injection Positions
Currently, the vast majority of patients receive treatment from two or more injection sites [24]. Under the empirical activity planning paradigm, the prescribed activity needs to be split proportionally to the volume of the perfused areas. There are several methods for calculating these proportions. The proportional volume of the right and left liver lobe can be assessed by manual delineation on CT or MRI, dividing the activity accordingly [25]. However, this assessment is complicated by the variable vascular anatomy in the liver. For instance, the middle hepatic artery supplying segment IV, may originate from the left or the right hepatic artery, or the proper hepatic artery [26]. The patient-specific arterial anatomy needs to be taken into account for delineation of the liver lobes.
A simplified method of determining patient-specific arterial anatomy has been presented by Kao et al., who developed an artery-specific method by calculating the volume of each flow area on catheter-directed CT with selective contrast injection from each injection position intended for treatment [27]. Each run of catheter-directed CT delineates the target arterial territory regardless of variant arterial anatomy, distortion by tumor, surgery or cirrhosis, cysts or ablation cavities, or arterial parasitization. The regular use of contrast-enhanced catheter-directed CT during work-up angiography for RE is not only useful to determine the target volume, but also to ascertain that the tumors lie within the target arterial territory [28].
Developments in Pre-treatment Dosimetry
An ideal particle for pre-treatment dosimetry follows a distribution pattern that is very similar to the distribution pattern of the microspheres used for treatment. This seems not to be the case for 99mTc-MAA and 90Y-microspheres, which may be due to the differences in the size, number, density, and morphology [29]. A recent study showed that substantial differences exist between intrahepatic 99mTc-MAA-distribution and subsequent 90Y-distribution [30]. In that study, at least one liver segment showed an under- or overestimation of ≥10 % in every single 99mTc-MAA-procedure. The mismatch between 99mTc-MAA and 90Y distribution increases with a mismatch in catheter tip position, injections close to bifurcations, and with injections in the main hepatic artery [30, 31]. Figure 3 shows an example of 99mTc-MAA and 90Y-distribution mismatch.
Example of intrahepatic biodistribution mismatch between technetium-99m macroalbumin aggregates (A) and resin yttrium-90 microspheres (B) while the catheter positions for both injections appear similar (C and D, respectively). In addition to the differences in particle properties, the position of the catheter tip close to the vessel wall may have contributed to the observed mismatch as well. Particles may have followed a laminar flow pattern instead of homogeneously spreading through the vessel lumen
Researchers have sought to develop a particle that is rheologically identical to the radioactive microspheres used for treatment. 99mTc-albumin spheres have been developed for this purpose (ROTOP-HSA microspheres B20, Rotop Pharmaka, Dresden, Germany). Just like 99mTc-MAA, these particles consist of 99mTc labeled to albumin, but are shaped spherically to better mimic the 90Y-microspheres. These 99mTc-albumin spheres have a mean diameter of 20 micron and 85 % is sized between 10 and 30 microns. The predictive value of these microspheres before 90Y-radioembolization is currently under investigation [32]. Several positron-emitting microspheres have been developed as well. Selwyn et al. developed a positron-emitting resin microsphere labeled with 18F that showed promising results regarding labeling efficiency and imaging capabilities [33]. The in vivo stability was, however, insufficient with significant leaching of 18F from these microspheres (15 % in 45 min). Other positron emitters labeled to resin or albumin microspheres have been investigated, of which yttrium-86, gallium-68, and zirconium-89 were most stably labeled and seem most useable for clinical applications [34–36]. Another type of particles, which are now under investigation in human patients, are holmium-166 (166Ho) loaded poly(L-lactic acid) microspheres for image-guided radioembolization [37]. The radioisotope 166Ho emits high-energy beta particles, used for tumor destruction, and gamma radiation, which allows for nuclear imaging (half-life 27 h; gamma energy 81 keV; maximum beta energy 1.8 MeV) [38]. Holmium is also a highly paramagnetic metal, and as such can be visualized on MRI [39]. Prior to administration of the therapeutic dose, a small scout dose of 166Ho-microspheres may be instilled to predict the distribution of the therapeutic dose. Since the particles in the scout dose are physically identical to the particles of the therapeutic dose, the biodistribution is expected to be more similar. A similar approach for 90Y is impeded by the longer physical half-life and the lower number of annihilation and Bremsstrahlung photons that are available for PET or SPECT imaging, respectively. A schematic overview of how the physical properties of 90Y and 166Ho can be used for imaging is presented in Fig. 4.
