Abstract
Renal cell carcinoma is a common malignancy causing significant mortality. In recent years abdominal imaging, often for alternate symptomatology, has led the trend toward the detection and confirmation of smaller renal tumors. This has permitted the greater use of localized and nephron-sparing techniques including partial nephrectomy and image-guided ablation. This article aims to review the current role of image-guided biopsy and ablation in the management of small renal tumors. The natural history of renal cell carcinoma, the role of renal biopsy, the principles and procedural considerations of thermal energy ablation, and the oncological outcomes of these minimally invasive treatments are discussed and illustrated with cases from the authors’ institution. Image-guided ablation, in particular, has changed the treatment paradigm and, by virtue of its increasingly evident efficacy and low morbidity, now favors the treatment of smaller tumors in patients previously unfit for surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCCa) is a common malignancy still associated with significant mortality. Over 200,000 new cases of kidney cancer are diagnosed in the world each year [1]. Of these, 63,300 new cases occur annually in the European Union (EU25), accounting for nearly 3% of all cancer cases in this region [2]. In the United Kingdom 7840 people were diagnosed with kidney cancer in 2006 and there were 3752 deaths in 2007 [3].
The incidence of RCCa has been increasing over several decades—with a 126% increase between 1950 and 2001 [4]. In parallel with this, there has been an increase in 5-year survival, from 51% in 1975–1977 to 67% in 1996–2004 [5]. This superficially suggests that the increase in disease is simply attributable to the increased incidental radiologic detection of smaller-volume tumors, which are of increasingly less clinical significance. However, many authors have noted an additional true background increase in RCCa incidence, contributed to by increasing rates of obesity [6] and a decreased mortality from cardiovascular disease.
The question still stands as to whether this is simply an increase in the numbers of small, clinically insignificant renal tumors. In fact, the Cancer Research U.K. data have shown an increase in mortality from renal cancer in the oldest birth cohort: for men and women aged 85 and older, death rates from RCCa more than doubled between 1971 and 2006, from 23.8 to 75.5 per 100,000 for men and from 12.8 to 30.5 per 100,000 for women [7]. This again testifies to the increasing relevance of RCCa in overall population cancer mortality. RCCa is now ranked as the fifth most common cause of cancer death, if the gender-specific cancers are set aside.
Modern cross-sectional imaging, often for other symptomatology, has increased the incidence of small renal tumors. The earlier detection and optimized surgery for these smaller renal tumors have both contributed significantly to improved outcomes for this disease, and this has been acknowledged by recent modifications to the TNM classifications. In 1997 sub-7-cm disease was deemed T1, and a subset of tumors <4 cm in diameter, T1a [8]. In particular, this T1a subgroup appears amenable to more localized or nephron-sparing techniques such as partial nephrectomy rather than radical nephrectomy. A nephron-sparing approach has importantly been shown to have an equivalent oncological outcome to radical nephrectomy, while minimizing the morbidity of more aggressive surgery that compromises renal function, in an often-elderly age group. In fact, renal function alone is increasingly acknowledged as an important overall prognostic indicator of cardiovascular morbidity and longevity in this age group [9]. Any means to decrease iatrogenic renal injury therefore seems likely to improve patient outcomes following management of small renal tumors. Radical nephrectomy has been shown to accelerate mild chronic renal failure in the remnant contralateral kidney through hyperfiltration injury [10], further compounding the issue, which again argues for a more conservative approach.
It needs to be acknowledged that in detecting increasing numbers of sub-4-cm tumors, careful radiological assessment is required to exclude entities such as complex, hyperdense cysts and fat-poor angiomyolipomas. As tumors become smaller at detection an increasing proportion will be benign at biopsy or extirpative surgery. Most authors have found that the proportion of benign tumors increases with diminishing size. It has been suggested that up to 25% of surgically resected sub-3-cm renal tumors are benign [11].
Smaller renal tumors (≤3 cm) tend to exhibit a relatively indolent growth pattern, with growth rates of 1–3 mm/year on average and very low rates of nodal positivity (at radical nephrectomy) and metastases [12–14]. However, much of the available natural history literature regarding small renal masses tends to average the growth rate of the observed tumors and a number of authors have noted that a significant cohort of renal tumors (~20%) appears to grow more rapidly [12, 14]. At present we have no reliable means of identifying this subset, with practical cytogenetic markers still being in development. The increasingly common clinical problem of incidental small renal tumors has again raised the case for watchful waiting or surveillance in some older patients, particularly those with other significant comorbidities. This is undoubtedly appropriate in some elderly or frail patients, as is an increased use of biopsy in a group of indeterminate patients.
The treatment options for small renal tumors have, however, changed dramatically in recent years, with the advent of robust laparoscopic and percutaneous image-guided ablation techniques. These procedures have changed the treatment paradigm and, by virtue of their increasingly evident efficacy and low morbidity, now favor the treatment of smaller renal tumors in older patients not previously amenable to open partial nephrectomy. There is now a clear case for image-guided ablation and/or laparoscopic partial nephrectomy as a primary treatment choice for smaller-volume renal disease across the entire patient spectrum. The current state of image-guided ablation and management of small renal tumors is discussed.
