Abstract
Purpose
To evaluate the incidence of each extrahepatic collateral pathway to hepatocellular carcinoma (HCC) and to assess technical success rates and complications of transcatheter arterial chemoembolization (TACE) through each collateral.
Methods
We retrospective evaluated extrahepatic collateral pathways to HCC on angiography in 386 procedures on 181 consecutive patients. One hundred and seventy patients had previously undergone TACE. TACE through extrahepatic collaterals using iodized oil and gelatin sponge particles was performed when a catheter was advanced into the tumor-feeding branch to avoid nontarget embolization.
Results
A single collateral was revealed in 275 TACE procedures, two were revealed in 74, and three or more were revealed in 34. Incidences of collateral source to HCC were 83% from the right inferior phrenic artery (IPA), 24% from the cystic artery, 13% from the omental artery, 12% from the right renal capsular artery (RCA) and left IPA, 8% from the right internal mammary artery (IMA) and right intercostal artery (ICA), and 7% from the right inferior adrenal artery (IAA). Technical success rates of TACE were 53% in the right ICA, 70% in the cystic artery, 74% in the omental artery, 93% in the left IPA, 96% in the right IPA, and 100% in the right RCA, right IMA, and right IAA. Complications included skin necrosis after TACE through the right IMA (n = 1), cholecystitis after TACE through the cystic artery (n = 1), and ulcer formation after TACE through the right gastric artery (n = 1), in addition to pleural effusion and basal atelectasis after TACE through the IPA and IMA.
Conclusion
Our study suggests that TACE through extrahepatic collaterals is possible with high success rates, and is also relatively safe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in the East Asian region. The prognosis of patients with inoperable HCC has improved with the development of therapeutic options such as percutaneous ethanol injection, microwave coagulation, and radiofrequency ablation, in addition to transcatheter arterial chemoembolization (TACE) [1–4]. Most HCC arises from liver cirrhosis with a poor hepatic functional reserve, and it is frequently synchronously or asynchronously multicentric; therefore, TACE still plays an important part in the treatment of inoperable HCC.
An extrahepatic collateral pathway to the liver is established in various conditions [5–7]. It mainly develops after interruption of the hepatic artery by surgical ligation, arterial injury induced by repeat TACE, or placement of a catheter. Adhesion between the liver and other organs exaggerates the degree of extrahepatic collaterals [5, 8]. An extrahepatic blood supply to HCC also develops in the anatomic location of HCC, although the hepatic arterial supply remains intact [8–12]. Extrahepatic collateral supplies can prohibit effective treatment by TACE. For transcatheter management of HCC to be effective, these collaterals should be adequately embolized [8–18].
In this report, we retrospectively analyzed the incidence of demonstrable extrahepatic collaterals feeding HCC on digital subtraction angiography (DSA), and the technical success rates and complications of TACE for each extrahepatic collateral pathway.
Materials and Methods
Patients
Between January 1994 and December 2003, 2329 TAE procedures were performed in 719 patients with inoperable HCC in our hospital. Of the 2329 procedures, TACE through the extrahepatic collaterals was attempted in 181 patients (25%) of 386 procedures. There were 135 men and 46 women, ranging in age from 35 to 86 years (mean 66 years). All patients had chronic hepatitis or liver cirrhosis. One hundred and thirty-nine patients had viral hepatitis C, 27 had viral hepatitis B, 4 had both viral hepatitis B and C, while in 2 liver disease was related to alcohol. The etiology was unknown in 9 patients. The diagnosis of HCC was made by nodular staining on DSA and nodular perfusion defect on computed tomography (CT) during arterial portography (CTAP), in addition to high serum levels of tumor markers (α-fetoprotein or protein induced in vitamin K absence II). One-hundred and seventy patients (94%) had previously undergone TACE for HCC. Ten patients (6%) had previously undergone surgical resection of HCC and 37 (20%) had previously undergone percutaneous therapies for HCC, in addition to TACE.