Schematic illustration of the physical properties and decay of 90Y (A) and 166Ho (B) in the liver. The upper row displays examples of the microsphere biodistribution in the liver using 90Y-bremsstrahlung SPECT (A upper left), 90Y-PET (A upper right), 166Ho-MRI (B upper left), and 166Ho-SPECT (B upper right)
Post-treatment Dosimetry
Although in most centers post-treatment dosimetry is not routinely performed, it may be useful for optimization of the toxicity profile and the efficacy of RE treatment. Post-treatment dosimetry can reveal tumors that have not received the planned radiation dose and require additional targeting [40]. Pre-treatment scout dose imaging or contrast-enhanced catheter-directed CT imaging does not guarantee that microspheres will reach and completely cover the tumor [41]. Therefore, post-treatment dosimetry remains a valuable tool to support treatment planning in selected patients that potentially benefit from a (super-selective) second treatment with radioembolization or other adjuvant modalities [42]. At the same time, it may prevent unacceptable toxicity whenever repeated treatment is warranted, since it has been shown that patients who receive a second treatment may have an increased risk of REILD [43].
Post-treatment dosimetry is also key for establishing dose-effect and dose-toxicity relations. This information should support general understanding of the radiobiological mechanisms involved in radioembolization and may elucidate important dosimetric parameters that are related to therapy outcome. Once these parameters are known, they should be included in the pre-treatment dosimetric model for activity planning.
Quantitative assessment of 90Y has long been considered impossible, due to the limited quality of the 90Y Bremsstrahlung SPECT images. Recent developments in hardware and image reconstruction software, however, have paved the way for quantitative 90Y-PET and 90Y-Bremsstrahlung SPECT imaging for accurate post-treatment dosimetry based on the actual microsphere distribution.
The wide range (0–2.3 MeV) and continuous nature of the 90Y-Bremsstrahlung photon energy spectrum complicate SPECT quantification of the local 90Y-activity with the reconstruction software that is currently available in the clinic [44]. The absence of a photopeak prohibits the use of simple energy window-based scatter rejection and correction techniques and hinders attenuation correction based on single photon energy. Furthermore, extensive compensation for penetration of high-energy photons through the collimator septa is required for accurate resolution recovery [45]. It was recently demonstrated, however, that quantitative 90Y-Bremsstrahlung SPECT imaging is possible with more elaborate reconstruction software, which includes energy-dependent models of the scatter and attenuation effects in the patient and of the photon interactions in the collimator-detector system [46, 47]. In a simulation study, Rong et al. demonstrated that the 90Y-activity in the liver can be quantified within 5 % error [46]. A study by Elschot et al. showed that quantitative 90Y-Bremsstrahlung SPECT may be used as an alternative to 90Y-PET for lesions larger than 25 mm [47].
90Y has long been considered a pure beta-emitter by the Nuclear Medicine community, despite the fact that the very low branch leading to positron emission (32 times per million decays) was already discovered in 1955 [48]. It was not until the introduction of the latest generation of 3D PET/CT scanners with time of flight (TOF) technology that the feasibility of 90Y PET was demonstrated [40]. An advantage of 90Y PET over Bremsstrahlung SPECT is that the advanced correction techniques for scatter, random, and attenuation effects developed for F18 PET are clinically available and can be directly applied to 90Y PET as well [40, 44]. Moreover, PET has a higher resolution than SPECT, because a mechanical collimator is not required, which results in better quantification of the 90Y activity in small lesions [44, 47]. A disadvantage of 90Y PET is the very low count rate, which results in images that are prone to deterioration by Poisson noise. The minimum detectable activity on 90Y PET images was estimated to be 1 MBq ml−1 by Carlier et al. [49]. Although this is probably good enough for intrahepatic dosimetry in most patients, it may impede detection of possible lung shunting on post-treatment 90Y PET images. Figure 4 shows the intrahepatic 90Y-biodistribution in a patient, visualized with 90Y-Bremsstrahlung SPECT and 90Y-PET.
