Abstract
The purpose of this study was to evaluate changes in vascular supply to hepatocellular carcinoma (HCC) located in the bare area of the liver in patients who were mainly treated with chemoembolization. Twenty-six patients with HCC showing a mean diameter of 3.1 ± 1.4 cm (mean ± standard deviation) were mainly treated with chemoembolization. All patients underwent 2.7 ± 2.3 chemoembolization sessions over 40.1 ± 25.2 months. Tumor feeding branches demonstrated in each chemoembolization session were retrospectively evaluated. Initially, 18 tumors (59.2%) were supplied by the hepatic artery (H) and 8 (30.8%) by both the hepatic and the extrahepatic arteries (H + C). Fourteen tumors (53.8%) recurred at the posterior aspect of the tumor and were supplied by H (n = 4), H + C (n = 5), and extrahepatic collaterals (C) (n = 5). Several tumors recurred despite repeated chemoembolization, and these were supplied by H (n = 1), H + C (n = 7), and C (n = 2) at the second recurrence, by H (n = 1), H + C (n = 2), and C (n = 3) at the third, by H + C (n = 2) and C (n = 2) at the fourth, by H + C (n = 2) and C (n = 2) at the fifth, and by H (n = 1) and C (n = 1) at the sixth. One tumor was supplied by H at the seventh and by H + C at the eighth recurrence. As the number of local recurrences increased, the feeding vessel shifted from H to C. Especially, the right inferior phrenic artery (IPA) and renal capsular artery (RCA) supplied the tumor early, while the small right RCAs, adrenal arteries, and intercostal and lumbar artery supplied late recurrences in turns. In conclusion, HCCs located in the bare area are frequently supplied by extrahepatic vessels initially, while recurrence after chemoembolization is mainly due to extrahepatic blood supply. The right IPA and RCA are common feeding vessels demonstrated early, while other extrahepatic collateral supply from the retroperitoneal circulation occurs in turns during the later course.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) frequently recurs after chemoembolization and development of extrahepatic collateral pathways is one of the causes of local tumor recurrence. Several extrahepatic collateral pathways supplying HCC have been reported [1–14].
At the level of the bare area of the liver, branches of the inferior phrenic arteries (IPAs) are in direct contact with the liver [3, 4]. In the event of hepatic artery damage, or even with a patent hepatic artery, these branches contribute substantially to the hepatic tumor vascular supply. In addition, IPAs usually anastomose with several arteries, such as the internal mammary artery and intercostal arteries [5, 6, 15]. Therefore, a tumor located in the bare area of the liver has the potential to receive collateral blood flow through several individual sources. Extrahepatic collateral supplies can inhibit the effectiveness of chemoembolization. For transcatheter management of HCC to be effective, these collaterals should be adequately embolized [1–14]. Therefore, interventional radiologists should have sufficient knowledge of extrahepatic blood supply to HCCs.
In this report, we retrospectively analyzed changes in vascular supply to HCCs located in the bare area of the liver during the subsequent clinical course of patients who were mainly treated by chemoembolization.
Materials and Methods
Patients
In the present study, we define “the bare area of the liver” as the posterior surface of Segment 7 demarcated by the right hepatic vein. Although we are well aware that “the bare area of the liver” also includes a part of lateral surface of Segment 8, the right-side margin is not anatomically outlined clearly. Between August 1999 and August 2008, we treated 26 consecutive HCC lesions in the bare area of the liver by chemoembolization. The patient profiles are summarized in Table 1. There were 14 men and 12 women and the mean patient age was 68.9 ± 7.5 years (mean ± standard deviation; range, 51 to 81 years). All patients had liver cirrhosis, which was associated with hepatitis C in 19 patients and hepatitis B in 6 patients, while in the remaining patient, the etiology was unknown. All patients had a single HCC lesion at the bare area, including 6 patients with 1 other HCC in addition to the tumor in the bare area and 2 patients with multiple HCCs but fewer than 5 lesions at other sites. The mean diameter of tumors in the bare area was 3.1 ± 1.4 cm (range, 1–6 cm). Diagnosis was established by imaging findings on computed tomography (CT) and/or magnetic resonance (MR) imaging, characteristic nodular enhancement during the arterial phase, and washout during the delayed phase images in all patients.
