Abstract
Purpose
90Y microspheres are used for intra-arterial treatment of liver tumours. In the patient preparation, a hepatic angiogram is performed and all arteries that could transport microspheres from the targeted liver vasculature to extrahepatic organs are blocked. 99mTc-labelled macroaggregated albumin (MAA) is injected intra-arterially to simulate the treatment and whole-body scintigraphy and single photon emission computed tomography (SPECT) of the abdomen are performed.
Methods
Various aspects of lung shunt fraction (LSF) estimation were studied: interobserver and intrapatient variability, influence of scan quality and underlying disease. Secondly, the interobserver variability in reading the MAA SPECT of the abdomen was investigated. We reviewed 90 whole-body scans and 20 SPECT scans performed at our institution. Readers were blinded to each other’s findings. Scoring the scan quality was based on the visualization of tracer degradation.
Results
The mean difference in LSF between the readers was 1%. In 1 of 23 patients who underwent repeated MAA injections a marked change in LSF was observed. No significant differences in LSF were recorded for primary versus secondary liver tumours. There was a correlation between scan quality and LSF, suggesting that low scan quality leads to overestimation of the LSF. Concordant results in ruling out the presence of extrahepatic tracer deposition were reached in 17 of 20 scans (85%).
Conclusion
Interobserver and intrapatient variability in LSF calculation was limited. LSF was clearly dependent on scan quality. The underlying disease had no significant impact on the LSF. Interobserver variability for reading the MAA SPECT scans was acceptable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary and secondary liver tumours are a major health problem [1]. In the past few years 90Y microspheres have been introduced in many cancer centres to treat hepatocellular carcinoma (HCC) or liver metastasis. Liver tumours that receive their blood supply mainly from the hepatic artery and appear hypervascular on contrast-enhanced morphological imaging are possible candidates for intra-arterial treatment strategies. Radiolabelled microspheres with a diameter small enough to gain entry into tumour vessels but too large to pass through the capillary bed (8–10 μm) into the venous circulation will become trapped when injected via the hepatic artery. Since the normal liver parenchyma receives its blood supply primarily via the portal vein, the normal tissue can be relatively spared whilst high doses are delivered to the tumour [2].
The physical half-life of 90Y is 64 h [3]. It is a high energy β-particle emitter: β- = 99.99%, \( {E_{\beta - \max }}:{2}{.3}\,{\hbox{MeV}} \). The electrons emitted by 90Y have a mean and maximum range of 2.5 and 10.5 mm in soft tissues, respectively, allowing the eradication of tumour cells that are not directly targeted [4]. Two types of 90Y-labelled microspheres are commercially available. SIR-Spheres® are non-biodegradable 90Y-labelled resin microspheres with a diameter of 20–60 μm (SIR-Spheres®, Sirtex Medical, Sidney, Australia). The standard activity provided by the company is 3 GBq. TheraSphere® (MDS Nordion, Ottawa, ON, Canada) is a medical device, consisting of 20 to 30 μm glass spheres. Vials of 3, 5, 7, 10, 15 and 20 GBq are available [2].
In the patient preparation for treatment with 90Y microspheres, the “macroaggregated albumin (MAA) procedure” is considered to be an essential step. First, a hepatic angiogram is performed and all vessels that could transport microspheres to non-target organs should be identified and blocked to prevent the spread of 90Y microspheres into the stomach, gall bladder, duodenum, pancreas, etc. Following thorough review of the angiogram and meticulous coil embolization, about 185 MBq 99mTc-labelled MAA is injected into the hepatic artery or one of its branches to simulate the treatment with 90Y microspheres. The distribution of 99mTc-MAA is visualized by gamma scintigraphy. A whole-body scan is carried out for assessing tracer activity in the lungs and single photon emission computed tomography (SPECT) of the abdomen is acquired to rule out tracer deposition in other non-target organs. The MAA procedure is considered of utmost importance in preventing toxicity from treatment with 90Y-labelled microspheres. It has been shown that excessive pulmonary activity of 90Y can induce potentially fatal lung toxicity [5]. Deposition of microspheres in the stomach, small intestine, pancreas or periumbilical abdominal wall can induce severe complications such as bleeding, ulceration, pancreatitis or skin necrosis.
This paper reviews our findings in 99mTc-MAA scintigraphy for treatment planning of intra-arterial administration of 90Y microspheres.
Aim
We retrospectively reviewed MAA scintigraphies that were carried out at our institution in patients possibly eligible for treatment by means of 90Y-labelled microspheres (TheraSphere® or SIR-Spheres®).
The following aspects were studied:
-
1.
Interobserver variability in estimating the lung shunt fraction (LSF)
-
2.
Influence of the scan quality on the estimated LSF
-
3.
Variability in LSF as a function of underlying disease (primary versus secondary liver tumours)
-
4.