Place of Image-Guided Biopsy
The majority of focal renal parenchymal lesions are clearly identifiable as simple, benign cysts and can usually be confidently characterized on imaging criteria alone [15]. Nevertheless, the increased rate of cross-sectional imaging in recent years has undoubtedly contributed to the incidence of problem cortical lesions requiring characterization and management [16]. Diligent radiologic technique is required if these lesions are to be appropriately characterized so as to exclude complex/hyperdense cortical cysts and fat-poor angiomyolipomas and reserve treatment for renal carcinomas. Accurate characterization at CT requires noncontrast imaging and careful region-of-interest comparison with appropriate thin collimation postcontrast imaging at CT and MR. Similarly, careful pixel density analysis at CT or fat suppression techniques at MR should help to highlight a number of angiomyolipomas. Despite this attention to technique, however, a number of sub-4-cm renal tumors prove to be benign at extirpative surgery. A study of 2770 adult patients undergoing radical or nephron-sparing surgery for solid renal tumors found a consistent increase in benignity with reducing tumor size: 25% of sub-3-cm tumors, 30% of sub-2-cm tumors, and 44% of sub-1-cm tumors were found to be benign [11].
The case for renal tumor biopsy in the presence of a concurrent malignancy has already been well made. Tissue diagnosis is essential in this situation to distinguish a RCCa, which can be effectively treated by resection or ablation, from a metastasis which is likely to be treated medically [17]. In the setting of small renal tumors, if a significant interventional procedure is to be invoked, and, in particular, with ablation, a nonextirpative technique, the case for pre- or periprocedural biopsy becomes self-evident.
Renal biopsy has been known for some years to yield variable results. A recent review paper reported an overall sensitivity for biopsy of malignancy of 80%–92% [17]. False-negative results often occur due to inaccurate placement of the needle tip in a small mass and/or sampling of, usually central, necrotic areas. As such, a negative biopsy result in the context of a radiologically suspicious mass should be viewed with caution. Specificity of biopsy for malignancy ranges from 83%–100% [17]. False positives are relatively unusual: most reported cases occurred prior to 1990, and the current specificity is likely to be higher with the up-to-date histological techniques.
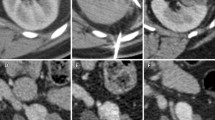
To increase yields the operator should seek to biopsy the more peripheral aspect of the tumor, avoiding the often central necrotic area, and achieve at least two adequate cores. Unfortunately renal tumor biopsy often incurs moderate but self-limiting, peritumoral hematoma, which can obscure the target for a second pass. This becomes particularly problematic if biopsy is performed immediately prior to ablation with the potential to obscure the target tumor prior to placement of ablation probes. If “periablational” tumor biopsy is to be performed, it is the authors’ practice to place the ablation probe(s) first and then biopsy alongside (or, on occasion, through a coaxial sheath) the ablation device. This risks slightly diminishing the positive biopsy yield but ensures that no obscuring hematoma subsequently compromises the planned ablation (Fig. 1).
The case for ablation probe placement prior to biopsy. An initial intraprocedural biopsy of a pedunculated 20-mm right lower pole renal tumor was undertaken. This caused considerable localized hematoma (A; arrow), making subsequent accurate placement of the RFA probe more difficult in this scoliotic patient and obscuring the duodenum. The patient presented 3 days later with a perforated duodenum which was subsequently repaired surgically (B)
The result of renal biopsy has been proven to have a significant impact on clinical management decisions in the majority of patients [18]. The timing of biopsy must, however, be measured against the morbidity of the planned procedure. If an onerous partial nephrectomy is planned, it will be more critical to ensure that the lesion is clearly malignant than prior to a straightforward ablation procedure. It remains mandatory, however, always to pursue biopsy proof of malignancy, particularly if a nonextirpative technique such as image-guided ablation is to be employed.
Thermal Energy Ablation Overview
Thermal ablative techniques have evolved rapidly in the last two to three decades. While cryoablation (CRA) has the longest published track record [19], heat-based techniques and their applications, e.g., radiofrequency ablation (RFA), microwave ablation (MWA), and electroporation, have developed rapidly in recent years. Ideally ablative techniques should yield a reproducible, contiguous, and rounded ablation zone, little modified by adjacent tissue perfusion or flowing vessels (“heat-sump” effect) [20]. Many devices were primarily used in the liver, reflecting the morbidity of liver resection, but during the 1990s it became apparent that smaller, rounded renal tumors within the retroperitoneum—and therefore relatively isolated from critical, temperature-sensitive structures—were ideally suited to a thermal ablative approach. The predominant techniques employed in renal tumor ablation have been RFA and CRA.