Initially, 22 tumors were treated by chemoembolization alone and 4 were treated by a combination of chemoembolization and radiofrequency ablation (RFA).
Chemoembolization Procedure
Written informed consent was obtained from each patient before the chemoembolization and RFA procedures. Institutional review board approval was not required at our institution for this retrospective study.
A 2-Fr tip (Progreat α; Terumo, Tokyo, Japan) was mainly used for all chemoembolization procedures. To navigate the microcatheter, a 0.016-in. guidewire (GT-wire; Terumo) was used. After the microcatheter was inserted into the feeding branch, 0.5 ml of 2% lidocaine (Xylocaine; Fujisawa, Osaka, Japan) was intra-arterially injected to prevent pain and vasospasm. First, a mixture of iodized oil (Lipiodol; Andre Guerbet, Aulnaysous-Bois, France) and anticancer drugs (epirubicin [Farmorbicin], Kyowa Hakko, Tokyo, Japan; mitomycin C [Mitomycin], Kyowa Hakko) was injected, and injection of gelatin sponge particles followed. Until December 2006, gelatin sponge particles (Gelfoam; Upjohn, Kalamazoo, MI, USA) cut into approximately 1-mm cubes were used. Since January 2007, 1-mm-diameter commercially available gelatin sponge particles (Gelpart; Nippon Kayaku, Tokyo, Japan) were used.
The embolized branches of the hepatic artery were minimized as selectively as possible in each patient. Extrahepatic collaterals were searched for with the use of a 4-Fr shepherd-hook catheter (Terumo) or cobra-shaped catheter (Hanako Medical, Kobe, Japan). When tumor stain was demonstrated through the extrahepatic vessel on angiography, the tumor-feeding branch was selected by the microcatheter. In total, 1–6 ml of iodized oil, 10–30 mg of epirubicin, and 2–6 mg of mitomycin C were used in a single chemoembolization session, depending on the size of each tumor.
RFA Procedure
RFA was performed 1–2 weeks after chemoembolization in the first 4 consecutive tumors (2.5–5 cm in diameter; mean, 3.3 ± 1.4 cm) in 4 patients. The procedure was performed using an expandable electrode (LeVeen; Radiotherapeutics, Sunnyvale, CA, USA) under ultrasonographic (US) guidance without injection of saline into the pleural cavity. However, RFA was not performed after the fifth patient because we were able to obtain a complete ablative margin in only 1 of the previous 4 tumors.
Follow-Up
Unenhanced CT was obtained 1 week after chemoembolization in all patients to check for iodized oil accumulation within the target tumor. In addition, dynamic CT was also performed the day after RFA. All patients were followed with dynamic CT and/or MR imaging obtained every 2–3 months after treatment to screen for any tumor recurrence. When tumor recurrence and/or newly developed tumors at other sites were detected, additional chemoembolization was performed, if possible.
Definition of Extrahepatic Collateral Supply to HCC
Several extrahepatic collaterals were searched when extrahepatic blood supply to the tumor was suspected. Initial extrahepatic blood supply to HCC was defined as follows: (i) an obvious tumor stain was demonstrated on arteriogram of the extrahepatic vessel during the initial chemoembolization; and (ii) arteriogram of the extrahepatic vessel during additional chemoembolization showed a stain corresponding to the tumor area that had not shown accumulation of iodized oil on CT obtained 1 week after the initial chemoembolization despite the lack of an obvious extrahepatic supply to HCC during the initial procedure (including cases that did not undergo arteriogram of extrahepatic vessels). Extrahepatic blood supply to recurrent HCC was confirmed when tumor stain was demonstrated on arteriogram of extrahepatic collaterals.
