Abstract
Renal cell carcinoma is a common malignancy with increasing incidence due to the incidental detection of non-symptomatic small renal masses on imaging. Management of these small tumors has evolved toward minimally invasive nephron-sparing techniques which include partial nephrectomy and image-guided ablation. Cryoablation and radiofrequency ablation are the most utilized ablation modalities with the former more suited for larger and central renal masses due to intra-procedural visualization of the ablation zone and reduced pelvicalyceal injury. In this article, we review the epidemiology and natural history of renal cell carcinoma, the role of biopsy, and the management options available—surgery, image-guided ablation, and active surveillance—with a focus on cryoablation. The clinical outcomes of the longer term maturing cryoablation data are discussed with reference to partial nephrectomy and radiofrequency ablation. Image-guided ablation has often been the management choice in patients deemed unfit for surgery; however, growing evidence from published series demonstrates image-guided ablation as a sound alternative treatment with equivalent oncological outcomes and minimal patient impact.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Renal cell carcinoma (RCC) is a common malignancy with increasing incidence. 338,000 new cases of kidney cancer were diagnosed worldwide in 2012 with 144,000 deaths [1]. The highest rates are in Northern America, Europe, Australia, and New Zealand. Kidney cancer is the fifth most common cancer in Europe excluding gender-specific cancers. 84,400 new cases were identified in the European Union (EU-27) in 2012, accounting for 3.2% of all malignancies [2]. This is an increase from 59,900 cases in 2004 (EU-25) [3]. There is roughly a 2:1 male-to-female ratio with a European incidence of 17.4 and 8.1 per 100,000, respectively, in 2012 [2].
The incidence of RCC has been increasing globally—with a 226% increase between 1975 and 2008 according to the SEER database [4]. The 5-year survival in this cohort has increased from 50.9% during 1975–1979 to 74.9% in 2007. A large proportion of this increase has been attributed to the detection of incidental renal masses on imaging, with 67% of renal masses discovered incidentally in a European cohort [5, 6]. This is also reflected in a stage migration pattern—with an increase in stage 1 RCC from 43% to 57%, over a 12-year period (1993–2004), and a decrease in all other stages [7]. Additionally, there was a reduction in mean size of stage 1 tumors from 4.1 cm in 1993 to 3.6 cm in 2003. Histological grade as assessed by Fuhrman classification is significantly lower in incidentally discovered masses than symptomatic counterparts, although this does not appear to be size matched [8, 9]. Despite this increase in early-stage small-volume disease, mortality rates have slowly risen since the 1970s. Cancer Research UK data show an increase in mortality rate from 2.9 to 4.5 per 100,000 in 1972 and 2012, respectively, but with a plateau in mortality rate more recently [10]. This rise is mirrored in the US, although the rise has been slower, with a recent downward trend in mortality rate which may reflect increased treatment of small-volume disease [4].
Two additional factors have to be considered for the increase in RCC incidence—age and obesity. The highest incidence of RCC are in those aged 75 and older [11]. The UK mortality rate in the 80 and older group has increased from 15 (1971–1973) to 48.4 (2010–2012) per 100,000 [10]. This is coupled with decreasing mortality from cardiovascular and cerebrovascular disease in the developed world. Obesity has been identified as an independent risk factor for developing RCC with the risk increasing 24% for men and 34% for women for every 5 kg/m2 rise in BMI [12]. However, tumors that develop in obese patients are less aggressive, lower stage at presentation, and have a reduced cancer-specific mortality [13, 14]. Age and obesity, are therefore, becoming increasingly important factors in an aging and overweight society where interventions have increasing risks.
The transition toward incidental detection on imaging has led to 70% of kidney cancer cases in 2008 presenting as small renal mass (SRM), which is defined as less than 4 cm in longest dimension and classified as a T1a lesion [15, 16]. Of these masses, 20–30% are benign and 10–20% are of higher oncological risk. In a study of 2770 excised solid renal tumors, the rate of RCC increased with size of the lesion. They identified that the RCC rate was 70% for less than 2 cm tumors, 78% between 2 and 2.9 cm, 80.1% between 3 and 3.9 cm, and 92.2% for those greater than 4 cm [17]. Additionally, the rate of RCCs being high grade increased with larger tumor size. Size and histological grade are therefore linked but smaller masses can still harbor the risk of high-grade tumors. A factor to consider is the high proportion of grade heterogeneity within SRMs with many high-graded tumors (Fuhrman grade 3–4) containing lower grade components (Fuhrman grade 1–2) [18]. Grade discordance increases with tumor size and higher grade tumors. This has an implication with biopsy assessment when considering active surveillance due to the risk of under sampling.
Role of biopsy
Accurate diagnosis of SRMs prior to management has been a contentious issue in the past. There are no specific radiological features that can separate indolent benign lesions from low- and high-grade RCCs [19, 20]. These benign lesions include fat-poor angiomyolipoma and oncocytoma as well as rare renal tumors such as metanephric adenoma, leiomyoma, and juxtaglomerular cell tumor. There have been recent advances in differentiating oncocytoma from RCC on multi-phase CT although this is rarely utilized in clinical practice with many equivocal SRMs [21, 22]. There has been historical concern with percutaneous renal mass biopsy in the ability to distinguish oncocytoma from RCC [23]. A key concern was regarding hybrid oncocytoma/RCC tumors as seen in a study by Schmidbauer et al. [24]. They identified 2 cases of hybrid tumors out of 13 cases (15.4%) with a preoperative diagnosis of oncocytoma on renal biopsy. However, a recent series has suggested that hybrid tumors are rare with only 3% of 147 excised oncocytomas or angiomyolipomas contained coexistent malignant tissue with no high-grade components [25]. Hybrid tumors are shown to have good oncological outcomes with little or no evidence of disease progression [25, 26]. This has led to a shift from a previous surgical management for oncocytomas to a conservative approach.