Methods
Arteriograms of the celiac and superior mesenteric arteries and CTAP were performed in all patients. An arteriogram of the right inferior phrenic artery (IPA) was routinely performed in patients who had an interrupted hepatic arterial circulation by previous treatment or in patients who had a tumor located near the diaphragm, even in the initial angiography. Other extrahepatic collateral pathways were sought when the tumor stain corresponding to HCC depicted by CTAP was not demonstrated on angiograms of these arteries. Findings of multiphasic CT obtained before TACE procedure also provided us with useful information. The individual vessels, which can feed HCC depending on the tumor location, were selected to determine whether collateral supply to the tumor was present. Aortography helped to locate the individual vessels arising from the aorta, if necessary.
TACE of the extrahepatic collaterals was attempted when an obvious blood supply to HCC from them was revealed on DSA. All patients were informed about the benefits and potential risks of the procedure, and informed consent was obtained from each patient. Institutional review board approval is not required at our institution for this type of study. TACE was performed with gelatin sponge particles (Gelfoam; Upjohn, Kalamazoo, MI, USA) after the injection of a mixture of 0.5–2 ml of iodized oil (Lipiodol; Andre Guerbet, Aulnay-sous-Bois, France), 10–20 mg of epirubicin (Farmorbicin; Kyowa Hakko, Tokyo, Japan), and 2–4 mg of mitomycin C (Mitomycin; Kyowa Hakko); in some circumstances, embolization with gelatin sponge particles alone was done. We did not perform infusion of anticancer drugs alone in any patient.
In the IPA, middle adrenal artery (MAA), inferior adrenal artery (IAA), and renal capsular artery (RCA), TACE using iodized oil and gelatin sponge particles was routinely performed. Iodized oil injection was stopped when the hepatic veins or pulmonary vessels were apparently demonstrated through the shunt. In the reconstructed IPA through other arteries, TACE using iodized oil and gelatin sponge particles was performed only when nontarget branches could be avoided. If this was impossible, the procedure was ended. In the internal mammary artery (IMA), intercostal artery (ICA), and lumbar artery (LA), iodized oil and gelatin sponge particles were used only when the microcatheter could be inserted into the tumor-feeding branch. If this was not possible, the artery was embolized by gelatin sponge particles alone. In the cystic artery, omental artery, pancreatic artery, gastric artery, and colic artery, TACE using iodized oil and gelatin sponge particles was performed only when the microcatheter could be navigated to avoid nontarget branches. The procedure was ended when the catheterization was unsuccessful.
In procedures performed between January 1994 and October 2002, a 2.4 Fr tip microcatheter (Microferret-18; Cook, Bloomington, IN, USA) was routinely inserted into the feeding artery by means of a coaxial method using a 0.016-inch guidewire (Radifocus GT wire; Terumo, Tokyo, Japan). In procedures carried out from October 2002 onwards, a 2 Fr tip microcatheter (Progreat α; Terumo) was routinely used. The microcatheter with its tip bent into a J shape by steam was used in almost all patients to facilitate insertion into small arteries that branched at acute angles. After the microcatheter was inserted into the feeding artery, 0.5 ml of 2% lidocaine (Xylocaine; Fujisawa, Osaka, Japan) was intra-arterially injected to prevent pain and vasospasm. TACE through the hepatic artery was added in the same fashion, if necessary.
We defined technical success as successful catheterization into the tumor-feeding branch of extrahepatic collaterals and delivery of TACE using iodized oil and gelatin sponge particles. CT was performed 1 week after TACE to check iodized oil accumulation within the tumor and nontarget area. Complications related to collateral TACE were retrospectively analyzed by laboratory tests and the CT findings, in addition to post-TACE symptoms.
Results
The following extrahepatic blood supply to HCC was demonstrated on DSA. A single extrahepatic collateral was revealed in 275 TACE procedures, two collaterals were revealed in 74 TACE procedures, and three or more collaterals were revealed in 34 TACE procedures. Results for each collateral pathway are summarized in Table 1.
Inferior Phrenic Artery
Blood supply from the right IPA was found in 145 patients (83%). All but 5 of these patients had previously undergone several TACE sessions for HCC (1–10 times, mean 3 times). The remaining 5 patients had HCC protruding into the bare area, and blood supply from the right IPA was demonstrated at the first TACE session. All tumors were located in the posterior segment, the dome of the right lobe of the liver, and the caudate lobe of the liver. Ten right IPAs were reconstructed via the retroperitoneal anastomosis [19].