Dosimetry During Treatment
The greatest disadvantage of post-treatment dosimetry is that irrespective of how accurate it estimates the absorbed dose, the activity has already been irreversibly administered, and although in some cases additional activity may be administered, it is impossible to rectify overdoses. Real-time dosimetry during treatment, on the other hand, would allow for feedback modifications to the treatment plan based on administered dose, distribution alterations and stasis, and extrahepatic deposition. Chemoembolization using ethiodated oil emulsion and real-time fluoroscopy is an example of a real-time feedback dosing mechanism, albeit fraught with its own limitations. Dosimetry during RE is even more technically challenging and has not yet been developed for clinical use. Two potential options for dosimetry during treatment involve the use of nuclear imaging and MRI.
MRI-Angiography
Perhaps the best-suited modality for dosimetry during treatment is MRI. MRI can provide anatomical and functional information at a high temporal and spatial resolution with excellent soft tissue contrast. The key ingredient is a type of microsphere that provides sufficient contrast on MRI. To our knowledge, there are currently two types of microspheres for MRI-guided RE under investigation. Both particles are based on a paramagnetic element to induce susceptibility artifacts on T2*-weighted MRI. One type consists of iron-oxide incorporated in or labeled onto glass microspheres [50, 51]. Studies in rats and rabbits have shown that the biodistribution of these microspheres can be accurately quantified with MRI but no clinical studies have yet been performed. The second type is the previously mentioned 166Ho-microspheres, which consist of holmium integrated in a matrix of poly(l-lactic acid). A recent study showed that the absorbed dose on both the tumor(s) and the normal liver can be accurately quantified post 166Ho-treatment using MRI [52]. A next step could be MRI-guided RE with these microspheres, as was previously demonstrated in an animal model [53]. However, as with all MRI-guided endovascular interventions, MRI-guided RE faces complicating factors such as a shortage of clinically available MR-compatible and trackable catheters, guide-wires and coils, limited operational space, and high costs [54].
Scintigraphy-Guided Angiography
Three-dimensional nuclear imaging is generally not associated with real-time imaging, because the scan duration is typically in the order of 30 min or longer, during which the camera rotates around a presumably static object. With the localized deposition of activity that is typical for liver RE (i.e. high activity concentrations), however, it may be possible to acquire good quality SPECT scans in 5–10 min. This may allow for dosimetry during treatment administration in the same angiography procedure, if a SPECT/CT scanner is available in the angiography room. An advantage would be that while the catheter tip can stay in position, quantitative SPECT/CT can guide further administration of the microspheres. Real-time tracking of the microsphere distribution during administration may further enhance the possibilities of nuclear imaging for treatment guidance. A hybrid imaging modality would give the interventional radiologist direct feedback, more control over the procedure, and the possibility to combine scout and therapy dose in one session.
Future Directions
A better understanding of the underlying mechanism of RE is needed to guide future developments. Radiobiological parameters such as dose rate, specific microsphere activity, and dose distribution, as well as clinical parameters such as tumor type, liver function, and disease burden, need to be related to dose–response relationships on treatment accuracy (i.e. efficacy and toxicity). In analogy to external beam radiation therapy, the concepts of normal tissue complication probability (NTCP) and tumor control probability (TCP) should be used for this purpose. Liver tolerance should then be explored prospectively using a dose-escalation protocol based on this information. This will involve issues with liver segmentation and compartmental dose calculation and definition, accounting for non-uniform dose distribution. Finally, the hypothesis that an optimized treatment planning methodology leads to improved outcome should be investigated, keeping in mind that a stepwise introduction of new methodology is expected to create the right balance between practicality and perfection.
Conclusion
Peri-procedural dosimetry, including both prediction and measurement of activity distribution, is the greatest current challenge for optimization of RE. Technical advances in imaging, development of imageable microspheres, new segmentation methods for partition modeling, and improved understanding of flow dynamics in relation to catheter positioning and embolic effects, will lead to more logical dose calculation and administration, and should ultimately result in further increase in efficacy and decrease in toxicity. Ultimately, real-time image guidance and distribution monitoring will enable further customization and optimization of treatment.