Renal mass biopsy technique has improved with technical failure around 5% and indeterminate or inaccurate pathological findings decreasing from 10% to 4% [27]. False-negative rates are often due to inaccurate placement of the needle tip in a SRM or sampling of usually central, necrotic areas [28]. Early difficulties in differentiating oncocytoma and chromophobe subtype RCC on core biopsy specimens are being resolved with modern immunohistochemical and molecular advances and interpretation, especially with assessment by experienced uropathologists [29]. These advances have increased specificity of biopsy and reduced technical failure leading to a reuptake in renal mass biopsy prior to management. The role of renal biopsy has made a significant clinical impact on the management of SRMs with unnecessary nephrectomy being avoided [30]. In the author’s institution, we largely perform a core needle biopsy in advance of definitive management.
Surgical management
The historical standard of treatment for RCC is radical nephrectomy (RN). The short-term morbidity associated with open radical nephrectomy, which include increased blood loss and transfusion, longer hospitalization, and later ambulation, have been reduced through the use of laparoscopic radical nephrectomy techniques [31]. Outcomes are similar with no difference in cancer-specific survival on long-term follow-up between the two groups [32]. The major morbidity associated with radical nephrectomy is renal dysfunction. In a randomized European trial assessing renal function after nephron-sparing surgery (NSS) vs. radical nephrectomy for sub-5 cm masses (EORTC 20904), the RN arm patients had moderate renal dysfunction of 85.7% (estimated glomerular filtration rate (eGFR) <60 mL/min/m2), severe renal dysfunction 10.0% (eGFR <30), and end-stage renal disease of 1.5% (eGFR <15) [33]. Renal dysfunction is an independent risk factor of death, cardiovascular events, and hospitalization [34]. This risk increases with worsening renal dysfunction. The associated renal dysfunction with RN has led to an evolution of nephron-sparing techniques which include NSS and ablation. This becomes even more relevant as 26% of patients with SRMs have chronic renal dysfunction at the outset [35].
The main surgical nephron-sparing technique is partial nephrectomy (PN), which has been shown to be as effective as RN with similar oncological outcomes at 10 years [36, 37]. In a randomized trial of PN and RN for sub-5 cm RCCs, PN was associated with a higher complication rate which includes severe hemorrhage, reoperation, and urinary fistulas [38]. Despite this, the trade-off is renal preservation and the international consensus is that PN should be the standard of care for SRMs with the European Association of Urology (EAU) guidance stating that PN should be offered for all T1a tumors and for technically feasible T1b tumors [39]. Interestingly, the randomized EORTC 20904 trial showed that renal function for the NSS arm was 64.7% moderate dysfunction, 6.3% severe dysfunction, and 1.6% end-stage renal disease [33]. The only perceivable beneficial impact of NSS vs. RN was with slightly reduced moderate renal dysfunction and there was no improved survival in this study population.
The advent of laparoscopic techniques has helped minimize the invasive approach of NSS for SRMs. The 10-year oncological outcomes of laparoscopic PN are similar to open PN with choice of operative technique (transperitoneal or retroperitoneal) dependent on surgeon preference and experience, as well as tumor location (anterior vs. posterior) [40, 41]. Laparoscopy remains a highly skilled technique which requires adequate hemorrhage control and judicious use of renal hilar clamping to reduce warm ischemic injury times. Preservation of renal function is multifactorial after PN and includes amount of parenchyma preserved, baseline function and warm ischemia injury times [42]. Warm ischemic injury of >25 min has been shown to be a predictor of short- and long-term renal compromise even adjusting for other factors. One technique employed is the use of cold ischemia with ice slush, which can be tolerated up to 2 h by the kidney with good nephron recovery [43]. Meticulous intracorporeal suturing and reconstruction of the renal parenchyma has been evolving to reduce ischemic time and decrease the complication rate [41]. Clampless techniques have also evolved where the renal artery is dissected up to the initial branches feeding the lesion with emerging vessels in the resection bed selectively coagulated during the procedure. This technique has seen the maximal nephron-sparing benefit in those patients with the poorest baseline renal function but with no long-term significant difference in those with normal baseline function [44, 45]. Robot-assisted partial nephrectomy aids accessibility during endosurgery and helps reduce the complexity of the surgery. Long-term oncological outcomes are still awaited and the main perceived benefits are reported as improved accessibility and reduction in warm ischemic time [46, 47].
Active surveillance
It is worth considering the role of active surveillance in the management of SRMs. The EAU guidelines reserve the use of active surveillance and limit it to those patients who are elderly and/or comorbid patients with limited life expectancy given the low evidence base [39]. A 2006 meta-analysis study of SRMs undergoing active surveillance revealed a mean growth rate of 0.28 cm/year and that initial tumor size and grade had no impact on growth rate [48]. There appears to be no difference in average growth between biopsy-proven RCC or benign tumors. Additionally, biopsy-proven RCC may not grow and up to 10% of these lesions may decrease in size which suggests that some RCCs may regress [49]. The risk to metastatic disease in the 2006 meta-analysis was 1% but this has been found to be as high as 6% for 3.1–4.0 cm lesions with risk related to larger tumor size and higher grade [48, 50].
Patients undergoing active surveillance will have serial imaging with ultrasound, CT, or MRI at 6–12 month intervals. The most readily definable marker of change on imaging is tumor growth but this is subject to inter-observer measurement error. Measurement error is particularly important as small linear measurements can impact significantly on volumetric growth. Additionally, we have seen that growth is a poor differentiator for benign vs. malignant disease, which is especially important if there is no biopsy-proven disease [48]. A renal biopsy is recommended prior to enrolment into surveillance and EAU recommends delayed intervention in those that show clinical progression [39]. The guidance of enrolment into active surveillance is not clear and is at clinical discretion and patient choice. Active surveillance appears to be preferentially utilized in elderly and comorbid patients but other factors such as racial and socioeconomic disparities have led to a health care inequality, although outcomes remain similar [51, 52]. In a pooled analysis of SRMs undergoing active surveillance, up to 45.4% of patients underwent delayed intervention with patient preference (57.2%) being the largest factor compared to improved medical condition (7.1%), and tumor growth (35.7%) [53]. At this point, it is worth mentioning the role of image-guided ablation (IGA) especially if extirpative surgery is not suitable or due to patient choice of intervention over active surveillance. The EAU guidance advises either ablation or active surveillance in patients with T1a lesions who are elderly and/or comorbid [39]. IGA can be offered as the primary intervention in such patients who often have slow-growing or relatively indolent disease.