Intrapatient variability in LSF in cases of multiple procedures
-
5.
Interobserver variability in assessing the presence of extrahepatic tracer deposition on SPECT of the abdomen
Materials and methods
For studying the LSF, we retrospectively analysed all MAA whole-body scans carried out in our hospital between November 2006 and February 2010. Whole-body acquisition was performed following injection of 185 MBq 99mTc-MAA into the hepatic artery or one of its branches. Scans were read by an experienced nuclear medicine physician and by a technologist in training.
The LSF was calculated by the formula below:
Regions of interest (ROIs) were drawn manually around the liver and the lungs on a geometric mean whole-body image (Hermes Medical Solutions, Stockholm, Sweden).
LSF <10% was categorized as “class A”. If the LSF was 10–14%, then it was classified as “B”. For LSF of 15–20% and LSF >20%, respectively, class “C” and “D” were attributed. According to the package insert of SIR-Spheres®, an LSF of class B implicates a reduction in planned activity of 90Y of 20%. If the LSF is class C, then the activity should be reduced by 40%. In patients with a class D LSF, treatment with SIR-Spheres® is contraindicated. Each reader was blinded to the LSF calculated by the other reader. We report in how many cases an experienced reader and an inexperienced reader reached a consensus on LSF classification.
Secondly, the quality of the MAA whole-body scan was scored. If adequate scaling of the whole-body image for assessing the liver resulted in visualization of the kidneys on the image, then quality was considered to be low. Quality was scored as moderate if pertechnetate (99mTcO4 −) uptake was evident in the thyroid. If no hallmarks of tracer degradation were present, scan quality was considered to be good. We report on the image quality distribution in the varying LSF classes and carried out a Spearman’s correlation test (SPSS 15.0 software package).
A third question concerns the potential influence of the underlying disease on the LSF. This is reported as descriptive statistics and a two-sided Student’s t test.
A fourth item we investigated concerns the reproducibility within a patient of the LSF estimation. In the subset of patients who underwent the MAA procedure more than once, we compared the LSF classes and linked this to possible causes of changes in LSF, such as scan quality and catheter position.
Besides whole-body scintigraphy, a SPECT scan of the abdomen was performed to rule out tracer deposition outside the liver. A subset of MAA SPECT images were retrospectively read by an experienced reader (nuclear medicine physician) and an inexperienced reader (resident in training). Readers were blinded to each other’s findings. Scans were interpreted side by side with a CT scan or MRI of the liver. For patients with colorectal cancer, 18F-fluorodeoxyglucose (FDG) PET scan was also available. Results were compared to the conclusions of another experienced nuclear medicine physician, who reported the scans at the time they were carried out. If side-by-side analysis was not sufficient, a software fusion (PMOD Technologies, Zurich, Switzerland) was allowed. We report on the level of consensus between the readers, as well as in how many cases software fusion was considered helpful.
If the SPECT scan was suspicious for inadvertent delivery of MAA outside the liver, a software fusion was considered essential to help pinpoint the culprit vessel to the radiologist.
Results
Whole-body scintigraphy
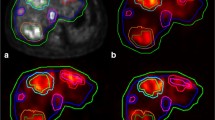
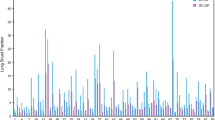
Ninety subsequent MAA whole-body scans were analysed. In 64% of assessable cases the activity was injected in the proper hepatic artery. In 24 and 12% the tracer was administered in the right hepatic artery (or its branches) and the left hepatic artery, respectively. An experienced nuclear medicine physician estimated the LSF: mean 7.5% (5.2% standard deviation), with a minimum of 1% and a maximum of 27%. The case with the highest recorded LSF in our series is depicted in Fig. 1a. An illustration of a normal case is added for comparison (Fig. 1b). Distribution of LSF is summarized in Fig. 2. Scans were also read by an inexperienced reader, a technologist in training. In 90% of cases the experienced reader and the inexperienced reader reached consensus, attributing the LSF to the same class. In cases where readers did not reach consensus in LSF category, it was often only marginally different when expressed in percentage. Overall the mean difference in LSF between readers was 1% (1.5% standard deviation). In 62% of the discordant cases, scan quality was suboptimal (low or moderate quality).
The quality of the images was scored by the nuclear medicine physician. Fifty-three scans were considered to be good quality scans, whereas 37 scans (41%) were considered to be of moderate or low quality (Table 1). In Fig. 3 we linked the LSF to the image quality. Low quality images tend to show higher LSF, and this is statistically supported by a Spearman’s correlation test (correlation coefficient 0.546, p value <0.000). This suggests that the high LSF are possibly an overestimation due to low image quality.