References
Rosenbaum CE, Verkooijen HM, Lam MG, Smits ML, Koopman M et al (2013) Radioembolization for treatment of salvage patients with colorectal cancer liver metastases: a systematic review. J Nucl Med 54:1890–1895
Kennedy A, Coldwell D, Sangro B, Wasan H, Salem R (2012) Radioembolization for the treatment of liver tumors general principles. Am J Clin Oncol 35:91–99
Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Nemcek AA Jr et al (2007) Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol 30:571–592
Hamami ME, Poeppel TD, Muller S, Heusner T, Bockisch A et al (2009) SPECT/CT with 99mTc-MAA in radioembolization with 90Y microspheres in patients with hepatocellular cancer. J Nucl Med 50:688–692
Liu D, Cade DN, Worsley D, Klass D, Lim H, et al (2013) Single procedure yttrium-90 (SPY90). Pilot study of consolidated single procedure selective internal radiation therapy (SIRT) utilizing yttrium-90 resin microspheres: preliminary results. CIRSE https://www.webges.com/cslide/presentations/cirse2013/62025/pdf/000001.pptx.pdf. Accessed 1 Feb 2014
Abdelmaksoud MH, Hwang GL, Louie JD, Kothary N, Hofmann LV et al (2010) Development of new hepaticoenteric collateral pathways after hepatic arterial skeletonization in preparation for yttrium-90 radioembolization. J Vasc Interv Radiol 21:1385–1395
Salem R, Parikh P, Atassi B, Lewandowski RJ, Ryu RK et al (2008) Incidence of radiation pneumonitis after hepatic intra-arterial radiotherapy with yttrium-90 microspheres assuming uniform lung distribution. Am J Clin Oncol 31:431–438
Lambert B, Mertens J, Sturm EJ, Stienaers S, Defreyne L et al (2010) 99mTc-labelled macroaggregated albumin (MAA) scintigraphy for planning treatment with 90Y microspheres. Eur J Nucl Med Mol Imaging 37:2328–2333
Hung JC, Redfern MG, Mahoney DW, Thorson LM, Wiseman GA (2000) Evaluation of macroaggregated albumin particle sizes for use in pulmonary shunt patient studies. J Am Pharm Assoc 40:46–51
Lenoir L, Edeline J, Rolland Y, Pracht M, Raoul JL et al (2012) Usefulness and pitfalls of MAA SPECT/CT in identifying digestive extrahepatic uptake when planning liver radioembolization. Eur J Nucl Med Mol Imaging 39:872–880
Sangro B, Gil-Alzugaray B, Rodriguez J, Sola I, Martinez-Cuesta A et al (2008) Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer 112:1538–1546
Flamen P, Vanderlinden B, Delatte P, Ghanem G, Ameye L et al (2008) Multimodality imaging can predict the metabolic response of unresectable colorectal liver metastases to radioembolization therapy with yttrium-90 labeled resin microspheres. Phys Med Biol 53:6591–6603
Gray B, Van Hazel G, Hope M, Burton M, Moroz P et al (2001) Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 12:1711–1720
Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ et al (2002) Body surface area and body weight predict total liver volume in Western adults. Liver Transpl 8:233–240
Sirtex (2014) SIR-Spheres Yttrium-90 Resin Microspheres Package Insert. Available at: http://www.sirtex.com/us/clinicians/package-insert/. Accessed 19 Dec 2014
Lam MG, Louie JD, Abdelmaksoud MH, Fisher GA, Cho-Phan CD et al (2014) Limitations of body surface area-based activity calculation for radioembolization of hepatic metastases in colorectal cancer. J Vasc Interv Radiol 25:1085–1093
BTG (2014) Therasphere yttrium-90 glass microspheres [package insert]. Available at: http://www.therasphere.com/physicians_us/package_insert.asp. Accessed 19 Dec 2014
Gulec SA, Mesoloras G, Stabin M (2006) Dosimetric techniques in 90Y-microsphere therapy of liver cancer: the MIRD equations for dose calculations. J Nucl Med 47:1209–1211
Ho S, Lau WY, Leung TW, Johnson PJ (1998) Internal radiation therapy for patients with primary or metastatic hepatic cancer: a review. Cancer 83:1894–1907
Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF et al (2011) Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys 79:163–171