The aim of RFA is to induce coagulative tumor necrosis by achieving a constant temperature of between 60 and 105°C throughout the target volume during treatment. Temperatures above 105°C result in tissue charring and gas formation, which can impair heat propagation and thus limit the ablation zone. RFA is achieved by applying a high-frequency (400- to 500-kHz) alternating current to the target lesion. The electrical circuit is formed by the tip of the RFA probe, the patient, and large grounding pads. Ionic agitation immediately adjacent to the probe tip consequently produces frictional heating, which is conducted to the adjacent target tissues. The effectiveness of these probes has been enhanced by clustering needle-like applicators into simple or expandable devices.
CRA has evolved considerably in recent years due to the development of finer 17-gauge argon cryoprobes that enable more subtle and accurate thermal ablation along with reduced risk of hemorrhage. CRA induces cellular destruction through rapid (central iceball) and slower (more peripheral iceball) ice formation. In the center of the ‘cryolesion,’ rapid intra- and extracellular ice formation causes direct cell injury. More peripherally there is extracellular ice formation and, as a result, osmotic denaturation of the cells. These effects are compounded by microvascular injury and ensured by a double freeze-thaw cycle [21]. The real merit of CRA, however, lies in the demonstrably contiguous nature of the iceball and its easy depiction by CT or MR. The operator must, however, ‘overtreat’ the lesion, in common with all thermal ablative techniques, to ensure that at least the −20°C isotherm effectively incorporates the target tumor. Critically, renal tumor cells appear to be contiguously and reliably destroyed if they fall within the approximate −30°C isotherm and are subjected to a double freeze-thaw cycle.
Procedural Considerations in Percutaneous Ablation
The practical implementation of percutaneous ablation varies greatly between institutions: in particular, in the use of conscious sedation versus formal general anesthesia and different imaging modalities. Below we describe a typical percutaneous ablation treatment at our institution:
-
A joint anesthetic and radiological preassessment is carried out 1 week prior to the planned procedure date. Blood samples are obtained for a coagulation screen, platelet count, and estimated glomerular filtration rate (eGFR), in addition to any supplemental investigations required prior to general anesthesia. Advice is given to stop any anticoagulant and antiplatelet drugs (typically) 1 week prior to the ablative treatment. Ablations are not performed on patients with an international normalized ratio (INR) >1.4 or a platelet count of <90 × 109/L.
-
A disposable enema is administered on the morning of the treatment. It has been the authors’ experience that this reduces the problem of a gas-distended colon becoming interposed between the flank and the posterolateral aspect of the kidney.
-
A single prophylactic dose of intravenous antibiotics (750 mg cefuroxime and 500 mg metronidazole) is administered 1 h prior to the procedure.
-
It is our practice to routinely perform percutaneous ablative treatment under general anesthesia. This offers several advantages including optimal on-table positioning irrespective of patient comfort and reproducible respiratory suspension for probe and biopsy needle placement. In addition, it is likely that ablations performed under general anesthesia are more aggressive than those performed under conscious sedation, and better outcomes likely result from this. Prone general anesthesia is, however, a particular anesthetic skill and due consideration needs to be given to risk of brachial plexus injuries, etc.
-
Following induction of general anesthesia, the patient is transferred to the CT table and into a prone-oblique position. This position is accentuated and supported by the use of ‘pillow bolsters’ under the abdomen. The prone-oblique position allows for better ‘presentation’ of the kidney to the operator and wider spacing of the posterior ribs.
-
A diagnostic renal CT is performed prior to probe placement. This includes noncontrast, arterial and venous phase sequences and provides critical pretreatment assessment of tumor enhancement, which will ultimately be the arbiter of treatment success. Clearly, due consideration of renal function and the risk of contrast-induced nephropathy (CIN) must be taken into account, and on occasion, ultrasound-guided procedures are performed with corroborating CT assessment in problem cases. A full diagnostic study is essential at this stage to assess any interval growth and the evolution of new tumors.
-
Probe placement is therefore usually carried out largely under CT guidance for CRA and under combined CT and ultrasound guidance for RFA.
-
In RFA a solitary probe is placed centrally in the small renal lesion. In our practice, RFA is usually reserved for tumors of <30 mm.
-
CRA allows for the placement of multiple cryoprobes into larger tumors and is usually utilized for tumors of >30 mm at our institution. Cryoprobes are designed to produce either a spherical or a cylindrical ablation zone. A combination of different probes can therefore be sited adjacently to form a composite iceball, which will incorporate any larger, more complex lesions. Further control of the shaped iceball can be obtained by connecting individual probes in different treatment clusters on the control panel of the cryosurgical trolley. This allows for the temperature of cryoprobe clusters to be controlled independently so that different areas of the expanding iceball can simultaneously remain static or continue to expand.
-
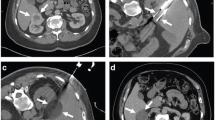
When placing multiple cryoprobes in a lesion it is clearly vital to ensure that the probes are suitably positioned to achieve the intended ablation zone. As with all thermal ablative techniques, treatment of the tumor margins is critical. Cryoprobes are placed in a ‘clock-face’ configuration approximately 10 mm from the tumor’s margin, approximately three probes to an average 35-mm renal mass. It is our routine practice to confirm probe placement with a limited coronal reformat prior to commencing ablation (Fig. 2).