When the LSF for patients suffering from HCC (n = 43, mean 7.3%, standard deviation 4.7%) was compared to those referred for treatment of colorectal liver metastasis (n = 38, mean 7.8%, standard deviation 5.7%), no significant difference was observed (p value 0.68).
In a subsequent analysis of the data set, we focused on intrapatient variability of LSF. This was assessable in 23 patients: 1 patient underwent 4 MAA procedures, 4 patients had 3 MAA scans and 18 patients underwent the procedure twice. Reasons for repeating the procedure were the presence of extrahepatic tracer deposition (12 patients), treatment of the other lobe in cases of bilobar disease (4), retreating a formerly treated lesion (3) or a combination of these (4). In 4 of 23 patients (17%) with multiple MAA scans a change in LSF classification was observed. In three cases a borderline change in classification was noticed: patients who were attributed an LSF category A had another scan in which the LSF was estimated to be 10 or 11%. In two cases the change might be attributable to a change in catheter position at the moment of MAA injection. There was one single case of more pronounced change in LSF: 7, 17 and 21%. On all occasions there was a good scan quality. The low LSF was obtained with the catheter position in the right hepatic artery, whereas both higher values occurred when the catheter was in the left hepatic artery. The latter interventions were problematic, in the sense that extrahepatic tracer deposition was seen in the abdomen.
SPECT scan of the abdomen
The last item we reviewed in our data set was the interobserver variability to assess the MAA distribution pattern on SPECT images of the abdomen. Twenty MAA SPECTS were analysed. The most frequent scenario was that both physicians agreed that no extrahepatic tracer deposition was present (13 cases, 65%). In four patients they both concluded that extrahepatic MAA deposition was present. Hence, reports were concordant in 17 of 20 cases (85%). In three cases no concordance was reached concerning the presence or absence of extrahepatic MAA deposition. Twice this was due to a faint activity below the liver, located in the anterior abdomen. This might be due to a patent falciform artery. In one case activity in the stomach was observed, attributed to free pertechnetate by the experienced reader and considered to be a contraindication for treatment by the inexperienced reader (Fig. 4). In 7 of 20 SPECT scans software fusion with morphological imaging was indicated to rule out or specify extrahepatic tracer deposition.
Discussion
We tested the interobserver variability for estimating the LSF, based on whole-body scintigraphy following intra-arterial injection of a tracer dose of 99mTc-MAA. In 90% of cases, LSF categorization was identical between an experienced and an inexperienced reader. Scans of suboptimal quality, characterized by tracer degradation, were overrepresented in the discordant LSF group. Subsequently we showed that there was a correlation between a higher LSF and inferior scan quality. This finding suggests that the tracer degradation leads to an overestimation of LSF.
To prevent tracer degradation, it is suggested that the 99mTc-MAA be prepared shortly before injection and the interval between injection and scanning be kept as short as possible.
The inferior scan quality also leads to discordant results in the interpretation of the SPECT images, since it is not always clear whether visualization of the stomach is due to the presence of free pertechnetate or rather due to misadministration of the tracer. Some authors [2] suggested prevention of uptake of free pertechnetate by preparing the patients with 600 mg perchlorate. The downside of the use of perchlorate is that it obscures the in vivo quality control by preventing pertechnetate uptake in the thyroid. As a consequence, the use of perchlorate could negatively affect the correct interpretation of the presence of activity in the lungs.
Overall, the role of LSF calculation seems limited. Of 90 scans, 21 (23%) showed an LSF of 10% or higher, of which 15 scans were of suboptimal quality. This implies that only six scans of satisfying quality suggested that a “significant” lung shunt was present. However, this leaves us with the discussion of what is considered to be significant for LSF estimations. Based on the package insert of Sirtex, one might consider an LSF of 10% or more to be clinically significant since an activity reduction is recommended in these patients. However, an expert panel recommended that the lung dose should not exceed 30 Gy in a single treatment [6]. Such a dose is obtained when about 610 MBq 90Y is delivered to the lungs. According to the TheraSphere® package insert, the activity should be reduced in order to bring the lung activity below 610 MBq 90Y. When reviewing the first 30 treatments carried out at our institution, we were only once confronted with such a situation. Since it concerned an MAA scan of moderate quality in a patient with normal lung function testing at baseline, no activity reduction was prescribed for the 90Y microspheres. In our retrospective review of follow-up data, no lung toxicity was encountered.
We also studied the variability of LSF within a patient. In 4 of 23 patients who underwent repeat MAA procedures, a different class of LSF was recorded. In three of them it was only a marginal difference when expressed quantitatively. So only in 1 of 23 patients was a possibly relevant change in LSF noticed when the procedure was repeated. However, this case was complicated by deposition of tracer in non-target organs intra-abdominally. This might explain the higher lung uptake in these procedures. In the vast majority of patients, LSF estimation seems reproducible despite differences in catheter position (left or right hepatic artery or proper hepatic artery).