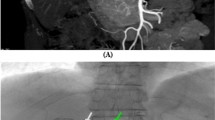
Two-hundred and eighty-five TACE procedures were attempted including repeated sessions. The first 10 attempts failed and intimal injury occurred in 1 procedure (0.3%). In 7 IPAs, TACE was retried and successfully performed after the second or third attempt via the left brachial approach (n = 1), using a catheter with a large side hole via the femoral approach (n = 3) (Fig. 1) or via the left brachial approach (n = 1), and isolation of the right IPA arising from the celiac axis using an occlusion balloon catheter and coil embolization of the left gastric artery (n = 2) [21]. The catheter with a large side hole was developed by us to select a branch arising from the proximate portion of the major artery at acute angles. A large side hole was created using a knife near the tip of a 4 or 5 Fr curved, shepherd-hook, or head-hunter angiographic catheter to face the orifice of the target branch and a microcatheter was advanced into it through the large side hole [20]. In the remaining 3 IPAs, catheterization was not retried. In the other 19 right IPAs derived from the proximate portion of the celiac axis, which could not be selected using a conventional coaxial technique, the catheter with a large side hole was successfully used at the first attempt. An additional 3 IPAs, 2 reconstructed IPAs from the dorsal pancreatic artery or left gastric artery with celiac occlusion and 1 IPA arising from the tortuous aorta, could not be selected. The overall technical success rate of TACE of the right IPA was 96%. Transient pleural effusion developed in 95 procedures (33%), and basal atelectasis mainly due to iodized oil accumulation in the lung was observed in 23 procedures (8%).
A Celiac arteriogram in a 60-year-old man shows the right inferior phrenic artery (arrow) arising from the proximate portion of the celiac artery. B Selective arteriogram of the right inferior phrenic artery using a catheter with a large side hole shows a tumor stain. C Spot radiograph obtained during TACE shows that the microcatheter is advanced into the right inferior phrenic artery through the side hole (arrow).
Blood supply from the left IPA to HCC was found in 22 patients (12%). The left IPA fed the tumor in the lateral segment of the liver in 20 patients, and it fed the tumor in the dome of the medial segment of the liver in 2 patients. Three left IPAs were reconstructed via the retroperitoneal anastomosis [19]. All but 1 patient with a tumor protruding to the diaphragm had previously undergone several TACE sessions for HCC (2–17 times, mean 7 times).
Thirty TACE procedures were attempted including repeated sessions and 28 TACEs were successful (93%). Two left IPAs were selected using the catheter with a large side hole. Two IPAs reconstructed through the dorsal pancreatic artery arising from the superior mesenteric artery could not be selected. Transient pleural effusion developed in 10 procedures (56%), and basal atelectasis was observed in 5 procedures (17%).
Cystic Artery
Blood supply from the cystic artery was found in 43 patients (24%) (Fig. 2). Almost all tumors fed by the cystic artery were located near the gallbladder bed, but 3 were located in the right lobe of the liver, while 1 was located in the medial segment of the liver distant from the gallbladder bed. Forty patients had previously undergone several TACE sessions (1–15 times, mean 4 times). In the remaining 3 patients with HCC protruding into the gallbladder bed, parasitization of the cystic artery was revealed at the initial angiography. A single branch was seen in 29 patients, and two branches were seen in 14 patients.
Sixty TACE procedures were attempted including repeated sessions and 42 attempts (70%) were successful. Extravasation due to perforation of the small branch of the cystic artery occurred in 2 procedures (3%) and gelatin sponge particles were injected to stop bleeding. Acute cholecystitis occurred after 1 procedure (2%) and was treated conservatively.