Definition of Tumor Recurrence
The tumor portion where iodized oil was not accumulated after the initial chemoembolization was defined as a “residual portion,” not a “recurrent portion.” Tumor recurrence was defined as the development of a viable tumor portion after the entire tumor was embolized by the initial or additional chemoembolization. The interval between the initial chemoembolization and the demonstration of recurrence on CT or MR imaging was defined as the period of tumor control.
Results
Findings at the Initial Chemoembolization
All tumors were partially or completely supplied by the hepatic arterial branches at the initial chemoembolization. The embolized hepatic artery was the superior posterior subsegmental artery (A7) (n = 12), posterior segmental artery (n = 7), superior anterior subsegmental artery (A8) and A7 (n = 5), inferior posterior subsegmental artery (A6) (n = 1), and A7 and the caudate artery (n = 1).
Arteriograms of extrahepatic collateral vessels were obtained in 18 patients (69.2%) during the initial chemoembolization procedure. Arteriogram of the right IPA was obtained in 12 patients, right IPA and right renal capsular artery (RCA) in 5, and right RCA and right middle adrenal artery in 1. In 2 patients, the right IPA was reconstructed through the dorsal pancreatic artery arising from the superior mesenteric artery (n = 1) or left gastric artery (n = 1) (Fig. 1). One right IPA could not be detected during the initial chemoembolization procedure because a vessel that arose from the left renal polar artery was demonstrated at the second chemoembolization when it was shown to supply the tumor (Fig. 2). Extrahepatic blood supply to the tumor at the initial procedure was observed in 8 tumors (30.8%), with a mean tumor diameter of 3.4 ± 1.4 cm (range, 1.6–6 cm), through the right IPA (n = 5), right IPA and right RCA (n = 2), and right RCA (n = 1). In 5 tumors, the extrahepatic collateral supply was demonstrated during the initial procedure and chemoembolization through these feeding branches was subsequently performed. In the remaining 3 tumors supplied by the right IPA (n = 2) and right RCA (n = 1), arteriograms of these vessels were not obtained during the initial procedure. Based on CT findings obtained 1 week after the procedure that showed a tumor area without iodized oil accumulation, additional chemoembolization was performed through these vessels 2–3 months after the initial chemoembolization.
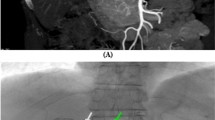
A 76-year-old man with a recurrent HCC located in the bare area of the liver after chemoembolization through the hepatic arterial branch. A Arteriogram of the left gastric artery shows the reconstructed right IPA and tumor stain (arrow). B The anasotomosis branch could not be selected, therefore embolization of the left gastric artery at the distal end from the anastomosis branch was performed, using metallic coils (arrow). C The tumor stain (arrow) is clearly depicted, without gastric wall staining after coil embolization. Chemoembolization was successfully performed, without any complications, through the left gastric artery
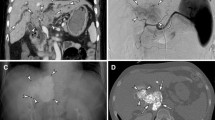
A 68-year-old man with an HCC located in the bare area of the liver. A CT obtained 1 week after chemoembolization through the hepatic arterial branch shows a defect of iodized oil accumulation at the posterior aspect of the tumor (arrow). B The right IPA is derived from the left upper renal polar branch. C Selective arteriogram of the right IPA shows a tumor stain corresponding to the defect of iodized oil (arrow)
Tumor Recurrence After Treatment