Fig. 2 A 55-mm renal clear cell carcinoma in a 72-year-old male (A; arrow). Treated by CRA (B); note that the multiple cryoprobes are out of the plane of the image. The cryoprobes are placed at intervals around the tumor and their relative positions are best confirmed by coronal MPR reconstruction (C). Follow-up CT at 1 month confirms complete ablation (D)
-
A minimum of 15-mm separation between the tumor capsule and injury-prone adjacent structures is usually recommended. Generous retroperitoneal fat may provide this ablative margin but supplemental ‘hydrodissection’ is often necessary. The colon is particularly susceptible to thermal injury and often needs to be displaced (Fig. 3), but small bowel, pancreas, liver, spleen, renal pelvis, and ureter may also encroach upon the intended ablation zone. Aliquots of 50–100 ml of contrast-tinted (1% contrast) 5% dextrose are instilled into the adjacent retroperitoneal space using an 18-gauge cannula needle. Up to 500 ml may be required to provide a safe margin. Some operators use carbon dioxide for the purposes of achieving this safety margin.
Fig. 3 A 46-mm exophytic left lower polar RCCa treated by CRA. Initial imaging (A) demonstrates close opposition of the adjacent descending colon (white arrow) to the target lesion (black arrow). Subsequent hydrodissection with contrast-tinted 5% dextrose (B; arrow) displaces the colon and allows for generous ablation margins without risking colonic injury
-
Finally, as previously described, a periablational biopsy is usually obtained prior to commencing treatment.
-
Specific treatment protocols vary according to equipment manufacturers. In the case of cryoablation, two freeze-thaw cycles are routinely completed.
-
Cryoablation allows for accurate monitoring of the ablation zone as demonstrated by the conspicuous iceball. The interface between iceball and renal parenchyma is particularly well demonstrated, although it should be noted that the interface between iceball and retroperitoneal fat may be less distinct (Fig. 4).
-
When treatment is completed the percutaneous probes are removed and sterile dressings applied. Following cryoablation it is important to allow the iceball to melt before attempting to remove the probes: failure to do so can result in significant renal injury and hemorrhage.
-
Patients are typically discharged after 24 h and imaging follow-up is carried out as described below.
Efficacy of Radiofrequency Ablation in Renal Tumors
Zagoria et al. recently published their experience with CT-guided percutaneous RFA of 125 biopsy-proven RCCs [22]. Overall 93% of renal tumors, ranging in size from 0.6 to 8.8 cm, with a mean of 2.7 cm, were successfully treated. Importantly, straightforward ablation was achieved in all tumors smaller than 3.7 cm. Smaller tumor size, but not tumor location (in terms of polarity or anterior-posterior location), was predictive of primary treatment success.
A study of the outcomes of RFA in 100 tumors undertaken by Gervais et al. found that 90% of tumors were successfully treated [23]. Importantly, all tumors of <4 cm and all exophytic tumors (67/100) were completely ablated. Multivariate analysis showed that both small tumor size and noncentral location were independent predictors of success.
A previous study at our institution demonstrated a similar overall success rate of 90.5% in image-guided RFA of 105 tumors [24]. The mean size of tumors completely ablated at a single sitting was 2.8 cm (range, 1.4–4.6 cm) and the mean size of those in whom initial treatment was subtotal was 4.1 cm (range, 1.8–6.8 cm). Logistic regression analysis demonstrated that tumors ≤37 mm in diameter were almost invariably treated straightforwardly in a single treatment session.
Typically studies investigating percutaneous RFA in small lesions, ranging in mean tumor size from 2.2 to 3.2 cm, have demonstrated an overall success rate of between 84% and 99% [22–26].
Efficacy of Cryoablation in Renal Tumors
Atwell et al. have reported their experience with treating 115 renal tumors in 110 patients by percutaneous cryotherapy [27]. Technical success was achieved in 112 tumors, a success rate of 97%. Of three treatment failures, two occurred in tumors of 4.0 and 4.3 cm. The success rate in larger tumors (≥4 cm) was therefore lower, at 93%.
A smaller concurrent study, treating 51 renal tumors in 46 consecutive patients by percutaneous cryotherapy, reported similar outcomes [28]. Of the 40 renal cell carcinomas treated, an overall complete response rate of 97.5% was achieved. In a subgroup of patients reaching 1-year follow up, a 100% complete response was reported. Their experience was also primarily in smaller-volume disease, with a median lesion size of 3 ± 1.9 cm.
Hot or Cold?