Omental Artery
Blood supply from the omental artery was found in 23 patients (13%) (Fig. 3). Four patients presented with active bleeding from the ruptured tumor fed by the omental artery. The tumor was located at the surface of the right lobe of the liver in 22 patients and at the lower edge of medial segment of the liver in one. All but 1 patient had previously undergone several TACE sessions (1–14 times, mean 6 times). In the remaining patient who had HCC seeded at the surface of the right lobe of the liver, blood supply from the omental artery was demonstrated at the initial TACE. A single omental artery was seen in 12 patients and two or more arteries were seen in 11 patients. Among them, 4 omental arteries were derived from the left gastroepiploic artery and 1 was derived from the dorsal pancreatic artery. The remaining arteries were derived from the right gastroepiploic artery.
A Celiac arteriogram in a 67-year-old man shows multiple tumor stain in the liver. The right hepatic artery is occluded by previous TACE procedures and a dilated omental artery is seen (arrow). B Selective arteriogram of the omental artery shows a tumor stain. C Selective arteriogram of the bile duct artery arising from the posterior superior pancreaticoduodenal artery also shows tumor stains. D Spot radiograph obtained during TACE of the bile duct artery.
Thirty-four TACE procedures were attempted including repeated sessions. TACE of these arteries was successfully performed in 25 procedures (74%) and bleeding was successfully stopped in all 4 patients. In 1 patient (3%) who had an omental artery derived from the left gastroepiploic artery, a small amount of iodized oil accumulation in the spleen was found on CT, but without symptoms.
Right Renal Capsular Artery and Right Adrenal Artery
Blood supply from the right RCA was found in 21 patients (12%) (Fig 4) and the blood supply from the right IAA was found in 13 patients (7%). Blood supply from the right MAA directly arising from the aorta was found in 7 patients (4%). All HCCs fed by these arteries were located in the posterior surface of the right lobe of the liver, in particular, near the right renal fossa. All but 2 patients had previously undergone several TACE sessions (2–11 times, mean 6 times). In the remaining 2 patients with a large HCC in the caudate lobe of the liver, the tumor was fed by both the right IPA and right RCA at the initial TACE. In 1 of them, the left gastric artery also supplied the tumor.
Forty-seven TACE procedures were attempted including repeated sessions and 46 TACE procedures were possible via the femoral approach. In the remaining patient with unsuccessful TACE of the right MAA and right IAA via the femoral artery, the procedure was successfully performed via the left brachial artery. The overall success rate of TACE for these arteries was 100%. Extravasation occurred during TACE of the right RCA in 1 procedure (4%) and it was stopped by injection of gelatin sponge particles. After another procedure (4%), a small amount of iodized oil accumulation in the upper pole of the right kidney, without symptoms, was observed because of backflow into the small renal branch. No other complications associated with the TACE procedure were observed in any patient.
Internal Mammary Artery
Blood supply from the right IMA was found in 15 patients (8%) and the blood supply from the left IMA was found in 1 patient (1%). All tumors supplied by the IMA were located at the surface of segments 3, 4, and 8, and all patients had previously undergone several TACE sessions (1–14 times, mean 6 times). Eleven patients had a history of TACE of the IPA.
Sixteen TACE procedures were attempted including repeated sessions, and were successful in all cases (100%). Skin necrosis in the subcostal region developed after one TACE procedure (6%) through the right IMA because of iodized oil backflow into small branches supplying the skin, and surgical repair was required. A small amount of left pleural effusion and basal atelectasis was observed after one procedure of the left IMA.
Intercostal Artery and Lumbar Artery
Blood supply from the right ICA was found in 14 patients (8%) (Fig. 5) and the blood supply from the right LA was found in 3 patients (2%). All patients had previously undergone several TACE sessions (2–17 times, mean 8 times) including TACE of the right IPA. A single artery supplied the tumor in 9 patients, and two or more arteries supplied it in 6 patients. All HCCs supplied by these arteries were located in the posterior or lateral surface of the right lobe of the liver. The collaterals arose at the T8 (n = 1), T9 (n = 4), T10 (n = 5), T11 (n = 3), T12 (n = 3), L1 (n = 1), and L2 (n = 1) levels.