All patients were followed for a mean of 40.1 ± 25.2 months (range, 8–116 months). In all 26 tumors, including 3 tumors that were treated with additional chemoembolization sessions, iodized oil was accumulated in the entire tumor. In 3 of 4 tumors treated with RFA after chemoembolization, the ablative area did not cover the entire tumor on CT and these tumors recurred at 14.3 ± 5.5 months (range, 9–20 months). In total, 14 tumors (53.8%), 11 of 22 tumors treated by chemoembolization alone and 3 of 4 tumors treated by a combination of chemoembolization and RFA, recurred 13.2 ± 9.0 months (range, 4–40 months) after the initial treatment. All 14 tumors recurred at the posterior aspect of the tumor arising in the bare area. Second recurrence was observed in 10 tumors 6.5 ± 3.1 months (range, 2–12 months) after treatment. Despite repeated chemoembolization, third recurrence was observed in 7 tumors after 5.7 ± 2.6 months (range, 3–11 months), fourth recurrence was observed in 6 tumors after 5.3 ± 2.2 months (range, 4–8 months), fifth recurrence was observed in 4 tumors after 9.8 ± 6.4 months (range, 5–19 months), and sixth recurrence was observed in 3 tumors after 4.7 ± 3.8 months (range, 2–9 months). Moreover, 1 tumor recurred at 10, 23, and 5 months after each additional chemoembolization session and the tumor was finally treated by irradiation. In total, 19 tumors (73.1%) could be controlled but 7 (26.9%) could not, despite repeated chemoembolization. Nine patients died after 42.9 ± 15.1 months (range, 18–69 months), including 3 who died of liver-unrelated causes (pneumonia [n = 1], traffic accident [n = 1], and pancreatic cancer [n = 1]). To date, 17 patients have survived 37.5 ± 29.0 months (range, 8–116 months) after the initial treatment, including 1 patient with a recurrent tumor in the bare area and 2 patients with a viable tumor at another site.
Extrahepatic Collateral Supply to Recurrent Tumors
All patients underwent 1–10 chemoembolization sessions (mean, 2.7 ± 2.3 sessions) for HCC in the bare area. During the subsequent treatment course, arteriogram of the right IPA was obtained for all but 1 patient (96.2%) whose tumor did not recur after the initial chemoembolization procedure. Arteriogram of the right RCA was obtained in 21 patients (80.8%), except for 4 patients without tumor recurrence after the initial chemoembolization and 1 patient whose recurrent tumor after the initial chemoembolization was controlled by chemoembolization through the right IPA. Arteriogram of the right intercostal and/or lumbar artery was obtained in 9 patients (34.6%), and arteriogram of the adrenal arteries directly arising from the aorta was obtained in 7 patients (26.9%). In 1 patient (3.8%), arteriogram of the right gonadal artery directly arising from the aorta was also obtained.
During follow-up, extrahepatic collateral supply to the tumor was observed in 18 patients (69.2%), including 8 (30.8%) in whom findings were observed at the initial treatment (Table 2). The tumor-feeding extrahepatic collateral was the right IPA (n = 13), right RCA (n = 7), right middle adrenal artery (n = 4), right lumbar artery (n = 4), right inferior adrenal artery (n = 4), right intercostal artery (n = 2), bile duct artery (n = 1), and dorsal pancreatic artery (n = 1). All tumor-feeding branches were embolized during each procedure. One feeding branch derived from the left gastric artery connected with the reconstructed right IPA could not be directly selected because of its small caliber and acute angulation. The left gastric artery was embolized using metallic coils (Tornado; Cook, Bloomington, IN, USA) at the distal level of the anastomosis branch to prevent nontarget embolization, and chemoembolization was successfully performed through the microcatheter placed in the left gastric artery proximal to the anastomosis branch (Fig. 1).