A meta-analysis of reported CRA or RFA of small renal masses was undertaken by Kunkle and Uzzo up to October 2007 [29]. Forty-seven studies and the ablation of 1375 renal masses were included in this analysis. The meta-analysis found that repeat ablation was performed more often after RFA (8.5% vs. 1.5%) and the rates of local tumor progression (which includes initial subtotal treatment and late local recurrence) were significantly higher for RFA compared with CRA, 12.9% vs. 5.2%, respectively. It remains important, however, to note that these data pool nonrandomized, case-series literature, with a tendency toward patients who are less fit and more with solitary kidneys and otherwise problematic lesions being referred to percutaneous RFA. The additional confounding factors of laparoscopic versus percutaneous procedures and general anesthesia versus conscious sedation compound the problems of taking away any clear message about the relative efficacy of RFA versus CRA.
CRA does, however, benefit from the radiographic and laparoscopic conspicuity of the intraprocedural ‘iceball.’ The 0°C ‘ice front’ acts as a proxy maker for the more critical −20 to −40°C isotherms within its margins. This isotherm region more reliably determines the zone at which a reliable 100% cell kill will be achieved by repeated freeze-thaw cycles. This form of intraprocedural monitoring likely enhances the operator’s control of the procedure and, notably, lends CRA to intraprocedural monitoring by CT or MR (Fig. 4).
CRA is, however, usually more time-consuming and expensive in requiring the placement of multiple probes to obtain an adequate therapeutic iceball. ‘Single-stick’ RFA is, in the authors’ experience, effective and straightforward for the treatment of sub-3-cm disease with excellent outcomes [22, 24].
Percutaneous or Laparoscopic Ablation?
The role of thermal energy ablation has been extensively evaluated as both a laparoscopic and a percutaneous technique. Hui et al. undertook a robust comparative meta-analysis evaluating percutaneous and surgical approaches to renal tumor ablation [30]. Forty-six studies between 1996 and 2006 were compared. They demonstrated that while the efficacy of primary ablation was slightly lower for a percutaneous than a laparoscopic technique—87% versus 94%—the efficacy of secondary ablation was equivalent, at 92%. Importantly, they also found that the major complication rate was significantly lower following percutaneous ablation: 3% versus 7%. This finding has been borne out by a more recent study that found that percutaneous cryoablation is superior to laparoscopic cryoablation in terms of reduced operative time, reduced requirement for transfusion, reduced opioid analgesic requirement, and shorter hospital stay [31]. This superior safety profile may compensate for the slightly increased requirement for retreatment.
It is also important to stress that several large studies have shown that retreatment by percutaneous ablation is usually successful [24–26, 32, 33]. Matin et al. conducted a multicenter review of patients undergoing ablative therapy for a renal mass [32]. Their review included both laparoscopic and percutaneous techniques, although the majority of RFAs (83%) were performed percutaneously. They found that although residual or recurrent disease was identified in 13.4% of patients following initial RFA, with salvage energy ablative therapy—both RFA and cryoablation—therapy failed in only 4.2%. They also found that survival in patients with recurrent or residual disease did not differ on the basis of either approach (laparoscopic vs. percutaneous) or initial ablative modality (RFA vs. cryoablation).
What Tumor Size for Ablation?
Zagoria et al. found that, following a single RFA session, tumor-free survival was achieved in only 47% of tumors larger than 3.7 cm, compared to 100% of tumors smaller than 3.6 cm [22]. Furthermore, they found that a 1-cm increase in tumor size increased the likelihood of recurrent disease following ablation by 2.19 times. A similar size threshold has been identified by other studies including our own data [24]. In attempting to treat larger tumors by RFA, other authors have explored a combined approach, utilizing RFA in combination with other minimal access therapies such as renal artery embolization and direct ethanol injection.
Yamakado et al. have published their experience of treating 12 large renal cell carcinomas in 11 patients using combined renal artery embolization and subsequent RFA [34]. Mean tumor size was 5.2 ± 1.7 cm (range, 3.5–9.0 cm). They performed selective tumor vessel embolization in nine patients and complete renal artery embolization in two patients. All tumors appeared to be completely ablated as judged by nonenhancement at subsequent CT and MR. It should be noted, however, that although 9 of 12 tumors required only one session of RFA, 2 of 12 required two sessions and 1 of 12 required four sessions to achieve a complete response.
Another novel approach to enhanced tumor treatment with RFA is the use of ethanol injection. The effectiveness of combined RFA and ethanol injection has previously been demonstrated in the liver, although the mechanism of this synergistic effect remains unclear. Possible explanations include either a reduction in perfusion related tissue cooling due to ethanol-induced small vessel thrombosis or improved thermal conduction through previously coagulated tissue due to ethanol. In a recent study 28 renal tumors were pretreated by injection with absolute ethanol into the tumor immediately prior to RFA [35]. This approach has the potential advantage of allowing treatment to be completed at one session, whereas renal artery embolization is usually carried out in a separate procedure 1–2 days prior to RFA. Predominantly smaller tumors were treated, with a mean size of 2.9 cm (range, 0.8–6 cm) and complete ablation was achieved in all tumors with either one or two sessions of RFA.
Hakime et al. have found, in mice, that RFA may be enhanced with the priming use of the antiangiogenic drug sorafenib [36]. The resultant reduction in tumor perfusion appears to enhance the volume of the ablation zone, with a clear potential for increased treatment efficacy.