TACE was attempted in 32 ICAs and 3 LAs including repeated sessions, and catheterization into the tumor-feeding branch was successfully performed in 17 ICAs (53%) and 3 LAs (100%); TACE using iodized oil and gelatin sponge particles was performed. In the remaining 15 right ICAs, TACE was performed with gelatin sponge particles alone because of unsuccessful catheterization into the tumor-feeding branch. No complications related to the TACE procedure were observed in any patient.
Miscellaneous Arteries
Blood supply from the left gastric artery (LGA) (Fig. 6), right gastric artery (RGA), bile duct artery (BDA) arising from the posterior superior pancreaticoduodenal artery (Fig. 3), and right colic or middle colic artery was found in 7 (4%), 5 (3%), 4 (2%), and 5 patients (3%), respectively. All patients but one had previously undergone several TACE sessions (1–15 times, mean 6 times). The remaining patient had a large tumor at the caudate lobe partially supplied by the LGA in addition to the right IPA and RCA. The RGA and LGA supplied the tumor in the lateral segment of the liver or caudate lobe of the liver, while the colic artery supplied the tumor in the inferior surface of the right lobe of the liver or medial segment of the liver. The BDA supplied the tumor in the caudate lobe or hepatic hilum. Two feeding branches were seen in 2 patients with collateral supply from the right colic artery (Fig. 7).
A Arteriogram of the left gastric artery in a 73-year-old woman shows a tumor feeding branch (arrow) and stain around the tumor in the left lobe of the liver that was previously treated. B The tumor-feeding branch of the left gastric artery was successfully selected. C Spot radiograph obtained during TACE.
TACE was attempted in 8 LGAs, 7 RGAs, 4 BDAs, and 6 colic arteries including repeated sessions, and it was possible in 5 (63%), 5 (71%), 3 (75%), and 4 (67%) procedures, respectively. In one TACE through the middle colic artery (13%), it was occluded by intimal injury, but this did not cause any symptom. Gastric ulcer developed after 1 (20%) of 5 successful TACE procedures through RGA and was treated by administration of an H2-blocker.
Discussion
In patients with HCC, various extrahepatic collateral vessels develop and supply the tumors [8–19]. This was observed in 25% of our patients, and almost all patients had previously undergone repeat TACE sessions; therefore, it was thought that the main cause of development of extrahepatic collaterals was attenuation of the hepatic arterial circulation by TACE. On the other hand, tumors protruding from the liver received the extrahepatic blood supply despite a patent hepatic artery. In a report by Chung et al. [9], right IPA parasitization was retrospectively suspected at the initial TACE in 80% of their patients, with blood supply to tumor from the right IPA demonstrated on a follow-up angiography. The incidence of right IPA parasitization in their report was extremely high compared with our study, but it may easily change according to each patient’s characteristics, in particular tumor location and size. Parasitization of arteries other than the right IPA at the initial angiography has also been reported. Hirota et al. [10] and Tanigawa et al. [11] reported a small HCC near the gallbladder fed by the cystic artery. Park et al. [12] reported two huge HCCs supplied by the right ICA at the initial angiography. In the present study, 11 patients (6%) with tumors located at the surface of the liver received the blood from extrahepatic collaterals including the right and left IPA, right RCA, LGA, omental artery, and cystic artery at the initial angiography.
The IPA, IMA, and ICA are known to communicate with branches of the hepatic arterial system through the diaphragm [9, 12–16, 19], and the IPA is the major source of diaphragmatic blood supply to the liver. The right RCA, MAA, and IAA run through the hepatorenal ligament and enter the liver. The branches of the right colic artery and middle colic artery may enter the liver through the right paracolic gutter [5], especially when adhesion between the liver and colon is present. The RGA and LGA anastomose with each other and enter the liver via the hepatoduodenal ligament [5]. The BDA also runs through this ligament. A deep branch of the cystic artery connects with the branch of a hepatic artery [11]. In addition, a small hepatic artery branches from the cystic artery and directly penetrates the liver through the gallbladder fossa [22]. The collaterals via the omental arteries probably enter the liver by direct adhesion of the omentum to the liver [5, 8].