Ho S, Lau WY, Leung TW, Chan M, Ngar YK et al (1996) Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur J Nucl Med 23:947–952
Lau WY, Kennedy AS, Kim YH, Lai HK, Lee RC et al (2012) Patient selection and activity planning guide for selective internal radiotherapy with yttrium-90 resin microspheres. Int J Radiat Oncol Biol Phys 82:401–407
Lam MG, Goris ML, Iagaru AH, Mittra ES, Louie JD et al (2013) Prognostic utility of 90Y radioembolization dosimetry based on fusion 99mTc-macroaggregated albumin-99mTc-sulfur colloid SPECT. J Nucl Med 54:2055–2061
Powerski MJ, Scheurig-Munkler C, Banzer J, Schnapauff D, Hamm B et al (2012) Clinical practice in radioembolization of hepatic malignancies: a survey among interventional centers in Europe. Eur J Radiol 81:e804–e811
Stuart JE, Tan B, Myerson RJ, Garcia-Ramirez J, Goddu SM et al (2008) Salvage radioembolization of liver-dominant metastases with a resin-based microsphere: initial outcomes. J Vasc Interv Radiol 19:1427–1433
Michels NA (1966) Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 112:337–347
Kao YH, Hock Tan AE, Burgmans MC, Irani FG, Khoo LS et al (2012) Image-guided personalized predictive dosimetry by artery-specific SPECT/CT partition modeling for safe and effective 90Y radioembolization. J Nucl Med 53:559–566
Louie JD, Kothary N, Kuo WT, Hwang GL, Hofmann LV et al (2009) Incorporating cone-beam CT into the treatment planning for yttrium-90 radioembolization. J Vasc Interv Radiol 20:606–613
Van de Wiele C, Maes A, Brugman E, D’Asseler Y, De Spiegeleer B et al (2012) SIRT of liver metastases: physiological and pathophysiological considerations. Eur J Nucl Med Mol Imaging 39:1646–1655
Wondergem M, Smits ML, Elschot M, de Jong HW, Verkooijen HM et al (2013) 99mTc-macroaggregated albumin poorly predicts the intrahepatic distribution of 90Y resin microspheres in hepatic radioembolization. J Nucl Med 54:1294–1301
Jiang M, Fischman A, Nowakowski FS (2012) Segmental perfusion differences on paired Tc-99m macroaggregated albumin (MAA) hepatic perfusion imaging and yttrium-90 (Y-90) bremsstrahlung imaging studies in SIR-sphere radioembolization: associations with angiography. J Nucl Med Radiat Ther 3
Ricke J (2010) Predictive Value of 99mTc- Albumin Spheres Before 90Y- SIR Therapy (EXPLOSIVE). Available at: NCT01186263 http://clinicaltrials.gov/ct2/show/NCT01186263?term=explosive+99mtc&rank=1. Accessed 19 Dec 2014
Selwyn RG, Avila-Rodriguez MA, Converse AK, Hampel JA, Jaskowiak CJ et al (2007) 18F-labeled resin microspheres as surrogates for 90Y resin microspheres used in the treatment of hepatic tumors: a radiolabeling and PET validation study. Phys Med Biol 52:7397–7408
Avila-Rodriguez MA, Selwyn RG, Hampel JA, Thomadsen BR, Dejesus OT et al (2007) Positron-emitting resin microspheres as surrogates of 90Y SIR-spheres: a radiolabeling and stability study. Nucl Med Biol 34:585–590
Maziere B, Loc’h C, Steinling M, Comar D (1986) Stable labelling of serum albumin microspheres with gallium-68. Int J Radiat Appl Instrum A 37:360–361
Schiller E, Bergmann R, Pietzsch J, Noll B, Sterger A et al (2008) Yttrium-86-labelled human serum albumin microspheres: relation of surface structure with in vivo stability. Nucl Med Biol 35:227–232
Smits ML, Nijsen JF, van den Bosch MA, Lam MG, Vente MA et al (2012) Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study. Lancet Oncol 13:1025–1034
Elschot M, Smits ML, Nijsen JF, Lam MG, Zonnenberg BA et al (2013) Quantitative Monte Carlo-based holmium-166 SPECT reconstruction. Med Phys 40:112502
van de Maat GH, Seevinck PR, Elschot M, Smits ML, de Leeuw H et al (2012) MRI-based biodistribution assessment of holmium-166 poly(l-lactic acid) microspheres after radioembolisation. Eur Radiol 23:827–835
Lhommel R, van Elmbt L, Goffette P, Van den Eynde M, Jamar F et al (2010) Feasibility of 90Y TOF PET-based dosimetry in liver metastasis therapy using SIR-Spheres. Eur J Nucl Med Mol Imaging 37:1654–1662