Nephrometry scoring
There are no specific radiological characteristics that help determine tumor grade or benignity of SRMs. However, attempts have been made to provide scoring systems to risk stratify treatment options [54]. The R.E.N.A.L. nephrometry scoring system is one such tool and provides a standardized way of quantitating renal masses [55]. Five variables are assessed which include: radius, exophytic/endophytic properties, proximity to collecting system, anterior/posterior location, and location relative to polarity. The scoring system helps objectify treatment decision-making for SRMs with lower scores tending toward minimally invasive techniques [56]. The usefulness of the scoring system also appears to apply to IGA techniques and is able to predict treatment efficacy and complications [57]. Scoring has been further adapted to form a modified R.E.N.A.L nephrometry score and the ABLATE renal ablation planning algorithm for IGA to help anticipate and mitigate potential complications [58, 59].
Role of IGA in the treatment of RCC
Image-guided ablation (IGA) is a useful tool in the management paradox of SRMs—i.e., the risk of leaving potentially aggressive disease vs. over treating potentially indolent disease with surgery and its consequent morbidity vs. watchful waiting. It is clear that patient preference is the major factor in those undergoing delayed intervention [53]. IGA provides a nephron-sparing treatment modality that reduces the morbidity associated with surgical resection but shows increasing evidence of good oncological outcomes [60]. It must be noted from the outset that there are no randomized trials assessing the efficacy of IGA against partial nephrectomy for T1a lesions. This forms a selection bias in most early IGA case series demonstrating data for those that are elderly and/or comorbid, and therefore, unsuitable for surgery.
There are several ablation techniques that are available for the treatment of renal tumors. The main focus of this review is in the role of cryoablation (CRA) in the treatment of RCC with occasional reference to radiofrequency ablation (RFA), as both these techniques are well established in the role of renal tumor ablation [28]. Before discussing CRA, we will briefly address the other ablation techniques and their utility in the treatment of renal masses.
Radiofrequency ablation (RFA) generates high-frequency alternating current (approximately 500 kHz) via an electrode placed in the targeted tumor [61]. This agitates ions within millimeters of the probe tip creating an intense frictional heat that conducts outwards causing coagulative necrosis in the adjacent tissues. This process is regulated to ensure optimal temperature control to ensure tumor cell death but not to char tissue which inhibits conduction [62]. RFA is the most widely used IGA treatment modality for RCC.
Microwave ablation (MWA) is also a heat-based thermal ablation technique that agitates water molecules in an oscillating electromagnetic field adjacent to the probe tip. Inefficiency of polar water molecule oscillation leads to heating resulting in coagulative necrosis and cell death. MWA has a theoretical advantage of a more predictable thermal profile over RFA and intermediate term results of MWA in the treatment of SRMs have demonstrated comparable cancer-specific survival and complication rate to laparoscopic radical nephrectomy [63].
Irreversible electroporation (IRE) is a non-thermal ablation modality in which there is irreversible cell membrane permeabilization from the application of rapid electrical pulses leading to cell necrosis. IRE is still in its infancy and current use is limited to research with the majority of in vivo treatments involving liver and pancreatic lesions [64]. High intensity focused ultrasound (HIFU) uses the principles of converting mechanical energy from focused ultrasound into heat leading to cavitation and coagulative necrosis. Results from small trials have been disappointing with technique limited by variable thermal profile in the target tissue as well as the targeting limitations of extracorporeal techniques which are sub-optimal compared to laparoscopic approach [65].
Cryoablation
CRA is a cold-based thermal ablation technique which has been recognized as a treatment modality for decades, but has experienced a recent clinical resurgence due to the advent of newer ‘third generation’ 17-gage cryoprobes suitable for percutaneous treatment [66]. The target tissue undergoes a phase change from liquid to solid during the freezing cycle, which is achieved through the closed-system circulation of a cryogen into the probe leading to the formation of a therapeutic ‘iceball’ around the tip. Centrally within this ‘iceball,’ there is rapid intra- and extracellular ice formation, while peripherally there is extracellular ice formation. Intracellular ice formation leads to damage of the cell membrane and intracellular proteins, while extracellular ice formation produces an osmotic gradient that leads to a fatal fluid shift out of cells. Additionally, microvascular injury secondary to freezing is thought to be synergistic with these effects [28, 67, 68]. The rate of cooling, the nadir tissue temperature, the duration of freezing, and thawing rate are all elements that lead to cellular injury and the process of tissue destruction is ensured by a double freeze-thaw cycle [69]. The cell lethal isotherm lies at −30 to 40°C and this is fully lethal to the target tissue during a double freeze-thaw cycle [70].
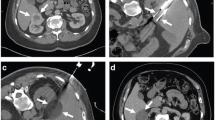
One of the key benefits of CRA is the intra-operative visualization of the ‘iceball’ (see Fig. 1B), which acts as good imaging surrogate for the ultimate ablation zone as seen in Fig. 1C. The visualization of frozen tissue in solid organs is seen as approximately 50 Hounsfield units less than that of unfrozen tissue on CT scan. The thin peripheral ice front (freeze margin) is not tissue-lethal with temperatures of around 0°C. However, this can act as proxy marker for the cell lethal zone and the visualized iceball should, in practice extend beyond the margin of the tumor by at least 5–6 mm [69, 71]. Visualization of the iceball is also possible with ultrasound and MRI. On ultrasound, the ice front is seen as a hyperechoic line and often casts acoustic shadowing which obscures visualization of the deep margin. On MRI, signal voids are seen on all sequences giving good delineation of the iceball. However, CT remains the most practical and utilized imaging modality both intra-procedural and during follow-up (Fig. 1).