In the present study, incidences of collateral source to HCC were 83% from the right IPA, 24% from the cystic artery, 13% from the omental artery, 12% from the right RCA and left IPA, 8% from the right IMA and right ICAs, and 7% from the right IAA. The right MAA, RGA or LGA, middle or right colic artery, BDA, and left IMA were also infrequent extrahepatic collateral pathways that were found in less than 4% of procedures. We estimate that the incidence of development of each collateral pathway depends on the size of the area that is attached to the liver in addition to the tumor location.
There was a close relationship between the tumor location and suspicious extrahepatic collaterals. Tumors located at the posterior surface of the right lobe and near the diaphragm were likely to be fed by the right IPA. The right ICA and LA also supplied them, especially when the IPA was attenuated by repeated TACE procedures. Blood supply from the right IMA was also seen when the tumor was located beneath the diaphragm or at the anterior chest wall. Tumors located near the right renal fossa were fed by the right RCA, MAA, and IAA. Tumors located at the anterior surface of the right lobe of the liver or at the lower edge of the medial segment of the liver were fed by the omental artery or colic artery. Tumors in the lateral segment of the liver were fed by the RGA or LGA in addition to the left IPA. The cystic artery mainly fed tumors located near the gallbladder fossa, but it infrequently supplied the tumor in the right lobe or medial segment of the liver at a distance from the gallbladder fossa when the hepatic artery was attenuated. Tumors arising in the caudate lobe tended to be fed by the right IPA, right RCA, and gastric artery. Several extrahepatic collaterals were connected to each other, and distribution of these vessels differed by individual.
Since a report by Soo et al. [13], embolization via extrahepatic collaterals has been reported as an alternative to achieve transcatheter management of hepatic tumors. TACE of extrahepatic collaterals is considered to be useful not only in control of the tumor but also in stopping hemorrhaging from the tumor [8, 17]. With advances in catheter and guidewire technology, it has become possible to introduce a microcatheter into not only small branches of the hepatic artery but also extrahepatic collaterals. In the present study, the successful TACE rates for each extrahepatic collateral ranged from 53% to 100%. Tumor-feeding branches arising from the ICA were the most difficult type of branch to catheterize, followed by tumor-feeding branches arising from the gastric, colic, and cystic arteries. These branches are usually of small caliber and branch at acute angles. Not only unsuccessful catheterization but also blockage of blood flow due to catheter insertion makes TACE of small feeding branches difficult. We introduced a 2 Fr tip microcatheter into TACE for HCC to facilitate catheterization into the small vessels and reduce the catheter’s mass effect. We believe that technical success rates of TACE through these small branches may improve with the introduction of thinner microcatheters. In addition, this 2 Fr tip microcatheter can be easily manipulated through a large side hole of the catheter [20]; therefore, we now use a 4 Fr catheter to create the large side hole.
When extrahepatic collaterals are embolized, there is a risk of nontarget branch embolization, which can lead to a variety of complications, depending on the location and magnitude of TACE. TACE of the IPA causes pleural effusion and basal atelectasis [23]. Paraplegia results from inadvertent embolization of spinal branches arising from ICA or LA [24]. Cutaneous complications occur as a result of embolization of vessels supplying the skin arising from the IMA, ICA, and LA [25]. We experienced skin necrosis in the subcostal region by TACE of the right IMA. Iodized oil containing anticancer drugs causes a cutaneous complication if it is injected into branches supplying the skin. Such embolic material should not be used in TACE of the IMA, ICA, or LA when the microcatheter is not advanced into the tumor-feeding branch. In cases with unsuccessful catheterization into the tumor-feeding branch, bland embolization without iodized oil may be a less risky alternative. Gallbladder infarction caused by inadvertent embolization of the cystic artery has also been reported [24, 26]. We experienced acute cholecystitis in one patient with HCC fed by the cystic artery. Ulcer formation occurs when branches supplying the alimentary tract are inadvertently embolized, as we experienced in one patient. In addition, we experienced arterial injury during catheter manipulation in 5 of 386 procedures (1%). In TACE of extrahepatic collaterals, embolic materials should be carefully injected so as not to reflux into nontarget branches nor flow excessively into the arteriovenous shunt, which is frequently seen on arteriography of the IPA [23]. In addition, the infused dosage of iodized oil and anticancer drugs should be properly reduced compared with TACE of the hepatic artery and complete blockage by gelatin sponge particles should be avoided.
