Abstract
The purpose of this study was to demonstrate the efficacy of bipolar radiofrequency ablation (RFA) using cooled-wet electrodes inducing coagulation in ex vivo bovine livers and in in vivo canine livers. In ex vivo experiments, 20 coagulations were created by monopolar (group A), and bipolar RFA (group B) using a 200 W generator (Valleylab) and one or two cooled-wet electrodes. In in vivo experiments, one coagulation was created by bipolar RFA in each of eight dogs via laparotomy. In ex vivo and in vivo experiments, RF was applied to one or two electrodes at 100 W for 10 min. The dimensions of the coagulations were compared in the two groups. In ex vivo experiments, the mean volumes of the coagulations produced in group B (54.0±16.5 cm3) were greater than those produced in group A (33.9±12.7 cm3) (P=0.007). In in vivo experiments, bipolar RFA produced a coagulation of 39.4±15.6 cm3 without a major complication. The present study showed that a RF electrode system using two cooled-wet electrodes in the bipolar mode created larger coagulation volumes than the monopolar mode, and this system can be used to create large coagulation without major complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiofrequency ablation (RFA) has emerged as a promising image-guided intervention for unresectable primary and secondary liver tumors [1–4]. Most RF systems are currently channeled in monopolar fashion, which involves intense heating at an active tip and a large grounding pad on the skin to complete the circuit [5]. One remaining challenge for the successful ablation of large tumors using a monopolar RF system is to increase the diameter of coagulation that can be created by a single radiofrequency application [6]. Indeed, RFA can be effective for the destruction of small tumors (<3 cm), but success for lesions larger than 3.5 cm in diameter has been less robust [7]. This limitation of monopolar RFA is related to the fact that in monopolar mode, the current density falls in an inverse square manner with respect to distance from the electrode, and therefore, tissue immediately adjacent to the electrode is exposed to highest current density [5]. In practice, this leads to tissue desiccation and char formation.
Several strategies have been devised to overcome inadequate volume of tissue coagulation, they include as a cluster cooled electrode (Valleylab, Burlington, Mass., USA), a wet electrode (Berchtold, Tuttlingen, Germany), an expandable-wet electrode (StarBurst Xli; RITA Medical Systems, Mountain View, Calif., USA), a multitined expandable electrode (LeVeen; Boston Scientific, Natick, Mass., USA) and bipolar electrodes [7–12]. Despite the development of recent devices that enable larger coagulations to be created, the treatment of liver tumors larger than 4 cm often requires multiple overlapping ablations [7, 13, 14]. However, because this approach is both time consuming and technically challenging, there is a need to increase the dimensions of coagulation using a single RF application [6, 14].
Based on results of previous studies [15–21], we postulated that bipolar RFA with electrodes allowing the simultaneous application of internal cooling and interstitial hypertonic saline (HS) infusion could be the most effective way of avoiding tissue carbonization and of delivering higher currents to target tissue. Recently, we designed and tested a bipolar RF electrode system equipped with cooled-wet electrodes, with interstitial HS infusion and internal cooling in ex vivo bovine liver [22, 23]. In the current study, we compared the performance of bipolar RFA versus monopolar RFA, with respect to the dimensions of coagulations in ex vivo liver tissue, and evaluated the in vivo efficacy and safety of our bipolar RF electrode system by creating controlled coagulation in a canine liver model.
Materials and methods
Ex vivo studies
RFA Settings
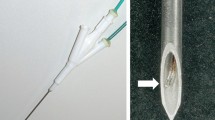
RFA was performed in ten freshly excised bovine livers weighing on average 7.5 kg. The livers were cut into several 10×10×10 cm3 blocks and dipped into a 50×20×20 cm saline (0.9%, room temperature)-filled bath. The RF electrode system used was composed of a 15-gauge cooled-wet electrode with a 3 cm active tip, and a 480 kHz generator (CC-3; Valleylab), which was used at 100 W. As described in a previous study [23], we developed a cooled-wet electrode to permit both electrode cooling and tissue HS infusion. We modified a 17-gauge cooled-tip electrode with a 3-cm active tip (Valleylab) by covering it with a 15-gauge electrically insulated metal outer sheath, up to 3.5 cm from the electrode tip (Fig. 1).
