Abstract
This experimental study was undertaken to compare radiofrequency (RF) ablation zones created by internally cooled (IC) and internally cooled wet (ICW) electrodes. IC and ICW electrodes with a 2-cm exposed active tip were used to induce 30 ablation zones in 10 explanted bovine livers with a 12-min ablation time, respectively. In addition, two kinds of electrodes produced 16 ablation zones in five living porcine livers, respectively. In explanted bovine livers using IC and ICW electrodes, the mean long-axis diameter, short-axis diameter, volume, and variable coefficient of long-axis diameters of the ablation zones were 3.01 cm, 2.62 cm, 11.08 cm3, 10%, 5.28 cm, 5.07 cm, 73.48 cm3, and 14%, respectively. In living porcine livers using IC and ICW electrodes, the corresponding values were 2.62 cm, 2.00 cm, 5.76 cm3, 15%, 3.84 cm, 2.89 cm, 18.50 cm3, and 25%, respectively. In both ex vivo and in vivo livers, long-axis diameters, short-axis diameters, volumes, and variable coefficients for the use of ICW electrodes were significantly greater than for the use of IC electrodes (each p < 0.05). ICW electrodes produced significantly larger ablation zones than IC electrodes in both ex vivo and in vivo livers, but the ablation zones induced by IC electrodes were more reproducible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiofrequency (RF) ablation has been accepted as a safe and effective technique for the treatment of unresectable hepatic tumors [1–3]. Many experimental and clinical studies have been undertaken in attempts to enlarge ablation zones [4–15]. These studies have included the use of internally cooled (IC), saline solution-enhanced, bipolar, expandable, and clustered electrodes [4–7, 11]. Use of an IC electrode was introduced to increase the volumes of RF ablation zones by the circulation of saline solution at temperatures of 2–5°C in two coaxial lumens situated in the electrode, which prevented carbonization around the active tip and maintained low impedance during treatment [12, 15]. The perfusion electrode was developed to increase the volume of the RF ablation zone by the infusion of saline solution into the tumor through side holes in its cannulated electrode before or during RF ablation [6, 9, 10].
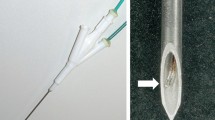
An IC perfusion (ICP) electrode was developed for RF ablation that allows simultaneous internal cooling and interstitial saline solution infusion by modification of an IC electrode [6–9, 13]. The conventional ICP electrodes have disadvantages, as the ablation zones are irregular in shape and the volumes are often difficult to predict due to uncontrolled diffusion of saline solution into the tissue. An experimental study found that RF ablation with the perfusion or conventional ICP electrode was accompanied by more severe surrounding parenchymal changes than RF ablation with an IC electrode [6]. Referring to previous reports on the drawbacks of the use of perfusion electrodes [9, 13], Ni et al. [14] first developed an IC wet (ICW) electrode with two coaxial lumens that enabled the circulation of cooling water and saline interstitial infusion through the side holes. We have selected a simpler design than the original ICW electrode. Our ICW electrode has only one lumen with two micro holes on the active tip (Fig. 1). The purpose of this study was to compare the volume, shape, and reproducibility of ablation zones after RF ablation in ex vivo bovine and in vivo porcine livers with the use of IC electrodes and ICW electrodes.
Materials and Methods
Radiofrequency Systems
The IC RF system is a 480-kHz generator (CC1; Valleylab, Burlington, MA, USA) capable of producing a maximum power of 200 W through a 17-gauge IC electrode with a 2- to 3-cm active distal part in a single device. Circuitry incorporated in the generator allows continuous monitoring of impedance between the active parts of the IC electrodes and the grounding pads. A thermocouple embedded in the electrode ensures constant monitoring of the temperature at the tip of the electrode. In our experiments, a peristaltic pump (RFP-200, variable-speed pump; RF Medical, Seoul, Korea) cooled the electrode internally by delivering chilled saline (2–5°C) in its electrode at a flow rate that was sufficient to maintain an electrode temperature below 20°C. RF power was emitted over 12 min per electrode insertion, with the generator set to deliver the maximum power in the impedance control mode [5].
