Abstract
Multidisciplinary treatment is recommended for the management of patients with advanced hepatocellular carcinoma (HCC). Some operative decollateralization of extrahepatic feeding arteries with laparotomy have been introduced for HCC. We herein newly develop laparoscopic devascularization (LDEV) to continue transarterial chemoembolization (TACE) for HCC with extrahepatic collateral arteries. A 74-year-old man with multiple huge HCC (4 tumors, 18 cm in diameter) and poor liver function (non-alcoholic steatohepatitis, Child–Pugh score 7) was treated with 6 times of chemoembolization in combination with LDEV, 3 times of ablation therapies, and lenvatinib therapy. His tumor markers were triple positive (AFP, 12,906.5 ng/ml; PIVKA-II, 491,743 mAU/ml; AFP-L3, 91.8%) before treatments; however, they all returned to normal limits. Complete response was achieved according to the modified RECIST criteria. Unfortunately, he died 6 months after the final treatment with no recurrence of HCC due to the postoperative complication of primary lung cancer. LDEV is a useful tool to continue effective TACE, and multidisciplinary treatment including chemoembolization and LDEV can cure advanced HCC patients with extrahepatic collaterals and impaired liver function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multidisciplinary treatment is recommended for the management of patients with advanced hepatocellular carcinoma (HCC) [1, 2]. Ablation therapy, transarterial chemoembolization (TACE), and molecular targeted therapy are recommended for unresectable HCC mainly according to tumor factors and liver functional factors. Currently, local ablation therapy consists of radiofrequency ablation (RFA) and microwave ablation (MWA) via percutaneous and laparoscopic approaches, which are well established [3,4,5]. Specifically, MWA using a cooling antenna has been developed to generate a large spherical ablation area in a short time [6]. As a first-line drug therapy for advanced HCC, lenvatinib has become available for use [7], and we have already reported a cured patient with a huge HCC that was converted to a resectable tumor with multidisciplinary treatment including lenvatinib [8]. TACE has spread worldwide as a standard treatment for intermediate HCC and can be applied to patients with large-sized and numerous HCC tumors and in those with impaired liver function [9,10,11]. Specifically, for large HCC, the long-term prognosis after TACE is poor [12, 13], and the development of extrahepatic collateral arteries is a serious problem that is encountered when TACE is continued [14, 15]. The main extrahepatic collateral arteries of HCC are the inferior phrenic, omental, and gastric arteries [16, 17]. Others include the gastroduodenal, superior mesenteric, internal mammary, renal, adrenal, and intercostal arteries. With advances in skills and devices used in the TACE procedure, TACE has been performed via collateral arteries and can provide good local control [18, 19]. Recently, TACE with drug-eluting beads (DEB-TACE) was used for large HCC to reduce symptoms and side effects related to TACE [20,21,22].
In the 1990s, the usefulness of so-called “wrap therapy” was reported: operative decollateralization of extrahepatic feeding arteries followed by wrapping of the liver with a silicone rubber sheet [23]. However, postoperative massive ascites often occurred, and thus, the procedure was never widely used. Recently, a unique procedure consisting of operative hepatic artery ligation and extrahepatic collaterals division (HALED) of the liver lobe containing a large HCC was reported [24]. According to the modified response evaluation criteria in solid tumors (mRECIST) [25], the complete response rate was 65%, major morbidity was 10%, and operative mortality was 5%. We have already reported the usefulness of laparoscopic devascularization (LDEV) for the continuation of conventional TACE [26]. LDEV has some advantages: (1) a laparoscopic approach enables the performance of additional TACE within a couple of days before recanalization of the feeding artery, (2) no wrap therapy is required, and (3) additional laparoscopic ablation and laparoscopic cholecystectomy are easily added.
Here, we report a patient with a large HCC and poor liver function who was successfully treated with a multidisciplinary approach consisting of conventional and DEB-TACE in combination with LDEV as well as percutaneous and laparoscopic ablation. He achieved a clinical complete response after the treatment series.