Instruments used for bipolar radiofrequency ablation. a Illustration shows cooled-wet electrode that allows simultaneous internal cooling and hypertonic saline infusion. b A photograph of two perfused-cooled electrodes used for parallel wet bipolar radiofrequency ablation. The arrow indicates a side hole for interstitial saline infusion
The 15-gauge cooled-wet electrode was advanced at least 4 cm into the liver tissue. To continuously measure the local tissue temperature during the procedure, a thermocouple was inserted at 15 mm from the electrode tip or at the midpoint between the electrode tips. Tissue impedance was monitored by the generator (Valleylab). A peristaltic pump (Watson-Marlow, Medford, Mass., USA) was used to infuse 0°C 0.9% saline solution into the lumen of the electrode at a rate sufficient to maintain a tip temperature of 20–25°C. The 6% HS solutions were infused through the cooled-wet electrodes using an infusion pump (Pilotec IS; Fresenius Medical Care, Alzenau, Germany) at a rate of 2 ml/min from 1 min before starting RFA and continuously during RFA. The applied current, power output, and impedance were continuously monitored during RFA, and recorded using a computer program (Real Time Graphics Software V 2.0; Valleylab). The technical aspects of the two RFA methods, including impedance and wattage changes, tissue temperature measured at 15 mm from the electrode tip, or at the mid-point between the electrode tips, and the dimensions of the RF-ablated areas were compared.
RF energy delivery modes
Ten ablation zones were created in monopolar mode (group A) and in bipolar mode (group B) at a same RF power setting of 100 W. In the monopolar mode, RF was applied to the perfused cooled electrode at an initial generator output of 100 W, and flowed from the electrode to a metallic dispersive pad, which was attached to one sidewall of the saline filled bath. The initial impedance was controlled at 80 Ω by altering the distance between the electrodes and the dispersive metallic pad. RF power was then manually increased to 100 W and held for a total of 10 min. Energy delivery was performed using an impedance-controlled algorithm that optimizes energy administration and tissue coagulation [24]. In this mode, power is automatically switched off for 15 s when the impedance rises by more than 10 Ω above the baseline value; subsequently, it is reapplied at the same or a lower level.
In the bipolar RF delivery mode, one electrode tip was connected to the RF generator output, and the other to the generator “ground”. In this mode, current flowed from one electrode to the other, and therefore a dispersive pad was not necessary [25]. The two cooled-wet electrodes were placed in the liver at an inter-electrode distance of 3 cm through an acrylic plate. RF power was then increased manually to 100 W, and held for a total of 10 min.
Lesion Size Measurement
Liver blocks containing RFA lesions, were sliced along an axial plane passing parallel to the axis of the electrode(s) (A-plane) and then these slices were cut transversely (T-plane). As the white central area of the RF induced ablated zone has been shown previously to correspond to the zone of coagulation necrosis [26], two observers measured the axial diameter (Dax) along the electrode of the ablated zone in the L-plane, and its transverse diameters (Dtr1, Dtr2) in the T-plane, respectively (Fig. 2). The axial diameter was defined as the distance between the proximal and the distal edge of the coagulation along the axis of the electrode, and the transverse diameters as the distances between two opposite edges of the coagulation perpendicular to the electrode shaft at the equator of the coagulation. The volumes of the ablated zones created were evaluated by approximating the lesion to a sphere using π(Dax×Dtr1×Dtr2)/6.