A 30-mm exophytic left anterior interpolar RCC. B Intra-procedural CT demonstrating an iceball around a cryoprobe. Note the bowel has been displaced by hydrodissection. C 2 weeks post-CRA demonstrating typical post-ablation appearance with complete devascularisation of the tumor. A crescent of high attenuation is seen at the anterior aspect which demonstrates a typical marginal rind seen with cryoablation. D 1 year post-CRA showing an 18-mm involuted devascularised ablation zone. E 3 years post-CRA demonstrating involuted scar tissue with no enhancing nodule. F 5 years post-CRA the ablation zone remains involuted with a central fleck of calcification
A disadvantage of thinner cryoprobes is a smaller ablation zone. To incorporate large tumors, multiple probes have to be sited with careful planning of in situ probe position. Different probe designs enable spherical, ellipsoid, or cylindrical ablation zones. Pre-procedural planning with a selection of various probes enables a larger ablation zone to form, with the probes working synergistically. There are a number of software packages to help plan more robust and predictable ablation zones [66]. Each cryoprobe can be regulated independently to precisely shape the ablation zone, which may prove useful for complex lesions or near critical structures and this makes CRA particularly suited for renal masses [72]. As a general guide, cryoprobes are placed in ‘clock-face’ configuration (as demonstrated in Fig. 2A) no greater than 1 cm from the tumor edge and the distance between adjacent cryoprobes should be less than 2 cm to prevent intra-tumoral clefts of sub-optimal thermal zones [73].
A Coronal reformat of a 57-mm left renal mass demonstrating the ‘clock-face’ arrangement of 7 cryoprobes. B Gas insufflation tracking around a stented ureter (solid arrow) creating some separation from the central right renal mass. An attempt at contrast-tinted hydrodissection is also seen. C Close proximity (approximately 1 cm) of a large exophytic left lower pole renal mass to the descending colon anterolaterally (horizontal arrow) and a loop of small intestine anteriorly. D Displacement of descending colon (vertical arrow) by contrast-tinted 5% dextrose solution instilled into the left anterior pararenal space increasing the space by several centimeters
CRA has shown to be less injurious to the pelvicalyceal system through collagen-sparing effects, as shown in porcine models and clinical experience [74, 75]. Blood vessels are also a critical structure that should be considered—with larger vessels providing a local ‘cold sink’ effect analogous to the ‘heat-sink’ effect seen with hyperthermic ablative modalities. CRA is less prone than RFA to perfusion-mediated thermodilution effects, however, to mitigate these effects, spacing between cryoprobes should be reduced near large vessels [73].
Imaging guidance
The two main methods of CRA delivery are imaging-based percutaneous technique and laparoscopy. A 2008 comparative meta-analysis by Hui et al. evaluated percutaneous and surgical approaches to renal tumor ablation by looking at tumor effectiveness and complication rates [76]. They identified that the primary efficacy rate was 87% for the percutaneous group vs. 94% for the surgical group with a similar secondary ablation efficacy rate of 92%. The major complication rate was significantly lower in the percutaneous group at 3% against 7% for surgical. It should be noted that techniques have evolved since early IGA techniques and with experience an improvement in outcomes and reduced complications are seen. Results may be partially influenced by preferential selection of surgical candidates. Two recent single-center studies have shown similar outcomes and complication rates between percutaneous and laparoscopic CRA with outcomes influenced by patient and tumor characteristics [77, 78]. Percutaneous CRA also benefits from reduced operative and anesthetic time, reduced analgesic requirements, reduced transfusion requirement, and shorter length of stay [79]. These benefits with equal oncological outcomes make percutaneous ablation a suitable treatment modality for primary treatment and secondary salvage treatment for residual or recurrent disease.
Procedural considerations
There are several benefits to performing percutaneous ablation under general anesthesia. The patient can be optimally positioned in a prone-oblique position which allows better access and visualization of the kidney through widening of the intercostal spaces. Additionally, respiratory suspension is reproducible enabling better probe placement. Ablation is painful and analgesic requirements are reduced with general anesthetic. It must be noted that CRA benefits from lower analgesic requirements than RFA due to the cooling analgesic effect on nerves [80].
Adjunctive maneuvers
Critical structures such as bowel (usually colon) and the renal pelvis are susceptible to injury with ablation. Prior to the advent of protective techniques, a major contraindication to IGA was proximity of the tumor to these structures. Simple maneuvers can assist in the displacing of bowel from the tumor such as patient positioning. In our local practice, the use of disposable enema the morning of the procedure helps reduce gas distension of the colon [28].
Hydrodissection is a technique which is commonly used to displace bowel away from the renal mass, using contrast-tinted fluid. This is usually achieved by percutaneous instillation via an 18–21G co-axial needle into the anterior pararenal space by imaging guidance. 5% dextrose in water is preferred for RFA as it is less ionic but this is not an issue with CRA and 0.9% saline can be used. The fluid is approximately 2% contrast-tinted to enable adequate visualization on CT, which is demonstrated in Figs. 2B–D. Aliquots of 50–100 mL are used but larger volumes may be required depending on the degree of displacement required and if there is any fluid spillage into the paracolic gutters or subhepatic space [81].
Gas insufflation is achieved by introducing sterilized carbon dioxide or air to displace bowel or other critical structures (Fig. 2B). Disadvantages of this technique are obscuration of view on ultrasound or MRI and larger quantities of gas required due to gaseous diffusion. Balloon interposition utilizes angioplasty or oesophageal dilation balloons to displace bowel away but can be poorly predictable. It is a second-line technique after other techniques fail. A major disadvantage is slippage of balloon and several balloons may be required. Torquing uses the ablation probes as a lever to displace the kidney. This can be used in addition to other techniques but caution is required to ensure that the probes are not deformed and there is no renal hilar or soft tissue injury—although no cases have been reported [82].
Pyeloperfusion with ureteral stenting is a method to prevent urothelial injury. This works on the principle of thermodilution within the renal pelvis by applying cold fluid (5% Dextrose in water) for RFA or MWA and warmed fluid for CRA [83]. CRA has the additional collagen-sparing benefit and is less injurious to the renal pelvis [74, 75].
Clinical outcomes
An increasing number of published case series demonstrate percutaneous CRA as an effective modality for the treatment of SRMs with longer follow-ups now filtering through on these larger case series. The outcomes and complication rates from these series can be seen in Table 1 which is limited to series with greater than 100 CRA [77, 84–91]. Smaller case series were excluded due to shorter follow-up and due to a relative inexperience in performing CRA. Breen et al. demonstrated the relevance of experience in 153 consecutive treatments that subtotal treatment significantly decreased in consecutive tertiles from 8/51 to 3/51 and subsequently 1/51 treatments [88].