Case report
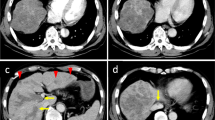
A 74-year-old man visited a primary care physician because of abdominal distension and anorexia. A computed tomography (CT) scan revealed a large tumor in the right liver, and he was subsequently referred to our hospital. He had a history of chronic atrial fibrillation, hypertension, dyslipidemia, and hyperuricemia. His body mass index was 26.8 kg/m2. He also had a history of alcohol assumption (20 g daily) and smoking (20 cigarettes daily) for 50 and 45 years, respectively. Laboratory data on admission are listed in Table 1. Hepatitis B virus surface antigen and hepatitis C virus antibody were both negative. He showed both liver and systemic inflammation, hyponutrition, and anemia. His Child–Pugh score was 7, and the indocyanine green retention rate at 15 min was 37.3%. The following liver fibrosis markers were elevated: the fibrosis-4 index (FIB-4) was 18.4, and the Mac-2-binding protein glycan isomer (M2BPGi) was 4.84. The levels of tumor markers were extremely high, and he was determined to be triple positive: alpha-fetoprotein (AFP) level was 12,906.5 (normal range ≤ 10) ng/ml, protein induced by vitamin K absence or antagonist-II (PIVKA-II) level was 491,743 (≤ 40) mAU/ml, and AFP-L3 fraction (AFP-L3) was 91.8% (≤ 10%). Contrast-enhanced CT on admission revealed a large hypervascular tumor 18 cm in diameter with central necrosis primarily in the anterior sector of the right liver and intrahepatic metastases in segments 4 and 7 (Fig. 1). He was diagnosed with HCC as a result of non-alcoholic steatohepatitis of the liver; the tumor was determined to be unresectable by a liver cancer multidisciplinary team at our institute.
Contrast-enhanced CT images on admission. CT images revealed a large low-density tumor in the right liver (a). Main tumor and 1st tumor in segment 7 (arrow) showed heterogeneous peripheral enhancement (b) and 2nd tumor in segment 7 (c) and tumor in segment 4 (d) (arrow heads) showed homogeneous enhancement in arterial phase. Main tumor and 1st tumor in segment 7 (arrow) revealed delayed washout in the equilibrium phase (e). Inferior vena caca was stretched by main tumor in portal phase in coronal image (f)
Initial DEB-TACE was divided into two procedures with a 2 week interval. A total dose of 80 mg of epirubicin containing microspheres (300–500 µm in diameter) was used in each procedure. The 3rd TACE was achieved with 30 mg of epirubicin containing microspheres (300–500 µm in diameter) for the main tumor in the right liver in combination with epirubicin (30 mg)-lipiodol emulsion for a satellite nodule in segment 4. After a month, LDEV was performed for the main tumor in the right liver, whereas laparoscopic RFA was performed for the surface of the main tumor and the viable HCC in segment 4. Staining of the large tumor and multiple extrahepatic collateral arteries of the HCC from the right gastroepiploic artery were observed on digital subtraction angiography before LDEV (Fig. 2a). After LDEV, extrahepatic collateral arteries of the HCC had disappeared (Fig. 2b). Intraoperative findings of LDEV are shown in Fig. 3. Two days after LDEV, a 4th TACE was performed as a conventional TACE (cTACE) with 35 mg of cisplatin suspended in lipiodol (CSL) plus gelatin sponge for residual tumor in the right liver [27]. Lipiodol accumulated substantially in most of the residual primary tumor. The other S7 HCC was suspected to feed from the right inferior phrenic artery, but selective cannulation was impossible. MWA using a cooling antenna [6] was then performed for the remnant 5 cm diameter HCC in segment 7. The 5th TACE with CSL (48 mg) plus gelatin sponge was performed for bilateral multiple small HCCs. Lenvatinib therapy was initiated and continued for 35 days (total dose: 280 mg). Six months after the 5th TACE, laparoscopic MWA was performed for suspicious residual tumor in the right liver, as assessed by intraoperative Sonazoid™-enhanced ultrasound. The 6th TACE was performed with CSL (50 mg) plus a gelatin sponge for a newly developed HCC in segment 3. Then, all tumors in the right liver, segment 4, and segment 3 were extremely decrease in size and were determined to exhibit a complete response according to the mRECIST criteria. Serial diagnostic images during multidisciplinary treatment were summarized (Fig. 4). Details of treatment and changes in the tumor marker levels are shown in Fig. 5. The levels of three tumor markers were within the normal range for 6 months.