Initial experience in the use of combining RFA with other minimal-access therapies is encouraging. It may be, however, that improved thermal ablative efficacy with energies such as CRA and MWA, along with enhanced image guidance and pretreatment tumor priming, are the main ways forward in enhancing treatment effectiveness.
Imaging Follow-up in Ablation
Various imaging protocols have been used to follow-up ablated renal tumors. Persistent contrast enhancement remains the only validated surrogate marker of residual viable tumor, and therefore pre- and postcontrast techniques are widely used.
Multiplanar CECT remains the most available and widely used technique, although subtraction MR imaging is also a useful technique, particularly in the context of renal impairment (eGFR <30) while bearing in mind the small risk of nephrogenic systemic fibrosis with gadolinium. Ultrasound appearances can be difficult to interpret in the global sense, although, again, there may be a role for contrast-enhanced ultrasound, particularly in patients with severely impaired renal function, which prohibits the use of CT and MR contrast agents.
It is our practice to perform an initial CT examination at approximately 2 weeks postablation to assess treatment adequacy. Subsequent CT assessment is then made at 6-monthly intervals up to 2 years. Unenhanced and arterial phase images of the chest and upper abdomen to include the tumor are supplemented with portal venous phase images of the abdomen and pelvis. Successful complete tumor ablation produces a characteristic sequence of CT appearances.
Perilesional hematoma can often occur as a result of probe placement or periablational biopsy. In addition, RFA or CRA often results in hemorrhagic necrosis and the degraded blood products cause patchy areas of increased attenuation within the ablation zone. Unenhanced images are therefore mandatory to distinguish the resultant areas of high-attenuation degraded blood products from residual tumor enhancement [37].
The true extent of the ablation zone often becomes better defined a few days after treatment, so ideally contrast-enhanced studies to assess treatment adequacy should be deferred for at least 2–3 days. Portal venous phase and delayed phase imaging may demonstrate a thin rim of contrast enhancement. This ‘penumbral’ zone between necrotic and viable tissue appears to represent injured tissue unable to eliminate contrast medium (Fig. 5). This needs to be distinguished from asymmetrical crescents of characteristic tumoral enhancement likely to represent residual viable disease (Fig. 6). During this early follow-up period, the ablated lesion may also appear larger than the original tumor at immediate follow-up [38].
A 29-mm right lower polar tumor, in a 36-year-old female with severely deforming spina bifida, successfully treated by CRA. Portal venous phase CT at 2 weeks postablation shows a thin hemorrhagic rind (white arrow) in addition to a less well-defined penumbral zone (black arrow). The common appearance of a penumbral zone is felt to represent injured tissue unable to eliminate contrast and is evident on late arterial phase and delayed imaging performed up to 2 weeks after ablation
A 52-mm RCCa (A; black arrow) with an incidental adjacent simple cyst (A; white arrow) in a 74-year-old male. Following RFA a thin crescent of residual disease is evident at late arterial phase imaging (B; arrow). This residual tumor was successfully treated at repeat RFA, with no evidence of disease at CT 2 weeks after this completion ablation (C)
At subsequent CT examinations a successfully ablated lesion will appear as an increasingly low-attenuation mass, without focal enhancement, typically decreasing in size over subsequent months (Figs. 7 and 8). Many operators have noted that cryoablated tumors appear to involute more rapidly than those treated by RFA, possibly as a result of disruptive rather than coagulative necrosis [39]. A postablation ‘halo’ is a common appearance around the treated tumor in the perirenal fat (Fig. 9). This is often seen with well-treated tumors.
Subtotal treatments are usually manifest as asymmetrical marginal crescents of residual enhancement. In the case of cryoablation a regular thin hemorrhagic rind, usually of no clinical consequence, has been seen to persist for a few weeks after treatment, around the ablation zone. On occasion, enhancing vessels have been seen by some authors deep within an ablation zone up to 2 weeks following ablation. These then usually involute and do not appear to signal tumor viability. Such appearances must therefore be carefully judged before being attributed to subtotal treatment but, if necessary, should be assessed by an interim CT study at 3 months.
It is critical that a clear distinction is drawn between subtotal treatments determined at the first postprocedural imaging and those tumors deemed fully treated but later showing an unexpected late local recurrence. Imaging follow-up and thereby oncological outcomes need to be judged against meticulous imaging technique and execution. Most authors advocate CT or MR follow-up at 6-monthly intervals to approximately 2 years and then annually to 5 years, during the initial years of an image-guided ablation program. If there is any equivocation regarding the completeness of treatment at the first postprocedural study, most radiologists will advise a further study at 3 months posttreatment.
Long-Term Oncological Outcomes
Short and intermediate outcome data following percutaneous thermal energy ablation have been extensively published and consistently demonstrate outcomes equivalent to surgery. Substantive, long-term, 5-year outcome data remain relatively sparse and are still awaited. Given the relative indolence of many smaller RCCa, large numbers of tumors will need to be followed to adequately power any findings and therefore discern any relative limitations in either ablative or resectional techniques. It remains important, however, that the technique utilized must be effective and commensurate with the disease process, not incurring undue renal injury or morbidity in this often elderly group of patients.