Primary local control is defined as an imaging absence of residual unablated disease, disease recurrence, or metastatic disease. This ranges from 85.4% to 98.5% (Table 1), although this figure improves to 92.7–99.3% with repeat ablation. Thompson et al. retrospectively compared the outcomes following PN, percutaneous RFA, and CRA. They identified a local recurrence-free survival rate at 3 years for T1a tumors of 98% in each group with no significant difference in the biopsy-proven RCC subset. Metastases-free survival at 3 years were similar in PN (99%) and CRA (100%) and significantly different to the RFA subset (93%). It must be noted that PN patients were younger (60.1 year (PN), 70.7 year (RFA), and 71.6 year (CRA)) and were significantly less comorbid compared to the ablation group. When comparing PN and CRA for T1b tumors, there was again no statistical difference for local recurrence-free survival or metastases-free survival, although numbers are small (n = 48) for the CRA group [90]. With increasing longer term data and experience with CRA, oncological outcomes are equipoise with standard PN for small renal masses.
The longer term data for PN and RFA for treatment of T1a RCC is well established with a 97.2% 5-year overall survival for both groups in an observational single-institution study with no statistical difference in cancer-specific survival and overall disease-free survival [92]. Chang et al. identified no statistical difference in 5-year clinical and oncological outcomes in both groups that were propensity-score matched [93]. The 5-year outcomes for CRA will be expectantly published in the coming years with maturation of the larger center databases but intermediate term data has been promising.
The reported time to local recurrence varies in the literature and this may be due to poor discrimination of primary subtotal treatment from local recurrence and that diligent treatment and assessment plays a role here. Recurrence occurs late in the post-ablation course as demonstrated by the 3 cases of local recurrence in the Thompson et al. study with recurrence times of 2.3–4.7 years [90]. Recurrence is often slow-growing, presenting as an enhancing nodule at the margin of the involuting ablation zone and is often not appreciated on imaging during the first 6 months. As such, we have adapted our local imaging follow-up with contrast-enhanced CT or MRI at 1 month post-treatment to assess for subtotal treatment and if satisfactory, follow-up imaging at 1, 3, and 5 years to exclude local recurrence as demonstrated in Fig. 1. If any concerns arise during the initial post-ablation study then an interim study can be performed at 3–6 months.
Complications
Complications in IGA techniques are mainly published using the Clavien–Dindo Complication Classification, which is a reliable classification system used for surgical procedures [94]. We have described significant complications as grade II and above. The majority of minor complications (grade I) include perinephric hematomas and pneumothoraces that require only conservative management. An underreported complication of ablation is injury to nerves within the abdominal wall which include intercostal, ilioinguinal, and genitofemoral nerves which can lead to chronic pain.
The significant complication rate is 0.7–7.4% (Table 1) and the vast majority of these are grade II. Table 2 demonstrates the described significant complications among the published series stratified according to Clavien–Dindo classification [77, 84–89, 91]. The pooled significant complication rate (>grade II) is 4.1% (53/1293 procedures) with the majority representing grade II complications. The most common reported significant complications include perinephric hematoma requiring blood transfusion, pelvicalyceal injury requiring stenting or nephrostomy, pneumothorax or pleural effusion requiring drainage, and intestinal injury that is managed conservatively. Life-threatening complications are uncommon (0.6%) and only 2 deaths have been reported among the studies—Mendelson syndrome and massive myocardial infarction [85]. In both cases, the patients had pre-existing comorbidities, which precluded surgery and neither case had local renal complications. Cryoshock is a cytokine-mediated response to CRA resulting in coagulopathy, shock, acute respiratory distress syndrome, and multiorgan failure [95]. Cryoshock is uncommon in the treatment of renal masses with only 1 case reported and is more commonly seen in the treatment of hepatic lesions.
Tumor characteristics are related to the risk of complications. Maximal tumor diameter and central tumor location are associated with an increase in major renal CRA complication [96]. In the same study, prior myocardial infarction and complicated diabetes mellitus was also related to an increase in complication rates. It has also been identified that upper pole lesions are more likely to have increased incidence of pneumothorax, whereas anterior or posterior location does not appear to have any significant difference in complication rate [88]. Only 5% of procedures in which there has been pleural transgression results in chest drain placement as identified by Georgiades et al. [89].
Direct comparison of complication rates between percutaneous RFA and CRA are difficult due to differing complexity of tumors treated. Schmit et al. identified a complication rate of 7.9% vs 2.9% (CRA vs RFA) but they attributed this to more complex and larger tumors treated by CRA with high RENAL nephrometry score [57]. When ablated masses are limited to less than 3.0 cm, there is no significant difference between RFA and CRA in complication rate and treatment success, although a higher likelihood of treatment failure is seen in RFA-treated central tumors due to ‘heat-sink’ effect [97]. Percutaneous RFA is therefore particularly suited to small peripheral exophytic lesions that can be treated by ‘single-stick’ ablation, whereas CRA should be utilized for larger complex tumors, especially if centrally located. IGA complication rates for SRMs are similar to those seen with PN. A 2012 UK audit, identified a complication rate of 4.9% (Clavien–Dindo >grade III) for those undergoing PN for T1a tumors with an overall complication rate of 17.4% [98]. A comparable pooled complication rate for CRA from the published series in Table 2 is 2.2% (Clavien–Dindo >grade III) with some series including small T1b masses within their data.
Patient groups particularly suited for cryoablation
The benefits of CRA have already been shown in several specific patient groups—elderly, obese, solitary kidney, heritable RCC syndromes, and local recurrence post-surgery. Outcomes of percutaneous renal CRA in elderly population (>80 years) has a technical success of 98.4% in 61 (33 biopsy-proven RCC) patients with no recurrence recorded and an 8.6% major complication rate [99]. Obese patients are often poor surgical candidates due to extensive comorbidities and technical surgical challenges. Percutaneous renal CRA may be an alternative option with complication rates and short-term outcomes in obese and morbidly obese patient similar to those in non-obese patients [100]. The major technical challenge for percutaneous ablation is increased skin-to-tumor depth which has shown to have a higher likelihood of treatment failure [86]. Patients with a solitary kidney require meticulous care to ensure minimal loss of renal function. Treatment of 38 tumors in 31 patients with a solitary kidney demonstrated a 92% local tumor control and caused minimal loss in renal function at follow-up with no patient requiring dialysis [101]. The nephron-sparing benefit is also suited for patients with pre-existing renal disease or patients with inheritable renal cancer syndromes such as von Hippel–Lindau disease. Additionally, percutaneous CRA is suited as a salvage therapy in patients with previous ipsilateral PN with acceptable oncologic outcomes, preservation of renal function, and relative preservation of renal function [102].