There are some limitations to the present study. We analyzed the extrahepatic collaterals in consecutive patients, including patients with advanced-stage tumors. In patients with multiple tumors, it is sometimes difficult to mach exactly the tumor stains on DSA and the defects on CTAP; therefore, partial blood supply to HCC from the branches arising from extra-celiac and superior mesenteric arterial circulation tends to be easily missed. We also excluded the equivocal staining on angiograms of extrahepatic collaterals. In addition, in old cases with atherosclerosis, screening of small branches directly arising from the aorta, such as the right RCA and MAA, may be incomplete. For these reasons, the incidence of blood supply from extrahepatic collaterals to the tumor may be underestimated in this study. The unified CT and angiography system may useful for precise evaluation of extrahepatic collaterals [27–29]. However, the equivocal collateral supply to the tumor may not be worth treating because the incidence of procedure-related complications may increase. Arora et al. [25] advocated that the extrahepatic collateral vessel obviously supplying the tumor was probably the vessel worth treating.
Tumors fed by extrahepatic collaterals may have multiple feeding arteries because the extrahepatic collateral vessel connects with not only the hepatic artery but also other extrahepatic collaterals [8]. This weakens the therapeutic effect of TACE. However, TACE is considered to be the only therapeutic option, especially in patients with multiple tumors. We believe that TACE of extrahepatic collaterals is worth performing to prolong the prognosis for inoperable patients, although we did not analyze the improvement in prognosis by TACE of extrahepatic collaterals in the present study.
In conclusion, extrahepatic blood supply to HCC frequently develops, in particular in patients who receive repeat TACE sessions. The right IPA is the most common extrahepatic collateral, followed by the cystic artery and omental artery. TACE through extrahepatic collaterals is possible at high success rates, and is also relatively safe under careful observation. It is important that radiologists suspect the presence of an extrahepatic blood supply to HCC when the hepatic artery is injured and/or when the tumor protrudes from the liver, and seek them according to the tumor location.
References
Dodd GD III, Soulen MC, Kane RA, et al. (2000) Minimally invasive treatment of malignant hepatic tumors: At the threshold of a major breakthrough. Radiographics 20:9–27
Yamada Y, Sato M, Kawabata M, et al. (1983) Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology 148:397–401
Uchida H, Ohishi H, Matsuo N, et al. (1990) Transcatheter hepatic segmental arterial embolization using lipiodol mixed with an anticancer drug and Gelfoam particles for hepatocellular carcinoma. Cardiovasc Intervent Radiol 13:140–145
Matsui O, Kadoya M, Yoshikawa J, et al. (1993) Small hepatocellular carcinoma: Treatment with subsegmental transcatheter arterial embolization. Radiology 188:79–83
Charnsangavej C, Chuang VP, Wallace S, et al. (1982) Angiographic classification of hepatic arterial collaterals. Radiology 144:485–494
Michels NA (1953) Collateral arterial pathways to the liver after ligation of the hepatic artery and removal of the celiac axis. Cancer 6:708–724
Koehler RE, Korobkin M, Lewis F (1975) Arteriographic demonstration of collateral arterial supply to the liver after hepatic artery ligation. Radiology 117:49–54
Miyayama S, Matsui O, Akakura Y, et al. (2001) Hepatocellular carcinoma with blood supply from omental branches: Treatment with transcatheter arterial embolization. J Vasc Interv Radiol 12:1285–1290
Chung JW, Park JH, Han JK, et al. (1998) Transcatheter oily chemoembolization of the inferior phrenic artery in hepatocellular carcinoma: The safety and potential therapeutic role. J Vasc Interv Radiol 9:495–500