Intraoperative findings of laparoscopic devascularization. Greater omentum and collateral arteries are seen in the laparoscopic view (a). The omentum near the surface of the tumor was cut with an energy device, and the feeding artery was ligated with clips (b). The tumor surface was recognized (c). Laparoscopic radiofrequency ablation was performed, and the tumor surface was well ablated (d)
Treatments and changes in the tumor marker level. TACE transarterial chemoembolization, LDEV laparoscopic devascularization, LRFA laparoscopic radiofrequency ablation, PMWA percutaneous microwave ablation, LMWA laparoscopic microwave ablation, PIVKA-II protein induced by vitamin K absence or antagonist-II, AFP alpha-fetoprotein
Unfortunately, the patient died of bronchial fistula after resection of the right upper lobe of the lung for primary lung cancer, although he did not experience recurrence of HCC for 18 months after the initial TACE.
Discussion
Here, we report a patient with bilateral large HCC and impaired liver function, who showed a complete response after multidisciplinary treatment. His tumor was determined to be unresectable, and he received three-staged DEB-TACE procedures. Targeted therapy for large HCC could cause tumor lysis syndrome, and thus, its initial use was avoided [28]. DEB-TACE was selected, because it is associated with fewer symptoms and side effects when used in large tumors [20,21,22]. Tumor lysis syndrome can occur even after DEB-TACE, and so we elected to perform staged DEB-TACE [29]. As multiple extrahepatic collateral arteries had developed in our patient, we thought that it was difficult to continue TACE via intra- and extra-hepatic feeding arteries. Further systemic therapy was not recommended for patients classified in Child–Pugh B [7]. TACE was an only way to continue multidisciplinary treatment; therefore, we decided to perform LDEV for dissection of extrahepatic collateral arteries [26]. We had previously reported 13 HCC patients treated with LDEV [26]. For all patients, we were able to perform effective additional TACE and/or hepatic arterial chemotherapy without no serious complication. A 4th TACE was immediately performed on postoperative day 2 and was quite effective, because extrahepatic feeders were completely dissected. cTACE was selected to achieve complete necrosis of the residual tumor [29]. cTACE results in a long-term prognosis equivalent to that of DEB-TACE, and its advantage is its ability to embolize arterioportal communication [20,21,22, 27].
Previously, “wrap therapy” was introduced with the concept of devascularization of collateral arteries by an open approach followed by TACE and provided a good tumor response. However, wrapping of the liver caused massive ascites and delayed the start of additional TACE [23]. LDEV was developed by our group as a less invasive procedure to enhance the effect of and to continue TACE [26]. The laparoscopic approach in combination with ablation of the tumor surface no longer requires wrapping and can allow for earlier TACE. Moreover, additional TACE can be performed within a few postoperative days. Under general anesthesia, the dissection of extrahepatic collateral arteries and performance of additional procedures only require a few hours. Laparoscopic ablation of the tumor on the liver surface can prevent recanalization of feeding arteries. If the tumor is too large, complete ablation of the entire tumor is usually difficult. Laparoscopic cholecystectomy can dissect the feeding arteries via the cystic artery and can prevent acute cholecystitis after TACE.
