Abstract
Background
Y90 transarterial radioembolization (Y90-RE) may improve clinical outcomes of unresectable intrahepatic cholangiocarcinoma (ICC); however, the optimal timing for Y90-RE is still debated. The purpose of this multicenter study was to retrospectively evaluate clinical outcomes of RE in patients with unresectable ICC, comparing three different settings: chemotherapy naïve patients (group A), patients with disease control after first-line chemotherapy (group B) and patients with progression after first-line chemotherapy (group C).
Materials and Methods
The study included 81 consecutive patients (49 male, mean age 62.4 ± 11.8 years): 35 (43.2%) patients were in group A, 19 (23.5%) in group B, and 27 (33.3%) in group C. Preprocedural clinical variables, tumour response according to RECIST 1.1 and overall survival (OS) were analysed and compared.
Results
Baseline demographic and clinical features did not differ significantly among groups, with the exception of prior surgical procedures that were significantly higher in group C patients, and macrovascular invasion that was more frequent in group B. Radiological response was available in 79 patients; objective response and disease control rates were 41.8% and 83.6%, respectively, without significant differences among groups. Median OS was 14.5 months (95% CI: 11.1–16.9) and was not significantly different among treatment groups. At multivariate analysis, tumour burden > 50%, neutrophil-to-lymphocyte (N/L) ratio ≥ 3 and radiological progression as best response resulted to be significant (P < 0.05) independent factors, negatively associated with OS.
Conclusion
Y90-RE is a valuable treatment option in unresectable ICC, irrespectively from the timing of treatment. Tumour extension, N/L ratio and radiological response affect post-treatment survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer [1], with an average annual incidence of about 2.1 per 100 000/year in Western countries [2] that has apparently increased over the last decades.
Surgery is the only potentially curative treatment option, with reported median survival of 36–39 months [3, 4]. However, only 30–40% of ICCs are resectable at initial diagnosis [2]. In unresectable ICC, prognosis remains poor, and treatment options are still limited [5]. Systemic chemotherapy, with gemcitabine and cisplatin, represents the standard of care in unresectable ICC [2], with a median overall survival (OS) of 11.7 months [6].
Loco-regional therapies represent a valid alternative to systemic therapy in patients with no or limited extrahepatic disease [2, 5]. Percutaneous ablation is indicated only in limited tumour burden (single lesion < 3 cm in size) [2], while intra-arterial therapies allow more extended treatments, with low toxicity and good tumour control [7, 8].
Among the different transarterial treatment options, yttrium-90 radioembolization (Y90-RE) has gained increasing interest. It allows to selectively deliver high radiation doses to the tumour, with low systemic and hepatic toxicity. A recent systematic review reported approximately 15 months median OS after Y90-RE that favourably compares to systemic chemotherapy [9]. However, debate is ongoing regarding the most proper timing for Y90-RE in primarily unresectable ICC.
The purpose of this retrospective multicentre study was to analyse tumour response and survival of Y90-RE in unresectable ICC, comparing three different clinical settings: chemotherapy naïve patients (group A), patients with radiological disease control after first-line chemotherapy (group B) and patients with radiological progression at first-line chemotherapy (group C).
Materials and Methods
The study conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the institution’s human research committee. Patients’ informed consent was waived. Patients provided written informed consent to the procedure.
The study included 81 consecutive patients (49 male; mean age 62.4 ± 11.8 years, range 38–83 years) with unresectable, biopsy-proven ICC, who underwent Y90-RE using resin spheres in three tertiary referral centres, between July 2008 and October 2017.
Treatment was decided after multidisciplinary tumour board evaluation. Patients were considered eligible for Y90-RE if they met the following criteria: (1) histologically proven ICC; (2) tumour considered unresectable by the liver surgeon; (3) liver dominant or liver only disease; (4) Eastern Cooperation Oncology Group (ECOG) performance status of 0–1; (5) adequate liver function (Child–Pugh class A-B8; bilirubin < 2.0 mg/dl; no clinically relevant ascites); (6) platelet count > 50x109 l−1 and (7) the absence of contraindications to angiography.