In a subsequent analysis 20 MAA SPECT scans were read by two physicians, one experienced in reading MAA scans and another one at the start of his learning curve. Results for assessment of tracer deposition in non-target organs were concordant in 85% of cases. This was considered to be fairly good. However, interobserver variability could be improved if the presence of free pertechnetate could be avoided. The results of both readers were compared with the reports made by an experienced nuclear medicine physician at the time the exam was carried out. For the experienced readers a concordance rate of 90% was achieved. Possibly this could be further improved by the use of hybrid SPECT/CT cameras. Hamami and coworkers compared the accuracy of planar imaging, SPECT and SPECT/CT hybrid imaging in 58 consecutive patients [9]. A combination of clinical and radiological follow-up data was used for establishing the gold standard in their series. Accuracy for detecting extrahepatic tracer deposition was 72, 79 and 96% for planar imaging, SPECT and hybrid SPECT/CT, respectively. To reduce the rate of false-positive readings of hybrid SPECT/CT further (3 of 58 patients) they recommend side-by-side reading with contrast-enhanced morphological imaging to rule out tracer deposition in intravascular tumour thrombus. Garin and coworkers also showed in their report on their initial experiences that SPECT/CT was superior when compared to planar images or SPECT [10].
A topic that is not well addressed in the literature is the intrahepatic tracer distribution. Only a few authors [6–8] make recommendations on how patient selection should be performed, taking into account adequate tracer accumulation in the targeted lesions. Flamen et al. [7] studied the predictive value of the MAA-based tumour to normal liver ratio for metabolic response of the lesion. Using a cut-off MAA uptake ratio of 1, the positive and negative predictive values of a good response on a lesion-by-lesion basis were 71% (17/24) and 87% (13/15), respectively. Thus, relatively cold lesions on MAA SPECT have a very low probability of showing a significant response on FDG PET scan.
In our experience assessing the tumour to normal liver ratio on MAA SPECT is rather subjective, if performed by side-by-side analysis with morphological imaging or FDG PET scan only. In our opinion, the consequent use of SPECT/CT might help us to better select patients and plan treatment.
Conclusions
We reviewed our experience with MAA scintigraphy for planning treatment with 90Y microspheres. We showed that interobserver variability is small for estimating the LSF on whole-body images and for assessment of tracer deposition in non-target organs on SPECT scan of the abdomen. Tracer degradation tends to negatively affect the concordance between readers and induces overestimation of the LSF. The intrapatient variability of the LSF when the MAA procedure was repeated was negligible in all cases but one. No difference in LSF was observed between patients referred for HCC compared to colorectal liver metastasis.
References
Parkin MD, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108.
Ahmadzadehfar H, Biersack HJ, Ezziddin S. Radioembolization of liver tumors with yttrium-90 microspheres. Semin Nucl Med 2010;40:105–21.
Annals of the ICRP. Publication 38: radionuclide transformations: energy and intensity of emissions. Oxford: Pergamon; 1983.
ICRU. Stopping powers for electrons and positrons report 37. Bethesda: International Commission on Radiation Units and Measurements; 1984
Ho S, Lau WY, Leung TW, Chan M, Johnson PJ, Li AK. Clinical evaluation of the partition model for estimating radiation doses from yttrium-90 microspheres in the treatment of hepatic cancer. Eur J Nucl Med 1997;24:293–8.
Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68:13–23.
Flamen P, Vanderlinden B, Delatte P, Ghanem G, Ameye L, Van Den Eynde M, et al. Multimodality imaging can predict the metabolic response of unresectable colorectal liver metastases to radioembolization therapy with yttrium-90 labeled resin microspheres. Phys Med Biol 2008;53:6591–603.
Sangro B, Bilbao JI, Boan J, Martinez-Cuesta A, Benito A, Rodriguez J, et al. Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2006;66:792–800.
Hamami ME, Poeppel TD, Müller S, Heusner T, Bockisch A, Hilgard P, et al. SPECT/CT with 99mTc-MAA in radioembolization with 90Y microspheres in patients with hepatocellular cancer. J Nucl Med 2009;50:688–92.
Garin E, Rolland Y, Boucher E, Ardisson V, Laffont S, Boudjema K, et al. First experience of hepatic radioembolization using microspheres labelled with yttrium-90 (TheraSphere): practical aspects concerning its implementation. Eur J Nucl Med Mol Imaging 2010;37:453–61.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lambert, B., Mertens, J., Sturm, E.J. et al. 99mTc-labelled macroaggregated albumin (MAA) scintigraphy for planning treatment with 90Y microspheres. Eur J Nucl Med Mol Imaging 37, 2328–2333 (2010). https://doi.org/10.1007/s00259-010-1566-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1566-2