Kao YH, Tan EH, Teo TK, Ng CE, Goh SW (2011) Imaging discordance between hepatic angiography versus Tc-99m-MAA SPECT/CT: a case series, technical discussion and clinical implications. Ann Nucl Med 25:669–676
Chang TT, Bourgeois AC, Balius AM, Pasciak AS (2013) Treatment modification of yttrium-90 radioembolization based on quantitative positron emission tomography/CT imaging. J Vasc Interv Radiol 24:333–337
Lam MG, Louie JD, Iagaru AH, Goris ML, Sze DY (2013) Safety of repeated yttrium-90 radioembolization. Cardiovasc Intervent Radiol 36:1320–1328
Elschot M, Vermolen BJ, Lam MG, de Keizer B, van den Bosch MA et al (2013) Quantitative comparison of PET and Bremsstrahlung SPECT for imaging the in vivo yttrium-90 microsphere distribution after liver radioembolization. PLoS ONE 8:e55742
Elschot M, Nijsen JF, Dam AJ, de Jong HW (2011) Quantitative evaluation of scintillation camera imaging characteristics of isotopes used in liver radioembolization. PLoS ONE 6:e26174
Rong X, Du Y, Ljungberg M, Rault E, Vandenberghe S et al (2012) Development and evaluation of an improved quantitative (90)Y bremsstrahlung SPECT method. Med Phys 39:2346–2358
Elschot M, Lam MG, van den Bosch MA, Viergever MA, de Jong HW (2013) Quantitative Monte Carlo-based 90Y SPECT reconstruction. J Nucl Med 54:1557–1563
Ford K (1955) Predicted 0 + level in 40Zr90. Phys Rev 98:1516–1517
Carlier T, Eugene T, Bodet-Milin C, Garin E, Ansquer C et al (2013) Assessment of acquisition protocols for routine imaging of Y-90 using PET/CT. EJNMMI Res 3:11
Gupta T, Virmani S, Neidt TM, Szolc-Kowalska B, Sato KT et al (2008) MR tracking of iron-labeled glass radioembolization microspheres during transcatheter delivery to rabbit VX2 liver tumors: feasibility study. Radiology 249:845–854
Li W, Zhang Z, Guo Y, Nicolai J, Reed A, Larson A (2013) SPIO-labeled 90Y microspheres permit accurate quantification of macroscopic intra-hepatic biodistribution. Paper presented at International Society for Magnetic Resonance in Medicine, Salt Lake City, UT
Smits ML, Elschot M, van den Bosch MA, van de Maat GH, van Het Schip AD et al (2013) In vivo dosimetry based on SPECT and MR imaging of 166Ho-microspheres for treatment of liver malignancies. J Nucl Med 54:2093–2100
Seppenwoolde JH, Bartels LW, van der Weide R, Nijsen JF, van het Schip AD et al (2006) Fully MR-guided hepatic artery catheterization for selective drug delivery: a feasibility study in pigs. J Magn Reson Imaging 23:123–129
Kos S, Huegli R, Bongartz GM, Jacob AL, Bilecen D (2008) MR-guided endovascular interventions: a comprehensive review on techniques and applications. Eur Radiol 18:645–657
Acknowledgments
The work of M. Smits was supported by the Foundation for Image-guided Cancer Treatments (in Dutch: Stichting Beeldgestuurde Behandeling van Kanker) and by the Alexandre Suerman MD/PhD grant of the University Medical Center Utrecht.
Conflict of interest
D. Sze is consultant for W.L. Gore, Inc., Guerbet, Inc., Codman/J&J, Inc., Covidien, Inc., Embolx, Inc., Amgen, Inc., and BTG, Inc., and is member of the scientific/medical advisory board of Surefire Medical, Inc., Koli, Inc., Treus Medical, Inc., RadiAction Medical, Inc., Lunar Design, Inc., and Northwind Medical, Inc. Y. Kao had previously received research funding from Sirtex Medical Singapore. J. Nijsen is co-inventor of 166-holmium PLLA-microspheres and the patents are assigned to University Medical Center Utrecht Holding BV. J. Nijsen is Chief Scientific Officer at Quirem Medical BV. The other authors declare that they have no conflict of interest.
Statement of Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smits, M.L.J., Elschot, M., Sze, D.Y. et al. Radioembolization Dosimetry: The Road Ahead. Cardiovasc Intervent Radiol 38, 261–269 (2015). https://doi.org/10.1007/s00270-014-1042-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-014-1042-7