The exclusion criteria included: (1) ECOG performance status ≥ 2; (2) extrahepatic uptake of 99mTc-labelled macroaggregated albumin (MAA) identified at SPECT-CT, due to arterial branches that could not be effectively excluded by coil embolization; and (3) estimated lung exposition to a radiation dose > 30 Gy in a single administration or 50 Gy cumulatively.
Patients were divided into three groups. Group A included chemotherapy naïve patients for whom Y90-RE was performed as first-line treatment at first diagnosis or at recurrence after surgery. Group B included patients submitted to Y90-RE as consolidation treatment after radiological disease control following first-line chemotherapy. Group C consisted of patients indicated to Y90-RE because of tumour progression after first-line chemotherapy.
Treatment Protocol and Follow-up
Y90-RE was preceded by a simulation procedure consisting of an angiographic study, to detect arterial feeders supplying the lesions and any extrahepatic branches requiring preventive embolization in order to avoid peri-procedural complications related to irradiation of non-target organs.
After the catheter was placed in its final position, 99mTc-labelled MAA was injected intra-arterially and SPECT-CT scan was performed to check the uptake of target lesions, to exclude extrahepatic uptake or to calculate hepato-pulmonary shunting.
The treatment was performed using resin particles (SirSphere®; Sirtex Medical Europe GmbH, Bonn, Germany) labelled with Y90. BSA (body surface area)-based method was used for dosimetry, according to the manufacturer’s recommendations. In case of bilobar disease, the treatment of each lobe was performed in different sessions, at an interval of 4–6 weeks.
Patients were followed by triphasic computed tomography (CT) and routine clinical and laboratory follow-up every 3 months. Tumour response was assessed according to Response Evaluation Criteria in Solid Tumours 1.1 (RECIST 1.1) [10]. At progression, patients were treated according to disease presentation after multidisciplinary tumour board discussion.
Data Analysis
Demographic and clinical data were retrospectively collected, including age, gender, presence and cause of cirrhosis, presence of ascites, ECOG performance status, laboratory data (liver function tests, complete blood count, coagulation profiles, albumin, total bilirubin, Ca 19.9), previous treatments and tumour extension (unilobar or bilobar, tumour number and maximum diameter, percentage of liver volume involved, presence of macrovascular invasion and/or metastases).
Biochemical toxicities occurring at any time after treatment were reported. The Common Terminology Criteria for Adverse Events of the National Cancer Institute were used to categorize toxicities [11].
Statistical Analysis
The primary outcome parameter of the study was OS. The secondary outcome parameters were radiological tumour response by RECIST 1.1 and time to progression after first Y90-RE.
Data were analysed using descriptive statistics (mean and standard deviation, SD) and compared with Chi-square or Fisher's exact test for categorical data and Student’s t test for paired data.
Survival time was calculated as the time between first Y90-RE and death or end of follow-up (28 February 2018). Survival curves were obtained by Kaplan–Meier analysis and compared using the log-rank test. For continuous variables, cut-off values were identified by ROC curve analysis. Factors significantly associated with OS at univariate analysis were included in the multivariate analysis. Statistical analysis was carried out with dedicated software (SAS, Cary, NC USA) considering a P value < 0.05 as statistically significant.
Results
Thirty-five (42.2%) patients were in group A, 19 (23.5%) in group B and 27 (33.3%) in group C. Indications adopted in each centre for Y90-RE were different; in particular, Y90-RE as consolidation treatment (group B) was more frequently considered in Hospitals 1 and 2 compared to Hospital 3 (Table 1). Patients’ clinical characteristics are presented in Table 1; 40 (49.4%) patients presented bilobar disease, while macrovascular tumour invasion was present in 34 (42%) patients (mainly represented by segmental or lobar portal vein thrombosis) and it was more frequent in group B patients. Extrahepatic metastases were identified in 20 (24.7%) patients, mainly represented by abdominal lymph nodes; in seven cases, a maximum of two lung nodules were detected (maximum diameter 16 mm), while in one patient the tumour infiltrated the right adrenal gland. A significantly higher number of group C patients had undergone surgery prior to Y90-RE and, as expected, had a longer interval between ICC diagnosis and Y90-RE. All the remaining baseline demographic and clinical features were not significantly different. However, a higher percentage of group A patients had liver cirrhosis (40%, versus 21% and 14.8% in group B and C, respectively), and group C patients presented more frequently with multifocal disease (51.9% versus 31.4% and 15.8% in group A and B, respectively).