Recently, a new treatment strategy for large HCC termed HALED was introduced [24]. This concept consisted of hepatic artery ligation and dissection of extrahepatic collateral arteries by open surgery. The efficacy and safety of HALED have been reported; however, hepatic artery ligation can interfere with additional hepatic arterial therapy. Actually, almost all patients were treated with percutaneous ethanol injection or targeted therapy by an oncologist. I believe that preservation of the hepatic artery is essential. TACE via the extrahepatic collateral arteries is a good option; however, complete embolization is difficult for patients with multiple extrahepatic feeders or multiple-site feeders. Recanalization of collateral arteries or the development of new collateral arteries can occur in this manner, but in contrast, LDEV can avoid such phenomena. Embolization of extrahepatic feeders can result in the ischemic injury of adjacent digestive organs including the omentum, stomach, and small intestine [16, 17]. LDEV can also be completed with minimal damage to adjacent organs.
In our patient, MWA with a cooling antenna [6] was performed for the other HCC in segment 7. The tumor (approximately 5 cm) was too large for conventional RFA, but a complete response was achieved by a single puncture using the percutaneous approach. Then, the 5th and 6th conventional TACE procedures were performed, which were accompanied by lenvatinib therapy. Lenvatinib has been reported to regulate tumor vessels and enhance the antitumor effect of TACE [7, 8].
HCC with triple-positive tumor markers is known to be highly malignant and is frequently a poorly differentiated type with vessel invasion [30]. Triple-positive tumor markers is a significant poor prognostic factor after both liver resection and ablation therapy [30]. Our patient had some highly malignant features (extra-large bilateral multiple HCCs and triple-positive tumor markers) and poor liver function. However, all tumor markers returned to normal limits, and a complete response was achieved according to the mRECIST criteria. Liver function did not deteriorate during the treatment period. His general condition had been good without recurrence or re-elevation of tumor markers for 6 months after the final HCC treatment until his death from primary lung cancer resection.
In conclusion, LDEV is a useful tool that can be used for the continuation of effective TACE, and multidisciplinary treatment consisting of TACE with LDEV, ablation, and targeted therapy can be expected to cure unresectable large HCC in patients with poor liver function.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- TACE:
-
Transarterial chemoembolization
- RFA:
-
Radiofrequency ablation
- MWA:
-
Microwave ablation
- DEB-TACE:
-
TACE with drug-eluting beads
- HALED:
-
Hepatic artery ligation and extrahepatic collaterals division
- mRECIST:
-
Modified response evaluation criteria in solid tumors
- LDEV:
-
Laparoscopic devascularization
- CT:
-
Computed tomography
- FIB-4:
-
Fibrosis-4 index
- M2BPGi:
-
Mac-2-binding protein glycan isomer
- AFP:
-
Alpha-fetoprotein
- PIVKA-II:
-
Protein induced by vitamin K absence or antagonist-II
- CSL:
-
Cisplatin suspended in lipiodol
References
Naugler WE, Alsina AE, Frenette CT, et al. Building the multidisciplinary team for management of patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2015;13:827–35.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14.
Doi K, Beppu T, Ishiko T, et al. Endoscopic radiofrequency ablation in elderly patients with hepatocellular carcinoma. Anticancer Res. 2015;35:3033–40.
Huo YR, Eslick GD. Microwave ablation compared to radiofrequency ablation for hepatic lesions: a meta-analysis. J Vasc Interv Radiol. 2015;26(1139–46):e2.
Vigano L, Laurenzi A, Solbiati L, et al. Open liver resection, laparoscopic liver resection, and percutaneous thermal ablation for patients with solitary small hepatocellular carcinoma (</=30 mm): review of the literature and proposal for a therapeutic strategy. Dig Surg. 2018;35:359–71.
Takahashi H, Kahramangil B, Berber E. Local recurrence after microwave thermosphere ablation of malignant liver tumors: results of a surgical series. Surgery. 2018;163:709–13.
Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73.
Sato N, Beppu T, Kinoshita K, et al. Conversion hepatectomy for huge hepatocellular carcinoma with arterioportal shunt after chemoembolization and lenvatinib therapy. Anticancer Res. 2019;39:5695–701.
Bruix J, Llovet JM, Castells A, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578–83.
Forner A, Llovet JM, Bruix J. Chemoembolization for intermediate HCC: is there proof of survival benefit? J Hepatol. 2012;56:984–6.