Comparison of radiofrequency-induced ablation regions created by applying radiofrequency in wet monopolar or wet bipolar modes ex vivo. a Photograph of cut section of the specimen along the electrode insertion axis from group A (wet monopolar mode). Arrow indicates the electrode insertion site. b Photograph of cut section of the same specimen (A) perpendicular to A (transverse plane). Dt1 and Dt2 indicate transverse diameters, which are the distances between two opposite edges of the coagulation perpendicular to the electrode shaft at the equator of the coagulation. c Photograph of specimen from group B (wet bipolar mode). Arrows indicate the electrode insertion sites. d Photograph of cut section from the same specimen as in (C) but perpendicular plan to C (transverse plane)
In vivo studies
Animals, anesthetics, and surgical technique
Following the guidelines of the animal research committee at our institution, eight domestic dogs (25–30 kg) were anesthetized using an intramuscular injection of 50 mg/kg ketamine hydrochloride (ketamine; Yuhan, Seoul, Korea) and 5 mg/kg xylazine (Rumpun; Bayer Korea, Ansan, Korea). Booster injections of up to one-half of the initial dose were administered as needed. Endotracheal intubation was performed and anesthesia was maintained with inhaled enfluorane (Gerolan; Choongwae Pharma Corporation, Seoul, Korea). Dogs were placed in the supine position, prepared and draped. The liver was exposed through a midline incision.
Radiofrequency procedure
A pair of 15 gauge cooled-wet electrodes were inserted at an inter-electrode distance of 3 cm into the livers of eight dogs. RF ablation was performed in bipolar mode at 100 W for 10 min with a 6% hypertonic saline infusion of 2 ml/min. The RF energy was concentrated between the two probes, thus creating a zone of targeted ablation. Energy delivery was performed using an automatic impedance controlled algorithm. The liver was allowed to cool to body temperature before being replaced, and the incision was closed using non-absorbable sutures. One ablation was performed per animal and thus eight ablation regions were created. To monitor the local tissue temperature during the procedure, a thermocouple was inserted midway between the two electrodes.
Assessment of coagulation necrosis
The dogs were followed and killed 4 days after the procedure. Livers containing lesions were sliced in the transverse plane at 5 mm intervals. Specimens were stained for mitochondrial enzyme activity by incubating them for 30 min in 2% 2,3,5,-triphenyl tetrazolium chloride, or TTC (Sigma, St Louis, Mo., USA), at 20–25°C. This test is used to determine irreversible cellular injury during the early stages of RF-induced necrosis [27]. Two observers measured using a caliper two diameters (Dtr1, Dtr2) of the central, white region of the RF-induced ablation zones in the slice showing maximal coagulation diameter. The axial diameter (Dax) was measured by the determining the number of slices showing an unstained area.
In addition, the slices were placed on an optical scanner (HP 4C/T; Hewlett-Packard, Palo Alto, Calif., USA), and images were saved to image management software (PhotoShop; Adobe, San Jose, Calif., USA). Area analysis was performed on a computer equipped with NIH Image J software (National Institutes of Health; http://rsb.info.nih.gov/ij/) [28]. The area of the coagulation within the perimeter, on each slice was calculated using the computer program, and volumes were calculated by multiplying area by slice thickness and summed to obtain total lesion volume.
Lesion shape was evaluated using a rough estimate of lesion “roundness” in two dimensions by computing the isoperimetric ratio for each lesion in the most representative slice [29]. This value was computed using the following formula: R=4πA/l2 where R is the isoperimetric ratio, A is the area of the measured zone, and l the perimeter of the lesion; the closer this value is to 1, the more circular the shape [30]. Values for A and l were obtained using the computer program NIH image J [28]. The RF-induced coagulations of three animals were fixed in 10% formalin for routine histologic processing and were finally processed by paraffin sectioning and hematoxylin-eosin staining for light microscopic study.
Statistical analysis
The dimensions of the thermal ablation areas and the technical parameters of the two groups were averaged and compared using Student t-test. Values are expressed as means±SD. The mean value of R (isoperimetric ratio) for each group was compared using factorial analysis of variance. For all statistical analyses, a P-value of less than 0.05 was considered significant. Statistics analyzed using the Instat program (GraphPad Software, Inc., San Diego, Calif., USA).