In 22 cases (27.2%), bilobar treatment in two different sessions was required; the remaining cases were treated in a single session. In two patients, treatment did not include the entire tumour volume; in one of these cases a second Y90-RE was performed six months later to complete the treatment, while the other case could not be completed due to rapid extrahepatic progression.
The median administered activity was 1.56 GBq (IQR, 1.1–1.85), and the median tumour absorbed dose was 109 Gy (IQR, 55–192), with 43 (53.7%) cases receiving at least 100 Gy (Table 2). In three cases, the procedure was prematurely interrupted because of arterial spasm associated with pain; all three cases occurred in earlier years, when sterile water was used to inject the particles.
As per hospitals’ policies, patients were discharged 36–48 h after the procedure. No major adverse events were recorded. Post-embolization syndrome (low-grade fever, abdominal pain, nausea and vomiting) was registered in 12 (14.8%) patients, lasting a maximum of seven days with no sequelae for the patients.
Radiological Tumour Response
One patient died within two months for unrelated causes (complications after a major trauma), and one patient was lost to radiological follow-up. Thus, radiological tumour response was available in 79 patients. According to RECIST 1.1, the best responses were: complete response in 4 cases (5.1%), partial response in 29 cases (36.7%), stable disease in 33 cases (41.8%) and progression in 13 cases (16.4%). Thus, the objective response and disease control rates were 41.8% and 83.6%, respectively (Table 2).
Median time to local and distant intrahepatic progressions was 295 days (95%CI, 222–646) and 222 days (95%CI, 170–368), respectively (Table 2). Extrahepatic tumour progression was observed in 40 patients (49.4%) at a median time of 368 days (95%CI, 222–1123; Table 2).
Radiological responses and times to progression did not differ significantly among treatment groups (Table 2).
Overall Survival
Mean follow-up from Y90-RE was 14.6 ± 12.6 months (median 11.1 months; range 2–64.2).
On follow-up, three patients were downstaged to resection (3.7%), while 12 patients underwent other loco-regional therapies targeting in 11/12 cases the same volume included in the first Y90-RE; loco-regional treatments included Y90-RE (seven patients), transarterial (chemo)embolization (four cases), radiofrequency ablation (one case) and endobiliary brachitherapy (one case); finally, 43 (53.1) patients were submitted to first- and/or second-line chemotherapy. No significant differences were observed in treatments after first Y90-RE comparing groups A, B and C (Table 2).
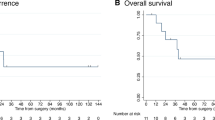
Median OS from Y90-RE was 14.5 months (95% CI: 11.1–16.9), with no significant difference comparing treatment groups (Table 2, Fig. 1). At univariate analysis, median OS was significantly (P < 0.05) affected by the tumour load, in terms of percentage of liver volume involvement, the baseline values of Ca19.9, the neutrophil-to-lymphocytes (N/L) ratio and the radiological tumour response (Table 3). At multivariate analysis, percentage of liver volume involvement, N/L ratio and radiological response were confirmed to be independently associated with OS (Table 3).
Discussion
Several series have described the results of Y90-RE in patients with unresectable ICC, with median OS ranging from 9 to 22 months (Table 4) [12,13,14,15,16,17,18,19,20,21,22,23]. However, in the absence of prospective randomized studies, indications to Y90-RE are still debated, and it is today difficult to understand how to insert Y90-RE in the treatment algorithm of unresectable ICC.