McDougal et al. followed a group of patients treated with RFA for a minimum of 4 years [40]. Sixteen patients with biopsy-proven RCC, ranging in size from 1.1 to 7.1 cm, were followed up. Five patients died of unrelated causes. Ten of the remaining eleven were free of recurrent and metastatic disease at 4 years. One patient showed progressive tumoral enlargement after initial treatment.
One of the largest series of long-term outcome data on percutaneous RFA was published by Levinson et al. [33]. They reported their findings in 31 patients undergoing 34 RFA treatments for tumors ranging from 1.0 to 4.0 cm. Mean follow-up in survivors was 61.6 months. Three recurrences were reported, at 7, 13, and 31 months. Overall recurrence-free survival rate was 90.3%, with a 100% metastasis-free and disease-specific survival rate. They cautiously concluded that RFA provides reasonable long-term oncological control.
CRA outcome data following MR-guided percutaneous cryotherapy have been published by Silverman et al. [41]. Twenty-six renal tumors ranging from 1.0 to 4.6 cm were treated with CRA in 23 patients. Follow-up with contrast-enhanced MR or CT imaging at a mean interval of 14 months (range, 4 to 30 months) showed successful ablation in 92% of tumors. Eighty-eight percent of tumors required only one treatment session.
Long-term clinical follow up has been described in patients undergoing laparoscopic and open CRA, although the radiological follow up is often less thorough than in equivalent percutaneous CRA studies. Davol et al., for example, followed up a group of 48 patients for a median period of 64 months (range, 36–110 months) [42]. They described a cancer-free survival of 87.5% after a single CRA procedure, increasing to 97.5% with a repeat CRA. Mean radiological follow-up, however, was only 36 months (range, 0–84 months).
It is anticipated that over the coming months and years, long-term data will be presented which will confirm these earlier results and further secure the curative role of RFA and CFA in small-volume renal tumors.
Conclusion
The efficacy of ablative techniques for small- to moderate-volume renal tumors appears assured by current intermediate-term outcomes in the literature. Enhanced periprocedural image guidance will be key to securing curative outcomes from thermal ablative techniques. In the case of renal tumors, CRA looks set to play an important role, certainly for the treatment of tumors ≥30 mm in diameter. Careful postprocedural imaging follow-up and, if necessary, retreatment will no doubt contribute significantly to the anticipated long-term efficacy of ablative techniques.
References
Lindblad A (2002) Textbook of cancer epidemiology. Oxford University Press, New York
Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P (2007) Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 18:581–592
Cancer Research UK Website. http://www.cancerresearchuk.org
Pantuck AJ, Zisman A, Belldegrun AS (2001) The changing natural history of renal cell carcinoma. J Urol 166:1611–1623
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Klinghoffer Z, Yang B, Kapoor A, Pinthus JH (2009) Obesity and renal cell carcinoma: epidemiology, underlying mechanisms and management considerations. Expert Rev Anticancer Ther 9:975–987
Quinn MJ, Babb P, Brock A, Kirby L, Jones J (2001) Cancer Trends in England and Wales 1950–1999. Office for National Statistics (ONS), London
Hermanek H (1997) TNM Atlas. Springer Verlag, New York
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351:1296–1305
McKiernan J, Simmons R, Katz J, Russo P (2002) Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology 59:816–820
Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H (2003) Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 170:2217–2220
Bosniak MA, Birnbaum BA, Krinsky GA, Waisman J (1995) Small renal parenchymal neoplasms: further observations on growth. Radiology 197:589–597
Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, Uzzo RG (2006) The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 175:425–431
Oda T, Miyao N, Takahashi A, Yanase M, Masumori N, Itoh N, Tamakawa M, Tsukamoto T (2001) Growth rates of primary and metastatic lesions of renal cell carcinoma. Int J Urol 8:473–477
Silverman SG, Israel GM, Herts BR, Richie JP (2008) Management of the incidental renal mass. Radiology 249:16–31
Jayson M, Sanders H (1998) Increased incidence of serendipitously discovered renal cell carcinoma. Urology 51:203–205
Silverman SG, Gan YU, Mortele KJ, Tuncali K, Cibas ES (2006) Renal masses in the adult patient: the role of percutaneous biopsy. Radiology 240:6–22
Maturen KE, Nghiem HV, Caoili EM, Higgins EG, Wolf JS, Wood DP (2007) Renal mass core biopsy: accuracy and impact on clinical management. AJR Am J Roentgenol 188:563–570