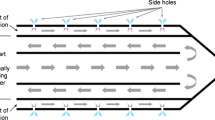
The ICW RF system uses the same generator and peristaltic pump as the IC electrode. A 17-gauge ICW electrode has two microholes (0.03 mm in diameter) at the exposure area so that 99% of the chilled 0.9% isotonic saline is used for cooling and 1% of the saline is used for infusion to the tissue (Fig. 1). Micro holes were made using a helium LASER with CO2 gas. An ICW electrode would be expected to make a much larger ablation zone than the usual IC electrode while creating a more regular ablation shape than a perfusion electrode. The use of an ICW electrode requires a precise hole-making process to provide constant spillage of the smallest amount of saline. In the electrode with a 2-cm active tip, microholes are located 7 and 9 mm from the distal end in the opposite side. The tissue infusion rate was 1 ml/min using this system. In the electrode with a 3-cm active tip, microholes were located 7 and 18 mm from the distal end in the opposite side. The tissue infusion rate was 1.2 ml/min. This ICW electrode system was developed in many pilot experimental studies.
Study Design
A series of RF ablation zones was induced in explanted bovine livers and in vivo in porcine livers. The IC electrodes and ICW electrodes with 2- and 3-cm exposed active tips were used to induce 30 ablation zones in 10 explanted bovine livers with a 12-min ablation time at room temperature (20°C), respectively. A total of 120 (4 electrodes ×30) RF ablation zones were induced. The electrodes were inserted at least 7 cm inside the liver parenchyma, and one ground pad was placed more than 20 cm from the distal tip of the electrode.
For the in vivo study, ten 1-year-old Yorkshire pigs were used. The pigs had free access to food and water before the experiments. All experiments were performed under clean conditions at our animal research center facility. The institutional animal care and use committee at our institution approved all of the experiments and surgical procedures.
All pigs were initially anesthetized with an intramuscular injection of 20 mg/kg body weight ketamine hydrochloride (Ketara; Yuhan, Seoul, Korea) and 2 mg/kg body weight xylazine (Rompun; Bayer Korea, Ansan, Korea). A 22-gauge ×1-in. intravenous catheter was inserted into the dorsal auricular vein. The pigs were intubated and anesthesia was maintained with inhaled enflurane gas (Gerolan solution; Choongwae Pharma, Seoul, Korea). We placed the pigs in the supine position after adequate anesthesia was achieved. Blood pressure, respiration, pulse, and ECG were monitored continuously. Both thighs of each pig were shaved for placement of a grounding pad, and the epigastric area was sterilized after being shaved. A median laparotomy was performed as a laparotomy allowed more accurate placement of the electrodes under sonographic guidance. Modification of blood flow to the liver was not attempted. Neither electrode-track ablation nor capsule cauterization was performed. A total of 32 ablation zones in 10 pigs were made with IC and ICW electrodes with 2-cm active tips, 16 ablation zones each. Three or four ablation zones were made in the liver of each pig. We carefully evaluated the ablation procedures by sonography (1- to 4-MHz convex probe; Acuson Sequoia 512; Siemens, Mountain View, CA, USA) to avoid large vessels and to prevent overlapping of the ablation zones.
Immediately after the experiments in eight pigs, the animals were sacrificed and the livers were explanted. The remaining two pigs, which had RF ablation procedures using ICW electrodes, were sacrificed immediately after CT examinations. Specimens were cut along the electrode axis and were macroscopically evaluated by measuring the two longest dimensions with calipers—one dimension along the electrode axis (D1; long-axis diameter) and the other dimension perpendicular to the electrode axis (D2; short-axis diameter). The calculated volume of the ablation zones was evaluated by approximating the volume to a sphere using the following formula: π(D1 × D2 × D2)/6. Although two diameters perpendicular to the electrode axis were not measurable for technical reasons, we used twice only one diameter measured perpendicular to the electrode axis, as the third diameter should have a similar measurement. In addition, we calculated the variable coefficient as the standard deviation of the long-axis diameters divided by the mean long-axis diameters [16]. This represents the reproducibility of the ablation zones of the long-axis diameters. Macroscopic changes in specimens have been reported to correlate well with coagulation necrosis as seen after a histopathological examination [6, 17]. Histopathological examinations were not performed.