Results
Ex vivo experiment
Technical parameters
With HS infusion, impedance values reduced to less than 60 Ω and were maintained at approximately 50 Ω (45∼55 Ω) during RFA in both groups. Moreover, no impedance rise to more than 100 Ω during RFA was observed in either group; Group A (wet monopolar) 49.5±2.7 Ω and B (wet bipolar) 51.5±2.3 Ω (P>0.05), and their mean current values were 1545±53.7 and 1520±89.6 mA, respectively (Table 1), which was not significant (P>0.05).
Effects of tissue ablation
The mean Dax of the RF induced central white zone on the L-plane measured in the gross specimens of the two groups, was as 4.6±0.4 cm in group A and 4.8±0.6 cm in group B (P>0.05) (Table 1). The respective mean Dt1 were 3.8±0.4 and 5.0±0.7 cm (P=0.0002), and the mean Dt2 of the ablated spheres on the T plane were 3.7±0.7 and 4.3±0.9 cm (P=0.006) (Fig. 3). Bipolar RF ablation tended to produce a more spherical coagulation than monopolar RF ablation, i.e. the Dax/Dt1 ratios were 1.2±0.1 in group A, and 0.96±0.2 in group B (P=0.003) (Fig. 2). Furthermore, the volumes of ablation zones obtained in monopolar and bipolar modes, were 33.9±12.7 and 54.0±16.5 cm3, respectively, which was statistically significant (P=0.007).
In vivo experiments
Technical parameters
In vivo impedance values during wet bipolar RFA varied within the range 50–100 Ω (mean 57±9 Ω). In six of the eight dogs, the impedance was well controlled to <60 Ω, but in the other two cases, rose intermittently to >80 Ω (mean 73.5±14 Ω), which induced pulsed RF application (Fig. 4). The mean current of the 10 min RF application was 1390±265 mA.
Graphic depiction changes in tissue impedance, RF current, and power during the wet bipolar radiofrequency ablation in the in vivo canine model. a In six of eight dogs, the tissue impedance was well controlled to <60 Ω during wet bipolar RFA using perfused cooled electrodes; approximately 1500 mA was delivered. b In the other two dogs, the tissue impedance rose frequently to >80 Ω during RF energy application, and this activated pulsed RF application mode
Effects of tissue ablation
The RFA regions created in all treated livers exhibited a characteristic central white zone surrounded by a red hemorrhagic zone. After staining with 2% 2,3,5-triphenyl tetrazolium chloride, the normal liver parenchyme and the peripheral hemorrhagic zone appeared pink, but the central white zone was unstained (Fig. 5). In the two cases that showed impedance rises during bipolar RF application, a large hepatic vessel (>3 mm) was located between the electrode insertion sites (Fig. 6), and the shape of the coagulation necrosis induced was irregular. The mean values of Dv, Dt1, and Dt2 in the central white zones in the ablated regions were 4.8±0.8, 3.7±0.5, and 4.1±0.5 cm, respectively, and the mean ablated volume induced by wet bipolar RFA was 39.4±15.6 cm3 (Table 2). The isoperimetric ratio (R) was 0.75±0.1.
Histopathologically, ablated regions in representative cases demonstrated a central necrotic zone surrounded by a peripheral hemorrhagic zone consisting of necrotic hepatocytes, interstitial hemorrhage, and polymorphonuclear (PMN) leukocyte infiltrates. Within the central necrotic zone, no viable cells were found, but within hemorrhagic lesions, areas of sinusoidal congestion and hemorrhage were accompanied by advanced necrotic changes and patches of living cells.