In this retrospective analysis, no differences in survival and tumour response were observed comparing chemo-naive patients to patients submitted to Y90-RE as consolidation or as salvage therapy, while other factors, such as tumour extension and inflammatory status, showed a significant correlation with post-treatment survival.
Y90-RE has been proposed as a valuable option in treatment naïve patients, with promising survival rates [14, 15, 17]. As opposite, in our series median OS was 15 months in both chemo-naïve and treatment naïve patients, with no differences compared to patients treated in the consolidation or in the salvage setting. The results in the first-line could improve combining systemic chemotherapy to Y90-RE, as recently proposed by Edeline et al. in a prospective phase II trial on 41 patients, obtaining 22 months median OS [24]. Of interest, the study reported a high rate (22%) of successful downstaging, followed by resection and excellent post-surgical outcomes [24]; the majority of downstaged patients were not cirrhotic and had unifocal disease confined to a single hemiliver [24], thus delineating a subgroup of potentially resectable patients that could benefit from a more aggressive first-line approach. In our series, 40% of group A patients had liver cirrhosis, only one-third had single lesions, and approximately 40% had bilobar disease and macrovascular invasion, representing a more advanced group of patients, unlikely to be ever able to undergo resection. These differences may at least partly explain the lower OS in group A patients compared to Edeline’s trial.
Y90-RE could be proposed as a consolidation treatment in unresectable patients with either partial response or stable disease after the first-line chemotherapy, to boost up local response while allowing patients to recover from systemic therapy. This approach was used in 19 patients in our series, obtaining 84% disease control rate, although the median OS was slightly lower (12 months) compared to group A and C patients, most likely as a result of the more extensive disease, with higher rates of bilobar disease, macrovascular invasion and metastases. Despite these unfavourable characteristics, the approach proved to be safe and the median OS in line with previous studies [9, 23].
Finally, in the salvage setting, Y90-RE can be proposed in chemo-refractory patients, with similar radiological responses and post-treatment survivals as in group A and B, provided that some selection criteria are carefully evaluated, such as tumour extension, presence of macrovascular invasion and/or distant metastases and tumour markers; specifically, in our series percentage of liver involvement and the inflammatory status reflected by the N/L ratio resulted to be significantly associated with OS at multivariate analysis.
Regarding the percentage of liver involvement, Mouli et al. reported a very poor prognosis (median OS, 1 month) in patients with tumour involving over 50% of the liver volume [15]. Our results confirm this finding, despite the longer OS (6 months); thus, tumour burden > 50% should be considered an exclusion criterion for loco-regional treatments, including Y90-RE.
Baseline N/L ratio has been extensively investigated as a useful prognostic indicator in several cancer types, including ICC [25,26,27]. Elevated N/L ratio is a reflection of the patient’s inflammatory status and indicates that the balance has been tipped in favour of pro-neoplastic inflammatory responses and is therefore associated with poor clinical outcomes. Our study confirms that a baseline-elevated N/L ratio represents a poor prognostic indicator in patients treated with Y90-RE, with a significantly lower median OS in patients with a baseline N/L ratio > 3 (11.1 vs. 17.1 months). Therefore, this biomarker could be used as a widely available predictive factor, possibly indicating the need for intensive combination therapies and close monitoring in patients with an elevated baseline N/L ratio.
Tumour response to treatment is a key factor not only for further treatment planning but also to define patients’ prognosis [14, 17, 18]; our results confirm that progressive disease after treatment is a poor prognostic indicator. The assessment of tumour response after Y90-RE is still a matter of debate. Several modified criteria have been proposed, such as Choi criteria [18] and modified RECIST and EASL (European Association for the Study of the Liver) criteria using the delayed-phase contrast enhancement to assess tumour vascularity [17, 28]. However, reproducibility of these criteria is arguable, and extensive validation is missing. Therefore, RECIST 1.1 should still be considered the standard of reference for assessing tumour response. The systematic review by Al-Adra et al. reported a weighted mean PR rate of 28% and SD rate of 54% [9], in agreement with our findings.