Uchida M, Imaide Y, Sugimoto K, Uehara H, Watanabe H (1995) Percutaneous cryosurgery for renal tumours. Br J Urol 75:132–136 discussion 136-7
Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK (2000) Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer 88:2452–2463
Hoffmann NE, Bischof JC (2002) The cryobiology of cryosurgical injury. Urology 60:40–49
Zagoria RJ, Traver MA, Werle DM, Perini M, Hayasaka S, Clark PE (2007) Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. AJR Am J Roentgenol 189:429–436
Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR (2005) Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol 185:64–71
Breen DJ, Rutherford EE, Stedman B, Roy-Choudhury SH, Cast JE, Hayes MC, Smart CJ (2007) Management of renal tumors by image-guided radiofrequency ablation: experience in 105 tumors. Cardiovasc Intervent Radiol 30:936–942
Varkarakis IM, Allaf ME, Inagaki T, Bhayani SB, Chan DY, Su LM, Jarrett TW, Kavoussi LR, Solomon SB (2005) Percutaneous radio frequency ablation of renal masses: results at a 2-year mean followup. J Urol 174:456–460 discussion 460
Matsumoto ED, Johnson DB, Ogan K, Trimmer C, Sagalowsky A, Margulis V, Cadeddu JA (2005) Short-term efficacy of temperature-based radiofrequency ablation of small renal tumors. Urology 65:877–881
Atwell TD, Farrell MA, Leibovich BC, Callstrom MR, Chow GK, Blute ML, Charboneau JW (2008) Percutaneous renal cryoablation: experience treating 115 tumors. J Urol 179:2136–2140 discussion 2140-1
Georgiades CS, Hong K, Bizzell C, Geschwind JF, Rodriguez R (2008) Safety and efficacy of CT-guided percutaneous cryoablation for renal cell carcinoma. J Vasc Interv Radiol 19:1302–1310
Kunkle DA, Uzzo RG (2008) Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer 113:2671–2680
Hui GC, Tuncali K, Tatli S, Morrison PR, Silverman SG (2008) Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol 19:1311–1320
Finley DS, Beck S, Box G, Chu W, Deane L, Vajgrt DJ, McDougall EM, Clayman RV (2008) Percutaneous and laparoscopic cryoablation of small renal masses. J Urol 180:492–498 discussion 498
Matin SF, Ahrar K, Cadeddu JA, Gervais DA, McGovern FJ, Zagoria RJ, Zagoria RA, Uzzo RG, Haaga J, Resnick MI, Kaouk J, Gill IS (2006) Residual and recurrent disease following renal energy ablative therapy: a multi-institutional study. J Urol 176:1973–1977
Levinson AW, Su LM, Agarwal D, Sroka M, Jarrett TW, Kavoussi LR, Solomon SB (2008) Long-term oncological and overall outcomes of percutaneous radio frequency ablation in high risk surgical patients with a solitary small renal mass. J Urol 180:499–504 discussion 504
Yamakado K, Nakatsuka A, Kobayashi S, Akeboshi M, Takaki H, Kariya Z, Kinbara H, Arima K, Yanagawa M, Hori Y, Kato H, Sugimura Y, Takeda K (2006) Radiofrequency ablation combined with renal arterial embolization for the treatment of unresectable renal cell carcinoma larger than 3.5 cm: initial experience. Cardiovasc Intervent Radiol 29:389–394
Fotiadis NI, Sabharwal T, Morales JP, Hodgson DJ, O’Brien TS, Adam A (2007) Combined percutaneous radiofrequency ablation and ethanol injection of renal tumours: midterm results. Eur Urol 52:777–784
Hakimé A, Hines-Peralta A, Peddi H, Atkins MB, Sukhatme VP, Signoretti S, Regan M, Goldberg SN (2007) Combination of radiofrequency ablation with antiangiogenic therapy for tumor ablation efficacy: study in mice. Radiology 244:464–470
Rutherford EE, Cast JE, Breen DJ (2008) Immediate and long-term CT appearances following radiofrequency ablation of renal tumours. Clin Radiol 63:220–230
Kawamoto S, Permpongkosol S, Bluemke DA, Fishman EK, Solomon SB (2007) Sequential changes after radiofrequency ablation and cryoablation of renal neoplasms: role of CT and MR imaging. Radiographics 27:343–355
Rukstalis DB, Khorsandi M, Garcia FU, Hoenig DM, Cohen JK (2001) Clinical experience with open renal cryoablation. Urology 57:34–39
McDougal WS, Gervais DA, McGovern FJ, Mueller PR (2005) Long-term followup of patients with renal cell carcinoma treated with radio frequency ablation with curative intent. J Urol 174:61–63
Silverman SG, Tuncali K, vanSonnenberg E, Morrison PR, Shankar S, Ramaiya N, Richie JP (2005) Renal tumors: MR imaging-guided percutaneous cryotherapy—initial experience in 23 patients. Radiology 236:716–724
Davol PE, Fulmer BR, Rukstalis DB (2006) Long-term results of cryoablation for renal cancer and complex renal masses. Urology 68:2–6
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Breen, D.J., Railton, N.J. Minimally Invasive Treatment of Small Renal Tumors: Trends in Renal Cancer Diagnosis and Management. Cardiovasc Intervent Radiol 33, 896–908 (2010). https://doi.org/10.1007/s00270-010-9892-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-010-9892-0