CT Examinations
Within 1 h after ablation using ICW electrodes, two pigs underwent CT examinations using a 64-channel multidetector-row CT (MDCT) scanner (LightSpeed VCT; GE Healthcare, Waukesha, WI, USA) with multiplanar reformation. After initial unenhanced images of the liver were obtained, 2 mL/kg of iopromide (Ultravist 300; Bayer HealthCare, Berlin, Germany) was infused. The following CT parameters were used: 150–200 mAs, 120 kV, 2.5-mm collimation, table speed of 65.63 mm/s, and pitch of 0.984. Images were acquired with precontrast and dynamic scans with an 8-s interval up to 3 min. After obtaining CT images, the pigs were sacrificed and livers were explanted. The size and shape of the ablation zones in gross specimens were correlated with the nonenhancing ablation zones as depicted on CT scans.
Statistical Analysis
The long-axis diameter, short-axis diameter, and calculated volumes for the RF ablation zones were recorded and are reported as the mean ± standard deviation. The diameters, calculated volumes, and ratios of long-axis to short-axis diameters of the ablation zones obtained with each system were compared using Student’s t-test. The variable coefficients of the long-axis diameters of ablation zones induced by the same systems were compared using the F-test for variance.
Results
Ex Vivo Studies
Thirty ablation zones were induced using each electrode. For ablation zones induced with the IC electrode, long-axis diameters ranged from 2.4 to 3.5 cm (mean, 3.01 cm) and 3.5 to 4.3 cm (mean, 3.94 cm) for 2- and 3-cm active tips, respectively. Short-axis diameters ranged from 2.0 to 3.4 cm (mean, 2.62 cm) and from 2.9 to 3.7 cm (mean, 3.25 cm) for 2- and 3-cm active tips, respectively. For ablation zones induced with the ICW electrode, the long-axis diameters ranged from 4.3 to 7.0 cm (mean, 5.28 cm) and from 4.8 to 7.0 cm (mean, 5.71 cm) for 2- and 3-cm active tips, respectively. Short-axis diameters ranged from 4.2 to 6.6 cm (mean, 5.07 cm) and from 4.8 to 7.3 cm (mean, 5.66 cm) for 2- and 3-cm active tips, respectively. Mean diameters of the long-axis and short-axis, calculated volumes, and D1/D2 ratio of the ablation zones induced with each system are reported in Table 1. The long-axis diameter, short-axis diameter, and calculated volume of the ablation zones were significantly larger for the ICW electrode than for the IC electrode (each p < 0.001). Variable coefficients of the long-axis diameters were 10% and 14% for the 2-cm-active-tip and 5% and 12% for the 3-cm-active-tip for the IC electrode and the ICW electrode, respectively. The variances of the long-axis diameters for 2- and 3-cm active tips were significantly larger for the ICW electrode than for the IC electrode (F-test, each p < 0.001). The D1/D2 ratios were significantly higher for the IC electrode than for the ICW electrode for 2-cm and 3-cm active tips (each p < 0.001).
In Vivo Studies
The 10 pigs tolerated the procedures well, with no major variation in blood pressure. A total of 16 ablation zones were induced in livers with the IC and ICW electrodes, respectively. For ablation zones induced with the IC electrode, long-axis and short-axis diameters ranged from 2.0 to 3.3 cm (mean, 2.62 cm) and from 1.6 to 3.0 cm (mean, 2.00 cm), respectively (Table 1, Fig. 2). For ablation zones induced with the ICW electrode, long-axis and short-axis diameters ranged from 2.2 to 6.0 cm (mean, 3.84 cm) and from 1.8 to 4.1 cm (mean, 2.89 cm), respectively. Mean volumes of the IC electrodes and ICW electrodes were 5.76 and 18.50 cm3, respectively. A 3.2-fold larger volume was ablated using the ICW electrode than using the IC electrode, with a statistically significant difference (p < 0.001). Variances of the long-axis diameters were statistically different (p = 0.015), and the variable coefficients were 15% and 25% for the IC electrode and ICW electrode, respectively. The D1/D2 ratios were 1.33 ± 0.21 for the IC electrode and 1.32 ± 0.21 for the ICW electrode, and did not show a significant difference (p = 0.988). Postcontrast CT scans obtained in the two pigs with RF ablation using ICW electrodes showed the same size and shape of six ablation zones with gross specimens (Fig. 3).