Conclusions
The increased use of cross-sectional imaging has identified an increasing number of SRMs leading to stage migration shift of kidney cancer toward smaller tumors. Kidney cancer remains an important cause of mortality and had previously provided a management conundrum of whether benign or low-grade tumors were being over treated. Pre-procedural biopsies have become more confident in characterizing SRMs, and many of those still carry a significant malignant potential. Urological guidelines still hold PN or RN as the gold standard, but IGA is now demonstrating equivalent oncological outcomes with minimal patient impact. This is emphasized by case series in what has been a comparatively older and more comorbid population. This has facilitated the option of IGA as a sound alternative treatment in patients especially those unsuitable for surgery. At present, there are no prospective randomized studies looking at percutaneous RFA or CRA directly against PN for SRMs. CRA, although technically more challenging, has been shown to be more suited toward larger SRMs and centrally located tumors than RFA.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49(6):1374–1403
Boyle P, Ferlay J (2005) Cancer incidence and mortality in Europe, 2004. Ann Oncol 16(3):481–488
Howlader N, Noone AM, Krapcho M, et al. (2015) SEER Cancer Statistics Review, 1975–2012, National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015
Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr (1999) Rising incidence of renal cell cancer in the United States. JAMA 281(17):1628–1631
Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR (2008) Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer 113(1):78–83
Laguna MP, Algaba F, Cadeddu J, et al. (2014) Current patterns of presentation and treatment of renal masses: a clinical research office of the endourological society prospective study. J Endourol 28(7):861–870
Gudbjartsson T, Thoroddsen A, Petursdottir V, et al. (2005) Effect of incidental detection for survival of patients with renal cell carcinoma: results of population-based study of 701 patients. Urology 66(6):1186–1191
Rabjerg M, Mikkelsen MN, Walter S, Marcussen N (2014) Incidental renal neoplasms: is there a need for routine screening? A Danish single-center epidemiological study. APMIS. 122(8):708–714
Cancer Research UK, http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer/mortality
Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F (2015) International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 67(3):519–530
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371(9612):569–578
Hakimi AA, Furberg H, Zabor EC, et al. (2013) An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst 105(24):1862–1870
Parker AS, Lohse CM, Cheville JC, et al. (2006) Greater body mass index is associated with better pathologic features and improved outcome among patients treated surgically for clear cell renal cell carcinoma. Urology 68(4):741–746
Gill IS (2009) Focal therapy for kidney and prostate cancer. Curr Opin Urol 19(2):125–126
Volpe A, Panzarella T, Rendon RA, et al. (2004) The natural history of incidentally detected small renal masses. Cancer. 100:738–745
Frank I, Blute ML, Cheville JC, et al. (2003) Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 170(6 Pt 1):2217–2220
Ball MW, Bezerra SM, Gorin MA, et al. (2015) Grade heterogeneity in small renal masses: potential implications for renal mass biopsy. J Urol 193(1):36–40
Woo S, Cho JY (2015) Imaging findings of common benign renal tumors in the era of small renal masses: differential diagnosis from small renal cell carcinoma: current status and future perspectives. Korean J Radiol 16(1):99–113
Gill IS, Aron M, Gervais DA, Jewett MA (2010) Clinical practice. Small renal mass. N Engl J Med 362(7):624–634
Bird VG, Kanagarajah P, Morillo G, et al. (2011) Differentiation of oncocytoma and renal cell carcinoma in small renal masses (< 4 cm): the role of 4-phase computerized tomography. World J Urol 29(6):787–792
Gakis G, Kramer U, Schilling D, et al. (2011) Small renal oncocytomas: differentiation with multiphase CT. Eur J Radiol 80(2):274–278
Blute ML Jr, Drewry A, Abel EJ (2015) Percutaneous biopsy for risk stratification of renal masses. Ther Adv Urol. 7(5):265–274
Schmidbauer J, Remzi M, Memarsadeghi M, et al. (2008) Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. Eur Urol 53(5):1003–1011
Ginzburg S, Uzzo R, Al-Saleem T, et al. (2014) Coexisting hybrid malignancy in a solitary sporadic solid benign renal mass: implications for treating patients following renal biopsy. J Urol 191(2):296–300
Waldert M, Klatte T, Haitel A, et al. (2010) Hybrid renal cell carcinomas containing histopathologic features of chromophobe renal cell carcinomas and oncocytomas have excellent oncologic outcomes. Eur Urol 57(4):661–665
Lane BR, Samplaski MK, Herts BR, et al. (2008) Renal mass biopsy—a renaissance? J Urol 179(1):20–27
Breen DJ, Railton NJ (2010) Minimally invasive treatment of small renal tumors: trends in renal cancer diagnosis and management. Cardiovasc Intervent Radiol 33(5):896–908
Lim A, O’Neil B, Heilbrun ME, Dechet C, Lowrance WT (2012) The contemporary role of renal mass biopsy in the management of small renal tumors. Front Oncol. 10(2):106
Maturen KE, Nghiem HV, Caoili EM, et al. (2007) Renal mass core biopsy: accuracy and impact on clinical management. AJR Am J Roentgenol 188(2):563–570
Acar C, Bilen C, Bayazit Y, et al. (2014) Quality of life survey following laparoscopic and open radical nephrectomy. Urol J. 11(6):1944–1950
Luo JH, Zhou FJ, Xie D, et al. (2010) Analysis of long-term survival in patients with localized renal cell carcinoma: laparoscopic versus open radical nephrectomy. World J Urol 28(3):289–293