Discussion
RFA has gained significant popularity as a safe and effective treatment for small liver malignancies during recent years, and its major advantage is reduced morbidity compared with surgical resection [1–5]. However, the most restrictive factor concerning monopolar RFA is the limited size of the RF-induced ablation zone [1, 6, 14, 31]. In our ex vivo study, bipolar RFA using the cooled-wet electrodes created larger coagulations than monopolar RFA: 54.0±16.5 versus 33.9±12.7 cm3. These larger coagulations created in bipolar mode could be attributed to higher electrical density of the bipolar mode and thus more heating [19, 20, 25]. Furthermore, in the bipolar mode one electrode is thermally shielded by the opposing second electrode, which also actively heats tissue in its proximity, therefore, since heat is trapped between the two electrodes, higher temperatures are achieved [25].
Our in vivo experiments showed that bipolar RFA using simultaneous intra-electrode cooling and interstitial perfusion created a coagulation of 39.4±15.6 cm3. This large volume of coagulation was achieved at 10 min without any procedure-related complication. In a previous study by Burdio et al. [20], physiologic saline was injected at a rate of 600 ml/h for bipolar RF ablation, and these workers demonstrated that bipolar RFA created a large region of coagulation (52.4±23.6 cm3). However, major complications, including bowel injuries, and hepatic vein thrombosis were described. Given that more saline was infused than in the present study, the risk of unexpected thermal injuries to adjacent vital structures and the loss of control of the RF-induced ablation area and shape were probably higher [20]. More recently, Burdio et al. [21] reported that HS-enhanced single probe bipolar RFA (coaxial electrode) with a 4 cm inter-electrode distance can achieve large ablation volumes (26 cm3) in perfused pig liver, with a fifth of the energy per coagulation volume achieved by other electrodes. In addition, Denys et al. [10] compared in vivo coagulation necrosis obtained with commercially available RF devices, and concluded that larger ablations were achieved with an expandable needle system than cooled electrode, and high complications rates with perfused needle. In their study, the largest in vivo coagulation volume was achieved using a RITA starbust XL electrode (39 cm3), and RF delivery time was 25 min. Compared with that previous study, we achieved similar in vivo coagulation volume with 10 min of RF application. This could be related to better energy efficacy of our bipolar system.
However, wet bipolar RFA using dual probes may have some disadvantages versus RFA using a single probe. First, in bipolar RFA with dual probes, the better efficacy could be achieved when the two electrodes placed at equal distance. However, in the clinical situation, to place two electrodes in parallel into target tissue is sometimes difficult. Secondly, in the bipolar mode there is no way to independently control the amount of heat generated in the vicinity of the probes, because both electrodes are active [32]. When the degree of cooling of the electrodes are different, for example, due to differences in perfusion, one probe may reach a higher temperature than the other and this can lead to boiling and a rapid increase in impedance. Third, though HS infusion during RFA improves the performance of RFA by increasing both thermal and electrical conductivity [33–35], it also induces some possible drawbacks, including a risk of unexpected thermal injury, irregular shape of ablation area and theoretical concerns of tumor seeding [10, 36]. Indeed, the standard deviation of diameters of RF-induced coagulation using the cooled-wet electrodes in our experiment was higher than that of other commercially available electrodes without using interstitial saline infusion, which means the diameter of the coagulation was less predictable.
Our experimental study has certain limitations. First, the extent to which the results of the present in vivo study can be transposed to the human liver is limited. Moreover, all ablations involved the normal liver parenchyma, and not tumor tissue. Despite these shortcomings, our model provides a reliable basis for testing the efficacy and safety of the wet bipolar RF mode for creating coagulation necrosis. Second, the size of canine liver is too small to test the maximal achievement of our wet bipolar system was not possible. We believe that the pig liver would be better for testing in vivo efficacy of the RF electrode system due its greater volume than the canine liver. Finally, in the present study using the cooled-wet electrodes, the bipolar RF mode was found to be more efficient than the monopolar mode, but whether this remains the case for other monopolar RF systems like the Radiotherapeutics and RITA systems, which have higher maximum power outputs, will require further study.
In conclusion, wet bipolar RF ablation using perfused cooled electrodes, showed better ex vivo efficacy than wet monopolar ablation for creating coagulation necrosis, created large regions of coagulation necrosis in the canine liver in vivo. Therefore, we believe that this technique could be applied to the RF ablation of larger tumors.