Change of the Feeding Vessel in Each Recurrence
At the initial chemoembolization, 18 tumors (69.2%) were supplied by the hepatic artery alone and 8 (30.8%) were supplied by both the hepatic and the extrahepatic arteries. Of 14 tumors showing first recurrence, 4 tumors were supplied by the hepatic artery, 5 were supplied by both the hepatic and the extrahepatic arteries, and 5 were supplied by extrahepatic collaterals alone. Of 10 tumors showing second recurrence, 1 tumor was supplied by the hepatic artery alone, 7 were supplied by both the hepatic and the extrahepatic arteries, and 2 were supplied by extrahepatic collaterals alone. In 6 of 7 tumors showing third recurrence, 1 tumor was supplied by the hepatic artery alone, 2 were supplied by both the hepatic and the extrahepatic arteries, and 3 were supplied by extrahepatic collaterals alone. Angiography was not performed for the remaining tumor because of poor hepatic function. In 4 of 6 tumors showing fourth recurrence, 2 tumors were supplied by both the hepatic and the extrahepatic arteries and 2 were supplied by extrahepatic collaterals alone. Angiography was not performed for the remaining 2 tumors because of poor hepatic function or dementia. In 3 of 4 tumors showing fifth recurrence, all tumors were supplied by extrahepatic collaterals alone. Angiography was not performed for the remaining tumor because of diffuse metastases. In 2 of 3 tumors showing sixth recurrence, 1 was supplied by both the hepatic and the extrahepatic arteries and 1 was supplied by extrahepatic collaterals alone. Angiography was not performed for 1 tumor because of diffuse metastases. At the seventh recurrence, the tumor was again supplied by the restored hepatic artery, and it was supplied by both the hepatic and the extrahepatic arteries at the eighth recurrence. As the number of tumor recurrences increased, the tumor-feeding vessels shifted from the hepatic artery to extrahepatic collaterals (Fig. 3). Table 3 shows the tumor-feeding branches at each recurrence in patients who underwent 3 or more chemoembolization procedures. In particular, other or small right RCAs, right adrenal arteries, and right lumbar and intercostal arteries fed the tumor in turns after the right IPA or right RCA had already been embolized (Figs. 4, 5, 6).
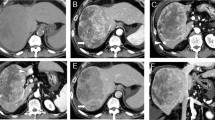
A 63-year-old woman with an HCC located in the bare area of the liver. A Arterial phase CT shows an enhancing tumor in the bare area of the liver. B Arteriogram of the proper hepatic artery shows tumor stain supplied by the posterior superior subsegmental artery of the liver. The branch was selected and chemoembolization was performed. C Arteriogram of the right IPA obtained simultaneously during the initial chemoembolization does not show any tumor stains. In this patient, RFA was added after chemoembolization. D CT obtained 20 months after the initial treatment shows a recurrent tumor (arrow). E Arteriogram of the right IPA shows a tumor stain in turn (arrow). Chemoembolization was performed; however, the tumor recurred again after treatment. F At the third recurrence (2 years 11 months after the initial treatment), the tumor is supplied by the right 11th intercostal artery. The tumor-feeding branch (arrow) was selected and chemoembolization was performed. The posterior branch of the right hepatic artery, right IPA, and right RCA were simultaneously embolized (not shown). G At the fourth recurrence (3 years 4 months after the initial treatment), the tumor is supplied by another right RCA (arrow), in addition to the right IPA supply
A 68-year-old woman with an HCC located in the bare area. A At the initial treatment, the residual tumor after chemoembolization of the hepatic arterial branch (arrow) is fed by the right RCA. B The recurrent tumor 1 year after the procedure is supplied by the hepatic arterial branch (not shown). At the second recurrence (1 year 8 months after the initial treatment), the tumor is fed by two right inferior adrenal arteries (arrows). Chemoembolization was performed through these vessels. C At the third recurrence (2 years 5 months after the initial treatment), the tumor is fed by 3 small RCAs (arrows)
A 71-year-old woman with an HCC located in the bare area. A Arterial phase CT at the third recurrence 4 years 2 months after the initial treatment shows a tumor protruding to the bare area (arrow). B The tumor was initially supplied by the hepatic arterial branch and was supplied by the right IPA at the first and second recurrences (not shown). At the third recurrence, the tumor (arrow) is supplied by a small branch arising from the right first lumbar artery (arrowhead). C The tumor-feeding branch was selected and chemoembolization was successfully performed
Discussion
Extrahepatic collateral pathways to the liver and HCC are established under various conditions. These pathways mainly develop after interruption of the hepatic artery by surgical ligation, arterial injury induced by repeated chemoembolization procedures, or placement of a catheter. Adhesion between the liver and the other organ exaggerates the degree of extrahepatic collaterals [7]. An extrahepatic blood supply to HCC also develops in the anatomical location of a tumor, although the hepatic arterial supply remains intact [3, 14]. In particular, it is thought that extrahepatic collateral supplies develop early in the bare area of the liver [14].