Scosyrev E, Messing EM, Sylvester R, Campbell S, Van Poppel H (2014) Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol 65(2):372–377
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351(13):1296–1305
Huang WC, Levey AS, Serio AM, et al. (2006) Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 7(9):735–740
Van Poppel H, Da Pozzo L, Albrecht W, et al. (2011) A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 59(4):543–552
Fergany AF, Hafez KS, Novick AC (2000) Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol 163(2):442–445
Van Poppel H, et al. (2007) A prospective randomized EORTC intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 51(6):1606–1615
Ljungberg B, Bensalah K, Canfield S, et al. (2015) EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67(5):913–924
Lane BR, Campbell SC, Gill IS (2013) 10-year oncologic outcomes after laparoscopic and open partial nephrectomy. J Urol 190(1):44–49
Porpiglia F, Volpe A, Billia M, Scarpa RM (2008) Laparoscopic versus open partial nephrectomy: analysis of the current literature. Eur Urol 53(4):732–742
Thompson RH, Lane BR, Lohse CM, et al. (2012) Renal function after partial nephrectomy: effect of warm ischemia relative to quantity and quality of preserved kidney. Urology 79(2):356–360
Becker F, Van Poppel H, Hakenberg OW, et al. (2009) Assessing the impact of ischaemia time during partial nephrectomy. Eur Urol 56(4):625–634
Porpiglia F, Bertolo R, Amparore D, et al. (2015) Evaluation of functional outcomes after laparoscopic partial nephrectomy using renal scintigraphy: clamped vs clampless technique. BJU Int 115(4):606–612
Shah PH, George AK, Moreira DM, et al. (2016) To clamp or not to clamp? Long-term functional outcomes for elective off-clamp laparoscopic partial nephrectomy. BJU Int 117(2):293–299
Froghi S, Ahmed K, Khan MS, Dasgupta P, Challacombe B (2013) Evaluation of robotic and laparoscopic partial nephrectomy for small renal tumours (T1a). BJU Int 112(4):E322–E333
Shiroki R, Fukami N, Fukaya K, et al. (2016) Robot-assisted partial nephrectomy: superiority over laparoscopic partial nephrectomy. Int J Urol 23(2):122–131
Chawla SN, Crispen PL, Hanlon AL, et al. (2006) The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 175(2):425–431
Jewett MA, et al. (2011) Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol 60(1):39–44
Lee H, Lee JK, Kim K, et al. (2015) Risk of metastasis for T1a renal cell carcinoma. World J Uro doi:10.1007/s00345-015-1659-4
Maurice MJ, Zhu H, Kiechle JE, Kim SP, Abouassaly R (2015) Nonclinical factors predict selection of initial observation for renal cell carcinoma. Urology 86(5):892–900
Patel HD, Kates M, Pierorazio PM, Allaf ME (2014) Race and sex disparities in the treatment of older patients with T1a renal cell carcinoma: a comorbidity-controlled competing-risks model. Urol Oncol. 32(5):576–583
Smaldone MC, Kutikov A, Egleston BL, et al. (2012) Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer 118(4):997–1006
Hayes MC, Breen DJ (2015) Excision versus ablation in renal cancer: optimising outcome and minimising risk. Eur Urol.
Kutikov A, Uzzo RG (2009) The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 182(3):844–853
Canter D, Kutikov A, Manley B, et al. (2011) Utility of the R.E.N.A.L. nephrometry scoring system in objectifying treatment decision-making of the enhancing renal mass. Urology. 78(5):1089–1094
Schmit GD, Thompson RH, Kurup AN, et al. (2013) Usefulness of R.E.N.A.L. nephrometry scoring system for predicting outcomes and complications of percutaneous ablation of 751 renal tumors. J Urol 189(1):30–35
Gahan JC, Richter MD, Seideman CA, et al. (2015) The performance of a modified RENAL nephrometry score in predicting renal mass radiofrequency ablation success. Urology. 85(1):125–129
Schmit GD, Kurup AN, Weisbrod AJ, et al. (2014) ABLATE: a renal ablation planning algorithm. AJR Am J Roentgenol 202(4):894–903
Yin X, Cui L, Li F, et al. (2015) Radiofrequency ablation versus partial nephrectomy in treating small renal tumors: a systematic review and meta-analysis. Medicine (Baltimore). 94(50):e2255
Zlotta AR, Wildschutz T, Raviv G, et al. (1997) Radiofrequency interstitial tumor ablation (RITA) is a possible new modality for treatment of renal cancer: ex vivo and in vivo experience. J Endourol 11(4):251–258
Khiatani V, Dixon RG (2014) Renal ablation update. Semin Interv Radiol 31(2):157–166
Yu J, Zhang G, Liang P, et al. (2015) Midterm results of percutaneous microwave ablation under ultrasound guidance versus retroperitoneal laparoscopic radical nephrectomy for small renal cell carcinoma. Abdom Imaging 40(8):3248–3256
Philips P, Hays D, Martin RC (2013) Irreversible electroporation ablation (IRE) of unresectable soft tissue tumors: learning curve evaluation in the first 150 patients treated. PLoS ONE 8(11):e76260
Klingler HC, Susani M, Seip R, et al. (2008) A novel approach to energy ablative therapy of small renal tumours: laparoscopic high-intensity focused ultrasound. Eur Urol 53(4):810–816
Breen DJ, Lencioni R (2015) Image-guided ablation of primary liver and renal tumours. Nat Rev Clin Oncol. 12(3):175–186
Hoffmann NE, Bischof JC (2002) The cryobiology of cryosurgical injury. Urology. 60(2 Suppl 1):40–49 (Review)
Webb H, Lubner MG, Hinshaw JL (2011) Thermal ablation. Semin Roentgenol 46(2):133–141
Baust JG, Gage AA, Robilottto AT, Baust JM (2009) The pathophysiology of thermoablation: optimizing cryoablation. Curr Opin Urol 19(2):127–132
Gage AA, Baust JG (1998) Mechanisms of tissue injury in cryosurgery. Cryobiology 37:171–186
Lubner MG, Hinshaw JL, Brace CL, Lee FT Jr (2013) Cryoablation. In: Dupuy DE, Fong Y, Mcmullen WN (eds) Image-guided cancer therapy: a multidisciplinary approach. New York: Springer, pp 61–78