References
Gazelle GS, Goldberg SN, Solbiati L, Livraghi T (2000) State of the art: tumor ablation with radio-frequency energy. Radiology 217:633–646
McGahan JP, Dodd GD (2001) Radiofrequency ablation of the liver: current status. Am J Roentgenol 176:3–16
Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F (1999) Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg 230:1–8
Lencioni RA, Allgaier HP, Cioni D Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE, Bartolozzi C (2003) Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 228:235–240
Goldberg SN (2001) Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound 13:129–147
Dodd GD, Frank MS, Aribandi M, Chopra S. Chintapalli KN (2002) Radiofrequency thermal ablation: computer analysis created by overlapping ablations. Am J Roentgenol 177:777–782
Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS (2000) Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology 214:761–768
Goldberg SN, Solbiati L, Hahn PF, Cosman E, Conrad JE, Fogle R, Gazelle GS (1998) Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology 209:371–379
Curley SA, Izzo F, Ellis LM, Vauthey JN, Vallone P (2000) Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg 232:381–391
Denys AL, De Baere T, Kuoch V, Dupas B, Chevallier P, Madoff DC, Schnyder P, Doenz F (2003) Radio-frequency tissue ablation of the in vivo and ex vivo experiments with four different systems. Eur Radiol 13:2346–2352
Pereira PL, Trubenbach J, Schenk M, Subke J, Kroeber S, Schaefer I, Temy CT, Schmidt D, Brieger J, Claussen CD (2004) Radiofrequency ablation: in vivo comparison of four commercially available devices in pig livers. Radiology 232:482–490
McGahan JP, Gu WZ, Brock JM, Tesluk H, Jones CD (1996) Hepatic ablation using bipolar radiofrequency electrocautery: preliminary investigation. Acad Radiol 3:418–422
Giorgio A, Tarantino L, de Stefano G, Coppola C, Ferraioli G (2004) Complications after percutaneous saline-enhanced radiofrequency ablation of liver tumors: 3-years experience with 336 patients at a single center. Am J Roentgenol 184:207–211
Goldberg SN, Dypuy DE (2001) Image-guided radiofrequency tumor ablation: challenges and opportunities–part 1. J Vasc Interv Radiol 12:1021–1032
Miao Y, Ni Y, Yu J, Marchal G (2000) A comparative study on validation of a novel cooled-wet electrode for radiofrequency liver ablation. Invest Radiol 35:438–444
Ni Y, Miao Y, Mulier S, Yu J, Baert AL, Marchal G (2000) A novel ‘cooled-wet’ electrode for radiofrequency ablation. Eur Radiol 10:852–854
Leveillee RJ, Hoey MF (2003) Radiofrequency interstitial tissue ablation: wet electrode. J Endourol 17:563–577
Lee JM, Han JK, Kim SH, Shin KS, Lee JY, Park HS, Hur H, Choi Bi (2004) Comparison of wet radiofrequency ablation with dry radiofrequency ablation and radiofrequency ablation using hypertonic saline preinjection: ex vivo bovine liver. Korea J Radiol 5:258–265
Lee JM, Han JK, Kim SH, Sohn KL, Lee KH, Ah SK, Choi BI (2003) A comparative experimental study of the in-vitro efficiency of hypertonic saline-enhanced hepatic bipolar and monopolar radiofrequency ablation. Korean J Radiol 3:163–169
Burdio F, Guemes A, Burdio JM, Navarro A, Sousa R, Castiella T, Cruz I, Burzaco O, Guirao X, Lozano R (2003) Large hepatic ablation with bipolar saline-enhanced radiofrequency: an experimental study in in vivo porcine liver with a novel approach. J Surg Res 110:193–201