The right IPA is the major source of diaphragmatic pathways and is the most frequent extrahepatic collateral vessel of HCCs [12–14]. There is close contact between the posterior portion of the liver and the diaphragm in the bare area, and branches of the IPA are in direct contact with the liver [3, 4]. Takeuchi et al. [16] clearly demonstrated blood supply to the liver from the right IPA immediately after balloon occlusion of the proper hepatic artery using a unified CT and angiography system. Chung et al. [3] also reported that right IPA parasitization was retrospectively suspected at the initial chemoembolization in 80% of their patients, with blood supply to tumors from the right IPA demonstrated on follow-up angiography.
The RCA system distributes to the renal capsule and perirenal space, and it is composed of three basic pathways: superior, medial, and inferior RCAs. These vessels usually arise from or together with the adrenal arteries. The system may also originate from the main renal artery or from the gonadal artery. In addition, perforating capsular arteries (small RCAs) arise from the arcuate and interlobular arteries, which are branches of the renal artery [17]. The perirenal space is anatomically connected with the bare area on the right side [18], therefore, the right RCA also distributes into the bare area.
The intercostal artery and internal mammary artery usually connect with the right IPA and support the diaphragmatic pathways [5]. There are also fine retroperitoneal networks between the right IPA, right RCA, dorsal pancreatic artery, adrenal artery, left gastric artery, and right lumbar artery [6]. Therefore, these vessels also infrequently act as a supportive diaphragmatic pathway [5, 19]. Because of such overlapping vascular territories, a tumor located in the bare area may potentially be fed by several separate extrahepatic collateral vessels, in addition to the hepatic arterial branch. When one vessel is attenuated by chemoembolization, other vessels may in turn replace the blood supply to the recurrent tumor at the next chemoembolization session. We named this sequence “the march of extrahepatic collaterals” (Fig. 7).
Schematic representation of “the march of extrahepatic collaterals.” At the beginning of the treatment, the tumor located in the bare area of the liver is mainly supplied by the hepatic arterial branch and partially supplied by major extrahepatic collaterals, such as the right IPA and right RCA. After damage to these vessels by repeated chemoembolization, minor extrahepatic collateral vessels of retroperitoneal circulation, such as the small right RCAs, adrenal arteries, posterior intercostal artery, and lumbar artery, feed the recurrent tumor in turns
As the tumor size increases, the prevalence of an extrahepatic supply increases [13]. Chung et al. [14] reported that prevalence of extrahepatic blood supply at the initial chemoembolization session in a tumor <4 cm in diameter was <3%; this increased to 63% when the tumor was >6 cm in diameter. In the present study, however, 30.8% of tumors with a mean diameter of 3.4 cm were receiving an extrahepatic blood supply at the initial chemoembolization. This suggests that extrahepatic parasitization easily develops even for a small tumor located in the bare area.
At the beginning of treatment, a tumor located in the bare area of the liver was mainly supplied by the hepatic arterial branch and partially supplied by major extrahepatic collaterals, such as the right IPA and right RCA. After damage to these vessels by repeated chemoembolization, minor extrahepatic collateral vessels of the retroperitoneal circulation, such as small right RCAs, adrenal arteries, posterior intercostal artery, and lumbar artery, fed the recurrent tumor in turns. Adhesion between the liver and the diaphragm after repeated chemoembolization and RFA may also exaggerate the development of such minor extrahepatic collaterals. These small vessels may make it more difficult to control the tumor by chemoembolization after the tumor has recurred several times.