Safety of Y90-RE has been confirmed by all studies, with low peri-procedural morbidity and mortality and 0-17% grade > 3 adverse events (Table 3) [12,13,14,15,16,17,18,19,20,21,22]. No major complications or deterioration of liver function were observed in our series. However, appropriate patient selection and accurate diagnostic work-up are the key to avoid major complications. Also, adequate dosimetry is essential to reduce liver toxicity and deliver sufficiently high activity to the tumour. In recent years, the correlation between dosimetry and clinical outcomes has been demonstrated [29, 30], and efforts are made to produce more accurate models for personalized dosimetry. In our series, only in approximately 50% of patients the tumour absorbed dose was > 100 Gy, reflecting the fact that the series included patients treated over a wide period of time, when knowledge on dosimetry was still limited. Despite the lack of association between dosimetry and OS in our series, it is clear that the results of Y90-RE in the future could improve by implementing specific dosimetric models.
The main limitations of our study are related to its retrospective design; patients were enrolled through a long period of time, with heterogeneous clinical histories; limited number of patients and pre-selection bias may explain why some well-known prognostic factors did not result to be associated with OS in the present series. Finally, the vast majority of patients were submitted to different therapies before and after Y90-RE; thus, isolating any survival effect achieved by Y90-RE alone is not possible.
Considering the relatively low incidence of this disease, its biological heterogeneity and even the heterogeneity in the definition of unresectability, a randomized controlled trial represents a challenge. An international trial has been initiated randomizing treatment naïve, unresectable ICC patients to standard chemotherapy alone or Y90-RE followed by chemotherapy (SIRCCA trial; ClinicalTrials.gov Identifier:NCT02807181); the trial was prematurely stopped after 3 years, due to slow recruitment. Moreover, in this complex scenario, the potential role of emerging molecular-targeted therapies and immune checkpoint inhibitors should not be neglected [31,32,33,34], and additional efforts are needed to analyse the possible interactions between these drugs and Y90-RE.
In conclusion, Y90-RE is a valuable treatment option in unresectable ICC, irrespectively from the timing of treatment. Inflammatory status, tumour extension and radiological tumour response are significantly associated with survival. In particular, N/L ratio is a widely available and inexpensive biomarker that could be routinely used to guide therapeutic decisions.
References
Massarweh NN, El-Serag HB. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control. 2017;24:1073274817729245.
Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–89.
Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96.
Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an eastern and western experience. JAMA Surg. 2014;149:432–8.
Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–79.
Valle J, Wasan H, Palmer DH, et al. ABC-02 trial investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81.
Hyder O, Marsh JW, Salem R, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol. 2013;20:3779–86.
Wright GP, Perkins S, Jones H, et al. Surgical resection does not improve survival in multifocal intrahepatic cholangiocarcinoma: a comparison of surgical resection with intra-arterial therapies. Ann Surg Oncol. 2018;25:83–90.
Al-Adra DP, Gill RS, Axford SJ, et al. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol. 2015;41:120–7.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Cirillo M, Venturini M, Ciccarelli L, et al. Clinician versus nurse symptom reporting using the national cancer institute-common terminology criteria for adverse events during chemotherapy: results of a comparison based on patient’s self-reported questionnaire. Ann Oncol. 2009;20:1929–35.
Saxena A, Bester L, Chua TC, et al. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17:484–91.
Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol. 2013;36:440–8.
Hoffmann RT, Paprottka PM, Schön A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35:105–16.
Mouli S, Memon K, Baker T, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol. 2013;24:1227–34.
Edeline J, Du FL, Rayar M, et al. Glass microspheres 90Y selective internal radiation therapy and chemotherapy as first-line treatment of intrahepatic cholangiocarcinoma. Clin Nucl Med. 2015;40:851–5.
Mosconi C, Gramenzi A, Ascanio S, et al. Yttrium-90 radioembolization for unresectable/recurrent intrahepatic cholangiocarcinoma: a survival, efficacy and safety study. Br J Cancer. 2016;115:297–302.