Song JE, Kim DY. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol. 2017;9:808–14.
Prajapati HJ, Kim HS. Treatment algorithm based on the multivariate survival analyses in patients with advanced hepatocellular carcinoma treated with trans-arterial chemoembolization. PLoS ONE. 2017;12:e0170750.
Xue T, Le F, Chen R, et al. Transarterial chemoembolization for huge hepatocellular carcinoma with diameter over ten centimeters: a large cohort study. Med Oncol. 2015;32:64.
Zhao Y, Fang Z, Luo J, et al. Evaluation of extrahepatic collateral arteries in hepatocellular carcinoma in three independent groups in a single center. Exp Ther Med. 2015;10:2366–74.
Ibukuro K, Fukuda H, Tobe K, et al. The vascular anatomy of the ligaments of the liver: gross anatomy, imaging and clinical applications. Br J Radiol. 2016;89:20150925.
Wang YL, Li MH, Cheng YS, et al. Influential factors and formation of extrahepatic collateral artery in unresectable hepatocellular carcinoma. World J Gastroenterol. 2005;11:2637–42.
Moustafa AS, Abdel Aal AK, Ertel N, et al. Chemoembolization of hepatocellular carcinoma with extrahepatic collateral blood supply: anatomic and technical considerations. Radiographics. 2017;37:963–77.
Huang Y, Jia Z, Tu J, et al. Supplemental conventional transarterial embolization/chemoembolization therapy via extrahepatic arteries for hepatocellular carcinoma. J Cancer Res Ther. 2017;13:720–4.
Lokken RP, Fidelman N, Kolli KP, et al. Safety and efficacy of doxorubicin drug-eluting embolic chemoembolization of hepatocellular carcinoma supplied by extrahepatic collateral arteries. J Vasc Interv Radiol. 2016;27:1698–704.
Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Interv Radiol. 2010;33:41–52.
Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255–64.
Facciorusso A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: current state of the art. World J Gastroenterol. 2018;24:161–9.
Sasaki Y, Imaoka S, Shibata T, et al. Decollateralization with silicone rubber sheeting for advanced hepatocellular carcinoma: a preliminary report. Surgery. 1990;108:840–6.
Elsanousi OM, Mohamed MA, Salim FH, et al. Selective devascularization treatment for large hepatocellular carcinoma: stage 2A IDEAL prospective case series. Int J Surg. 2019;68:134–41.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Beppu T, Doi K, Matsuda T, et al. A promising new procedure: laparoscopic devascularization for hepatocellular carcinoma with extra-hepatic feeding arteries. Gan To Kagaku Ryoho. 2002;29:2247–51.
Beppu T, Ohara C, Yamaguchi Y, et al. A new approach to chemoembolization for unresectable hepatocellular carcinoma using aclarubicin microspheres in combination with cisplatin suspended in iodized oil. Cancer. 1991;68:2555–60.
Huang WS, Yang CH. Sorafenib induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. 2009;15:4464–6.
Katiman D, Manikam J, Goh KL, et al. Tumour lysis syndrome: a rare complication of trans-arterial chemo-embolisation with doxorubicin beads for hepatocellular carcinoma. J Gastrointest Cancer. 2012;43(Suppl 1):S187–90.
Beppu T, Nakagawa S, Nitta H, et al. The number of positive tumor marker status is beneficial for the selection of therapeutic modalities in patients with hepatocellular carcinoma. J Clin Transl Hepatol. 2017;5:165–8.
Author information
Authors and Affiliations
Contributions
KY and TB designed and drafted the manuscript. TB, NS, KK and SA performed the operation. TB, HY, TM, HM and TO contributed to the treatment protocol. HY and EO assisted in the preparation of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamamura, K., Beppu, T., Sato, N. et al. Huge hepatocellular carcinoma with extrahepatic collateral arteries successfully treated by multidisciplinary treatment including laparoscopic devascularization: a case report. Clin J Gastroenterol 14, 251–257 (2021). https://doi.org/10.1007/s12328-020-01286-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-020-01286-2