RFA is one of the most effective techniques for local treatment of inoperable HCCs. It is also useful to treat a tumor located in the bare area, however, several thoracic complications, such as diaphragmatic perforation (intrathoracic hernia, abscess extending to the thorax, and bronchobiliary fistula), right shoulder pain, and pleural effusion, have been reported [20–22]. In a report by Kang et al. [22], RFA of a tumor abutting the diaphragm was less effective with regard to technical success and local tumor control because of the difficulty in targeting the tumor by US. In addition, the operator’s concern regarding thermal injury of the adjacent diaphragm may lead to incomplete ablation because it is very difficult to monitor the ablative zone and the diaphragm in the subphrenic area with a poor sonic window and ill-defined hyperechoic zone during ablation. Conversely, Takaki et al. [23] reported that there was no significant difference in tumor progression rates between tumors adjacent to the diaphragm and tumors apart from the diaphragm after CT-guided RFA combined with chemoembolization. In our small number of patients treated with a combination of chemoembolization and US-guided RFA, however, the ablative margin did not cover the entire tumor in 3 of 4 cases, and these 3 tumors subsequently recurred. CT guidance may allow the operator to easily target tumor abutting the diaphragm by the RF electrode, although the risk of thermal injury to the diaphragm remains [21].
Local recurrence rates of HCCs located in the bare area treated with chemoembolization were higher than those of HCCs treated with RFA in a report by Kang et al. [22]. We speculate that differences in local recurrence rates in each study may involve a different tumor location rather than different therapeutic modalities. In the present study, all tumors were located in the bare area. However, in their report, not only tumors abutting the diaphragm but also tumors located within a 5-mm-wide area near the diaphragm were included in the “abutting group.” In addition, the hepatic segment of the tumor location was not provided. We speculate that tumors originating in the bare area may be more difficult to treat by a combination of chemoembolization and RFA than tumors in other segments abutting the diaphragm.
There are some limitations to the present study. We excluded equivocal staining on angiograms of extrahepatic collaterals. In addition, in elderly patients with atherosclerosis, screening of small branches arising directly from the aorta, such as the middle adrenal artery, may be incomplete. For these reasons, the incidence of blood supply from extrahepatic collaterals to the tumor may have been underestimated in this study. In addition, “tumor recurrence” in the present study may include small residual tumor tissue following the initial or additional chemoembolization. Therefore, the prevalence of extrahepatic blood supply to HCC at the initial chemoembolization may also have been underestimated.
In conclusion, HCCs located in the bare area are frequently supplied by extrahepatic vessels in addition to the hepatic artery even at the initial treatment. Recurrence of such tumors after chemoembolization is mainly due to the extrahepatic blood supply. The right IPA and RCA are common feeding vessels demonstrated early, while other extrahepatic collateral supply from the retroperitoneal circulation, such as the small right RCAs, adrenal arteries, and intercostal and lumbar artery, occurs in turns during the later course. Interventional radiologists should be familiar with the clinical features of HCCs located in the bare area and the complex vascular sequence supplying such tumors.