Ahmed M, Brace CL, Lee FT Jr, Goldberg SN (2011) Principles of and advances in percutaneous ablation. Radiology 258(2):351–369
Littrup PJ, Ahmed A, Aoun HD, et al. (2007) CT-guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol 18(3):383–392
Nakada SY, Lee FT Jr, Warner T, Chosy SG, Moon TD (1998) Laparoscopic cryosurgery of the kidney in the porcine model: an acute histological study. Urology. 51(5A Suppl):161–166
Janzen NK, Perry KT, Han KR, et al. (2005) The effects of intentional cryoablation and radio frequency ablation of renal tissue involving the collecting system in a porcine model. J Urol 173(4):1368–1374
Hui GC, Tuncali K, Tatli S, Morrison PR, Silverman SG (2008) Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol 19(9):1311–1320
Zargar H, Samarasekera D, Khalifeh A, et al. (2015) Laparoscopic vs percutaneous cryoablation for the small renal mass: 15-year experience at a single center. Urology. 85(4):850–855
Kim EH, Tanagho YS, Saad NE, Bhayani SB, Figenshau RS (2014) Comparison of laparoscopic and percutaneous cryoablation for treatment of renal masses. Urology. 83(5):1081–1087
Finley DS, Beck S, Box G, et al. (2008) Percutaneous and laparoscopic cryoablation of small renal masses. J Urol 180(2):492–498
Allaf ME, Varkarakis IM, Bhayani SB, et al. (2005) Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation–initial observations. Radiology 237(1):366–370
Farrell MA, Charboneau JW, Callstrom MR, et al. (2003) Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. AJR Am J Roentgenol 181(5):1315–1317
Ginat DT, Saad WE (2010) Bowel displacement and protection techniques during percutaneous renal tumor thermal ablation. Tech Vasc Interv Radiol. 13(2):66–74
Cantwell CP, Wah TM, Gervais DA, et al. (2008) Protecting the ureter during radiofrequency ablation of renal cell cancer: a pilot study of retrograde pyeloperfusion with cooled dextrose 5% in water. J Vasc Interv Radiol 19(7):1034–1040
Rodriguez R, Cizman Z, Hong K, Koliatsos A, Georgiades C (2011) Prospective analysis of the safety and efficacy of percutaneous cryoablation for pT1NxMx biopsy-proven renal cell carcinoma. Cardiovasc Intervent Radiol 34(3):573–578
Buy X, Lang H, Garnon J, et al. (2013) Percutaneous renal cryoablation: prospective experience treating 120 consecutive tumors. AJR Am J Roentgenol 201(6):1353–1361
Blute MLJr, Okhunov Z, Moreira DM, et al. (2013) Image-guided percutaneous renal cryoablation: preoperative risk factors for recurrence and complications. BJU Int. 111(4 Pt B):E181–E185
Kim EH, Tanagho YS, Bhayani SB, et al. (2013) Percutaneous cryoablation of renal masses: Washington University experience of treating 129 tumours. BJU Int 111(6):872–879
Breen DJ, Bryant TJ, Abbas A, et al. (2013) Percutaneous cryoablation of renal tumours: outcomes from 171 tumours in 147 patients. BJU Int 112(6):758–765
Georgiades CS, Rodriguez R (2014) Efficacy and safety of percutaneous cryoablation for stage 1A/B renal cell carcinoma: results of a prospective, single-arm, 5-year study. Cardiovasc Interv Radiol 37(6):1494–1499
Thompson RH, Atwell T, Schmit G, et al. (2015) Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol 67(2):252–259
Okhunov Z, Juncal S, Ordon M, et al. (2015) Comparison of outcomes in patients undergoing percutaneous renal cryoablation with sedation vs general anesthesia. Urology. 85(1):130–134
Olweny EO, Park SK, Tan YK, Trimmer C, Cadeddu JA (2012) Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol 61(4):1156–1161
Chang X, Liu T, Zhang F, et al. (2015) Radiofrequency ablation versus partial nephrectomy for clinical T1a renal-cell carcinoma: long-term clinical and oncological outcomes based on a propensity score analysis. J Endourol. 29:518–525
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Seifert JK, France MP, Zhao J, et al. (2002) Large volume hepatic freezing: association with significant release of the cytokines interleukin-6 and tumor necrosis factor a in a rat model. World J Surg 26(11):1333–1341
Schmit GD, Schenck LA, Thompson RH, et al. (2014) Predicting renal cryoablation complications: new risk score based on tumor size and location and patient history. Radiology 272(3):903–910
Atwell TD, Schmit GD, Boorjian SA, et al. (2013) Percutaneous ablation of renal masses measuring 3.0 cm and smaller: comparative local control and complications after radiofrequency ablation and cryoablation. AJR Am J Roentgenol 200(2):461–466
Fernando A, Fowler S, O’Brien T, British Association of Urological Surgeons (BAUS) (2015) Nephron-sparing surgery across a nation—outcomes from the British Association of Urological Surgeons 2012 national partial nephrectomy audit. BJU Int doi:10.1111/bju.13353
Miller AJ, Kurup AN, Schmit GD, et al. (2015) Percutaneous clinical T1a renal mass ablation in the octogenarian and nonagenarian: oncologic outcomes and morbidity. J Endourol 29(6):671–676
Schmit GD, Thompson RH, Boorjian SA, et al. (2013) Percutaneous renal cryoablation in obese and morbidly obese patients. Urology. 82(3):636–641
Weisbrod AJ, Atwell TD, Frank I, et al. (2010) Percutaneous cryoablation of masses in a solitary kidney. AJR Am J Roentgenol 194(6):1620–1625
Hegg RM, Schmit GD, Boorjian SA, et al. (2013) Percutaneous renal cryoablation after partial nephrectomy: technical feasibility, complications and outcomes. J Urol 189(4):1243–1248
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts to report.
Human and Animal Rights Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Patel, N., King, A.J. & Breen, D.J. Percutaneous image-guided cryoablation of small renal masses. Abdom Radiol 41, 754–766 (2016). https://doi.org/10.1007/s00261-016-0682-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0682-2