Hirota S, Matsumoto S, Fukuda T, et al. (1999) Solitary hepatocellular carcinoma fed by the cystic artery: Limitation of transcatheter arterial embolization. Cardiovasc Intervent Radiol 22:206–209
Tanigawa N, Sawada S, Okuda Y, et al. (1998) A case of small hepatocellular carcinoma supplied by the cystic artery. AJR Am J Roentgenol 170:675–676
Park S II, Lee DY, Won JY, et al. (2003) Extrahepatic collateral supply of hepatocellular carcinoma by the intercostal arteries. J Vasc Interv Radiol 14:461–468
Soo CS, Chuang VP, Wallace S, et al. (1983) Treatment of hepatic neoplasm through extrahepatic collaterals. Radiology 147:45–49
Kim JH, Chung JW, Han JK, et al. (1995) Transcatheter arterial embolization of the internal mammary artery in hepatocellular carcinoma. J Vasc Interv Radiol 6:71–77
Duprat G, Charnsangavej C, Wallace S, et al. (1988) Inferior phrenic artery embolization in the treatment of hepatic neoplasms. Acta Radiol 29:427–429
Nakai M, Sato M, Kawai N, et al. (2001) Hepatocellular carcinoma: Involvement of the internal mammary artery. Radiology 219:147–152
Kodama Y, Shimizu T, Endo H, et al. (2002) Spontaneous rupture of hepatocellular carcinoma supplied by the right renal capsular artery treated by transcatheter arterial embolization. Cardiovasc Intervent Radiol 25:137–140
Miyayama S, Matsui O, Nishida H, et al. (2003) Transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma fed by the cystic artery. J Vasc Interv Radiol 14:1155–1161
Miyayama S, Matsui O, Taki K, et al. (2004) Transcatheter arterial chemoembolization for hepatocellular carcinoma fed by the reconstructed inferior phrenic artery: Anatomical and technical analysis. J Vasc Interv Radiol15:815–823
Miyayama S, Matsui O, Akakura Y, et al. (2001) Use of a catheter with a large side hole for selective catheterization of the inferior phrenic artery. J Vasc Interv Radiol 12:497–499
Miyayama S, Matsui O, Taki K, et al. (2004) Combined use of an occlusion balloon catheter and a microcatheter for embolization of the unselectable right inferior phrenic artery supplying hepatocellular carcinoma. Cardiovasc Intervent Radiol 27:667–681
Komatsu T, Matsui O, Kadoya M, et al. (1999) Cystic artery origin of the segment V hepatic artery. Cardiovasc Intervent Radiol 22:165–167
Tajima T, Honda H, Kroiwa T, et al. (2002) Pulmonary complications after hepatic artery chemoembolization or infusion via the inferior phrenic artery for primary liver cancer. J Vasc Interv Radiol 13:893–900
Chung JW, Park JH, Han JK, et al. (1996) Hepatic tumors: Predisposing factors for complications of transcatheter oily chemoembolization. Radiology 198:33–40
Arora R, Soulen MC, Haskal ZJ (1999) Cutaneous complications of hepatic chemoembolization via extrahepatic collaterals. J Vasc Interv Radiol 10:1351–1356
Takayasu K, Moriyama N, Muramatsu Y, et al. (1985) Gallbladder infarction after hepatic artery embolization. AJR Am J Roentgenol 144:135–138
Takeuchi Y, Arai Y, Inaba Y, et al. (1998). Extrahepatic arterial supply to the liver: Observation with a unified CT and angiography system during temporary balloon occlusion of the proper hepatic artery. Radiology 209:121–128
Ishijima H, Koyama Y, Aoki J, et al. (1999) Use of a combined CT-angiography system for demonstration of correlative anatomy during embolotherapy for hepatocellular carcinoma. J Vasc Interv Radiol 10:811–815
Takayasu K, Muramatsu Y, Maeda T, et al. (2001) Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: Analysis of factors affecting local recurrence and survival rates. AJR Am J Roentgenol 176:681–688
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyayama, S., Matsui, O., Taki, K. et al. Extrahepatic Blood Supply to Hepatocellular Carcinoma: Angiographic Demonstration and Transcatheter Arterial Chemoembolization. Cardiovasc Intervent Radiol 29, 39–48 (2006). https://doi.org/10.1007/s00270-004-0287-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-004-0287-y