The graph shows the sizes of ablation zones induced by RF ablation with internally cooled (IC; filled diamond) and with IC wet (ICW; x) electrodes. Long-axis diameters are given on the x-axis, and short-axis diameters are located on the y-axis. Note the linear correlation of the two axes and significant difference between IC and ICW electrodes
A gross specimen and a CT image of ablation zones induced using the internally cooled wet (ICW) electrode. A Photograph of a gross specimen with an ablation zone induced by an ICW electrode with a 2-cm active tip in a living porcine liver. B A post-contrast CT scan with reformation along the course of the electrodes showing three nonenhancing ablation zones (arrows). Note the same size and shape of the ablation zone (middle) as for the gross specimen (A)
Discussion
RF ablation has shown excellent local tumor control rates and minimal morbidity in the treatment for small hepatic tumors [1–3, 18]. Successful tumor ablation is achieved when the tumor is completely destroyed by heat. The ideal tool for this purpose would create, during one session, an area of destruction 0.5 to 1 cm larger than the index tumor in a manner akin to surgical margins [1]. Based on a 3-cm thermal injury induced by each RF application, Dodd et al. [1] reported that tumors <2 cm can be treated during 1 RF application, tumors that range from 2 to 3 cm require 6 overlapping ablations, and tumors >3 cm require at least 12 overlapping ablations. However, the technical difficulty of accurate placement of electrodes is a challenge in clinical practice, and operators are prone to make placement errors in multiple positioning of the electrode. If a larger ablation zone can be induced during a single procedure, the number of sessions required to ablate a large tumor can be reduced. Consequently, the maximal size of the ablation zone induced by a single procedure is very important unless combination therapy with chemoembolization is adopted [19].
Previously developed “perfusion” and “cooled” electrodes represent effective but suboptimal solutions for enhancing RF ablation efficacy. Regarding the “perfusion” function, interstitial saline infusion increases the size of the ablation zone in two ways—by improving both the electrical and the thermal conductivity of the tissue. With properly high tissue conductivity, impedance can be lowered, and resistive heating near the electrode is decreased. Consequently, energy delivery is increased. Regarding the “cooled” function, lowering the tissue temperature around the electrode may prevent charring at the electrode-tissue interface. Without charring, proper low impedance can be maintained throughout the procedure and can improve energy delivery [13, 14, 20, 21].
Conventional ICP electrodes induced a larger ablation zone than IC electrodes but the shape was irregular and unpredictable because of uncontrolled diffusion of the saline solution [6]. In addition, the conventional ICP electrodes with an outer sheath and relatively larger holes showed the intrinsic drawbacks that they have a larger diameter (15-gauge) and the cooling effect could be decreased because of perfusion of the saline solution at a slow circulation rate using a syringe pump [7–9, 11]. In contrast, our ICW electrode obtained a better cooling effect as well as perfusion effect because two microholes maintained the controlled infusion despite the fast circulation rate (100–120 ml/min) of the chilled saline solution in the coaxial lumens situated in the electrode using a peristaltic pump.
In our study, the ICW electrode clearly induced significantly larger ablation zones than the IC electrode in all experiments. This difference can probably be attributed to delivery of greater power throughout the procedure due to low impedance caused by spilled saline around the electrode. Ablation zones induced by the IC electrode were significantly more reproducible in size than ablation zones induced by the ICW electrode. The possible causes of the low reproducibility using the ICW electrode can be considered in two ways. First, the distribution of spilled saline may not be uniform in a different surrounding tissue. Second, in some instances, a fragment of ablated tissue could obliterate the microholes and spillage of saline could be stopped. At that time, the ICW electrode acts as a usual IC electrode where the size of the ablation zone is the same as for an IC electrode.
RF ablation zones obtained with the IC electrode and ICW electrode were equally spherical for in vivo livers (1.33 ± 0.21 and 1.32 ± 0.21; p = 0.988) but were more elliptical than RF ablation zones obtained for ex vivo livers. However, the ICW electrodes were more spherical than the IC electrodes for ex vivo livers (p < 0.001). The lack of blood flow could help the sphericity of the ablation zone with the spilled saline of ICW electrode for ex vivo livers.
An in vivo experiment for the electrodes with a 3-cm active tip could not be performed for technical reasons. The porcine liver for experiments is smaller than the human liver. In addition, the deep fissures divide the main portion of the porcine liver into four lobes, which are too thin to locate electrodes with a 3-cm active tip. As the electrode with a 3-cm active tip is the most commonly used electrode in human subjects, the lack of an in vivo experiment with this electrode is one of the limitations of our study.