Beuzit L, Edeline J, Brun V, et al. Comparison of choi criteria and response evaluation criteria in solid tumors (RECIST) for intrahepatic cholangiocarcinoma treated with glass-microspheres yttrium-90 selective internal radiation therapy (SIRT). Eur J Radiol. 2016;85:1445–52.
Swinburne NC, Biederman DM, Besa C, et al. Radioembolization for unresectable intrahepatic cholangiocarcinoma: review of safety, response evaluation criteria in solid tumors 1.1 imaging response and survival. Cancer Biother Radiopharm. 2017;32:161–8.
Reimer P, Virarkar MK, Binnenhei M, et al. Prognostic factors in overall survival of patients with unresectable intrahepatic cholangiocarcinoma treated by means of yttrium-90 radioembolization: results in therapy-naïve patients. Cardiovasc Intervent Radiol. 2018;41:744–52.
Gangi A, Shah J, Hatfield N, et al. Intrahepatic cholangiocarcinoma treated with transarterial yttrium-90 glass microsphere radioembolization: results of a single institution retrospective study. J Vasc Interv Radiol. 2018;29(8):1101–8.
White J, Carolan-Rees G, Dale M, et al. Yttrium-90 transarterial radioembolization for chemotherapy-refractory intrahepatic cholangiocarcinoma: a prospective, observational study. J Vasc Interv Radiol. 2019;30(8):1185–92.
Zhen Y, Liu B, Chang Z, Ren H, Liu Z, Zheng J. A pooled analysis of transarterial radioembolization with yttrium-90 microspheres for the treatment of unresectable intrahepatic cholangiocarcinoma. Onco Targets Ther. 2019;12:4489–98.
Edeline J, Touchefeu Y, Guiu B, et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2019. https://doi.org/10.1001/jamaoncol.2019.3702.
Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124.
Taussig MD, Koran MEI, Mouli SK, et al. Neutrophil to lymphocyte ratio predicts disease progression following intra-arterial therapy of hepatocellular carcinoma. HPB (Oxford). 2017;19:458–64.
D’Emic N, Engelman A, Molitoris A, et al. Prognostic significance of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients treated with selective internal radiation therapy. J Gastrointest Oncol. 2016;7:269–77.
Camacho JC, Kokabi N, Xing M, et al. Modified response evaluation criteria in solid tumors and European Association for The Study of the Liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J Vasc Interv Radiol. 2014;25:256–65.
Bourien H, Palard X, Rolland Y, et al. Yttrium-90 glass microspheres radioembolization (RE) for biliary tract cancer: a large single-center experience. Eur J Nucl Med Mol Imaging. 2019;46(3):669–76.
Levillain H, Duran Derijckere I, Ameye L, et al. Personalised radioembolization improves outcomes in refractory intra-hepatic cholangiocarcinoma: a multicenter study. Eur J Nucl Med Mol Imaging. 2019;46(11):2270–9.
Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72(2):353–63.
Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular targeted therapies: ready for “prime time” in biliary tract cancer. J Hepatol. 2020. https://doi.org/10.1016/j.jhep.2020.03.007.
Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–84.
Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020. https://doi.org/10.1016/s1470-2045(20)30157-1.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Irene Bargellini received honoraria for Speaker activities from Sirtex Medical Europe GmbH, Biocompatibles UK LTD, Terumo Europe NV and for Advisory Boards from Sirtex Medical Europe GmbH. Gianluca Masi received honoraria for Speaker activities and Advisory Boards from Sirtex Medical Europe GmbH and Bayer Spa. The other authors declare no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was waived.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bargellini, I., Mosconi, C., Pizzi, G. et al. Yttrium-90 Radioembolization in Unresectable Intrahepatic Cholangiocarcinoma: Results of a Multicenter Retrospective Study. Cardiovasc Intervent Radiol 43, 1305–1314 (2020). https://doi.org/10.1007/s00270-020-02569-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-020-02569-4