References
Kim JH, Chung JW, Han JK et al (1995) Transcatheter arterial embolization of the internal mammary artery in hepatocellular carcinoma. J Vasc Interv Radiol 6:71–74
Nakai M, Sato M, Kawai N et al (2001) Hepatocellular carcinoma: involvement of the internal mammary artery. Radiology 219:147–152
Chung JW, Park JH, Han JK et al (1998) Transcatheter oily chemoembolization of the inferior phrenic artery in hepatocellular carcinoma: the safety and potential therapeutic role. J Vasc Interv Radiol 9:495–500
Duprat G, Charnsangavej S, Wallace S et al (1998) Inferior phrenic artery embolization in the treatment of hepatic neoplasms. Acta Radiol 29:427–429
Park SI, Lee DY, Won JY et al (2003) Extrahepatic collateral supply of hepatocellular carcinoma by the intercostal arteries. J Vasc Interv Radiol 14:461–468
Miyayama S, Matsui O, Taki K et al (2004) Transcatheter arterial chemoembolization for hepatocellular carcinoma fed by the reconstructed inferior phrenic artery: anatomical and technical analysis. J Vasc Interv Radiol 15:815–823
Miyayama S, Matsui O, Akakura Y et al (2001) Hepatocellular carcinoma with blood supply from omental branches: treatment with transcatheter arterial embolization. J Vasc Interv Radiol 12:1285–1290
Kodama Y, Shimizu T, Endo H et al (2002) Spontaneous rupture of hepatocellular carcinoma supplied by the right renal capsular artery treated by transcatheter arterial embolization. Cardiovasc Intervent Radiol 25:137–140
Miyayama S, Matsui O, Nishida H et al (2003) Transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma fed by the cystic artery. J Vasc Interv Radiol 14:1155–1161
Suh SH, Won JY, Lee DY et al (2005) Chemoembolization of the left inferior phrenic artery in patients with hepatocellular carcinoma: radiologic findings and clinical outcome. J Vasc Interv Radiol 16:1741–1745
Kim HC, Chung JW, Jae HJ et al (2006) Hepatocellular carcinoma: transcatheter arterial chemoembolization of the gonadal artery. J Vasc Interv Radiol 17:703–709
Kim HC, Chung JW, Lee W et al (2005) Recognizing extrahepatic collateral vessels that supply hepatocellular carcinoma to avoid complications of transcatheter arterial chemoembolization. Radiographics 25:S25–S39
Miyayama S, Matsui O, Taki K et al (2006) Extrahepatic blood supply to hepatocellular carcinoma: angiographic demonstration and transcatheter arterial chemoembolization. Cardiovasc Intervent Radiol 29:39–48
Chung JW, Kim HC, Yoon JH et al (2006) Transcatheter arterial chemoembolization of hepatocellular carcinoma: prevalence and causative factors of extrahepatic collateral arteries in 479 patients. Korean J Radiol 7:257–266
Miyayama S, Yamashiro M, Okuda M et al (2009) Anastomosis between the hepatic artery and the extrahepatic collateral or between extrahepatic collaterals: observation on angiography. J Med Imaging Radiat Oncol 53:271–282
Takeuchi Y, Arai Y, Inaba Y et al (1998) Extrahepatic arterial supply to the liver: observation with a unified CT and angiography system during temporary balloon occlusion of the proper hepatic artery. Radiology 209:121–128
Uflacker R (1997) Atlas of vascular anatomy: angiographic approach. Williams & Wilkins, Baltimore, p 418
Lim JH, Kim B, Auh YH (1998) Anatomical communications of the perirenal space. Br J Radiol 71:450–456
Miyayama S, Yamashiro M, Okuda M et al. (2009) Hepatocellular carcinoma supplied by the right lumbar artery. CardioVasc Intervent Radiol (in press)
Head HW, Dodd GD III, Dalrymple NC et al (2007) Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology 243:877–884
Yamakado K, Nakatsuka A, Takai H et al (2008) Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology 247:260–266
Kang TW, Rhim H, Kim EY et al (2009) Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol 10:34–42
Takaki H, Yamakado K, Nakatsuka A et al (2009) CT-guided RF ablation combined with chemoembolization for subphrenic hepatocellular carcinoma. J Vasc Interv Radiol 20:S10
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyayama, S., Yamashiro, M., Okuda, M. et al. The March of Extrahepatic Collaterals: Analysis of Blood Supply to Hepatocellular Carcinoma Located in the Bare Area of the Liver After Chemoembolization. Cardiovasc Intervent Radiol 33, 513–522 (2010). https://doi.org/10.1007/s00270-009-9697-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-009-9697-1