In conclusion, ICW electrodes produced significantly larger ablation zones than IC electrodes, but the ablation zones induced by IC electrodes were more reproducible. The ablation zones induced by ICW electrodes were more spherical (ex vivo) than those induced by IC electrodes or equally spherical (in vivo). The larger size of the ablation zones produced by the ICW electrodes may be attributed to the lower impedance resulting from a smaller amount of spilled saline.
References
Dodd GD III, Soulen MC, Kane RA et al (2000) Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics 20:9–27
Tateishi R, Shiina S, Teratani T et al (2005) Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 103:1201–1209
Choi D, Lim HK, Rhim H, Kim YS et al (2007) Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol 17:684–692
de Baere T, Denys A, Wood BJ et al (2001) Radiofrequency liver ablation: experimental comparative study of water-cooled versus expandable systems. AJR 176:187–192
Goldberg SN, Stein MC, Gazelle GS, Sheiman RG, Kruskal JB, Clouse ME (1999) Percutaneous radiofrequency tissue ablation: optimization of pulsed-radiofrequency technique to increase coagulation necrosis. J Vasc Interv Radiol 10:907–916
Kim SK, Gu MS, Hong HP, Choi D, Chae SW (2007) CT findings after radiofrequency ablation in rabbit livers: comparison of internally cooled electrodes, perfusion electrodes, and internally cooled perfusion electrodes. J Vasc Interv Radiol 18:1417–1427
Lee JM, Han JK, Kim SH et al (2005) Bipolar radiofrequency ablation using wet-cooled electrodes: an in vitro experimental study in bovine liver. AJR 184:391–397
Lee JM, Han JK, Kim SH et al (2005) Radiofrequency ablation in the liver using two cooled-wet electrodes in the bipolar mode. Eur Radiol 15:2163–2170
Lee JM, Han JK, Kim SH et al (2004) Optimization of wet radiofrequency ablation using a perfused-cooled electrode: a comparative study in ex vivo bovine livers. Korean J Radiol 5:250–257
Lee JM, Han JK et al (2004) Comparison of wet radiofrequency ablation with dry radiofrequency ablation and radiofrequency ablation using hypertonic saline preinjection: ex vivo bovine liver. Korean J Radiol 5:258–265
Lee JM, Han JK, Kim SH, Sohn KL, Choi SH, Choi BI (2005) Bipolar radiofrequency ablation in ex vivo bovine liver with the open-perfused system versus the cooled-wet system. Eur Radiol 15:759–764
Lorentzen T (1996) A cooled needle electrode for radiofrequency tissue ablation: thermodynamic aspects of improved performance compared with conventional needle design. Acad Radiol 3:556–563
Miao Y, Ni Y, Yu J, Marchal G (2000) A comparative study on validation of a novel cooled-wet electrode for radiofrequency liver ablation. Invest Radiol 35:438–444
Ni Y, Miao Y, Mulier S, Yu J, Baert AL, Marchal G (2000) A novel “cooled-wet” electrode for radiofrequency ablation. Eur Radiol 10:852–854
Solbiati L, Goldberg SN, Ierace T et al (1997) Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology 205:367–373
Burdio F, Guemes A, Burdio JM et al (2003) Bipolar saline-enhanced electrode for radiofrequency ablation: results of experimental study of in vivo porcine liver. Radiology 229:447–456
McGahan JP, Brock JM, Tesluk H, Gu WZ, Schneider P, Browning PD (1992) Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol 3:291–297
Lencioni R, Cioni D, Crocetti L et al (2005) Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 234:961–967
Yamakado K, Nakatsuka A, Ohmori S et al (2002) Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol 13:1225–1232
Hoey MF, Mulier PM, Leveillee RJ, Hulbert JC (1997) Transurethral prostate ablation with saline electrode allows controlled production of larger lesions than conventional methods. J Endourol 11:279–284
Livraghi T, Goldberg SN, Monti F et al (1997) Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology 202:205–210
Acknowledgment
This work was supported by a 2008 research grant from the Korean Liver Cancer Study Group.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cha, J., Choi, D., Lee, M.W. et al. Radiofrequency Ablation Zones in Ex Vivo Bovine and In Vivo Porcine Livers: Comparison of the Use of Internally Cooled Electrodes and Internally Cooled Wet Electrodes. Cardiovasc Intervent Radiol 32, 1235–1240 (2009). https://doi.org/10.1007/s00270-009-9600-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-009-9600-0