Burdio F, Guemes A, Burdio JM, Navarro A, Sousa R, Castiella T, Cruz I, Burzaco O, Lozano R (2003) Bipolar saline-enhanced electrode for radiofrequency ablation: results of experimental study of in vivo porcine liver. Radiology 229:447–456
Lee JM, Han JK, Kim SH, Lee JY, Choi SH, Choi BI (2004) Hepatic bipolar radiofrequency ablation using perfused-cooled electrodes: a comparative study in the ex vivo bovine liver. Br J Radiol 77:944–949
Lee JM, Han JK, et al (2005) Bipolar radiofrequency ablation in ex vivo bovine liver with the open-perfused system versed the cooled-wet system. Eur Radiol DOI: 10.1007/s00330-004-2375-4
Goldberg SN, Stein M, Gazelle GS, Sheiman RG, Kruskal JB, Clouse ME (1999) Percutaneous radiofrequency tissue ablation: optimization of pulsed-RF technique to increase coagulation necrosis. J Vasc Interv Radiol 10:901–916
Haemmerich D, Staelin ST, Tungjitkusolmun S, Lee FT, Mahvi DM, Webster JG (2001) Hepatic bipolar radiofrequency ablation between sperated multiprong electrodes. IEEE Trans BioMed Eng 48:1145–1152
Lee JD, Lee JM, Kim SW, Kim CS, Mun WS (2001) MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol 2:151–158
Goldlust EJ, Placzynski RP, he YY, Hsu CY, Coldberg MP (1996) Automated measurement of infarct size with scanned images of triphenyltetrazolium chloride-stained rat brains. Stroke 27:1657–1662
http://rsb.info.nih.gov/ij/, accessed November 20th, 2004-10-20
Chinn SB, Lee FT Jr, Kennedy GD, Chinn C, Johnson CD, Winter TC III, Warner TF, Mahvi DM (2001) Effect of vascular occlusion on radiofrequency ablation of the liver: results in a porcine model. Am J Roentgenol 176:789–795
Do Carmo MP (1976) Differential geometry of curves and surfaces. Prentice-Hall, Englewood, N.J., pp 31–35
Buscarini E, Buscarini L (2004) Radiofrequency thermal ablation with explandable needle of focal liver malignancies: complication report. Eur Radiol 14:31–37
Haemmerich D, Tungjitkusolmu S, Staelin ST, Tsai JZ, Webster JG, Lee FT Jr, Mahvi DM, Vorperian VR (2002) Finite-element analysis of hepatic multiple probe radio-frequency ablation. IEEE Trans Biomed Eng 49:836–842
Goldberg SN, Ahmed M, Gazelle GS, Kruskal JB, Huertas JC, Halpern EF, Oliver BS, Lenkinski RE (2001) Radiofrequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating, and coagulation-phantom and porcine liver study. Radiology 219:157–165
Lee JM, Kim YK, Lee YH, Kim SW, Li CA, Kim CS (2003) Percutaneous radiofrequency thermal ablation with hypertonic saline injection: in-vivo study in a rabbit liver model. Korean J Radiol 4:27–34
Ahmed M, Lobo SM, Weinstein J, Kruskal JB, Gazelle GS, Halpern EF, Afzal SK, Lenkinski RE, Goldberg SN (2002) Improved coagulation with saline solution pretreatment during radiofrequency tumor ablation in a canine model. J Vasc Interv Radiol 13:717–724
Boehm T, Malich A, Goldberg SN, Reichenbach JR, Hilger I, Hauff P, Reinhardt M, Fleck M, Kaiser WA (2002) Radio-frequency tumor ablation: internally cooled electrode versus saline-enhanced technique in an aggressive rabbit tumor model. Radiology 222:805–813
Acknowledgements
The authors thank John Roberts, PhD, for his editorial assistance and manuscript preparation. This study was supported by grant No. 04-2004-046-0 from the Seoul National University Hospital Research Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00330-005-2892-9
Rights and permissions
About this article
Cite this article
Han, J.K., Lee, J., Kim, S.H. et al. Radiofrequency ablation in the liver using two cooled-wet electrodes in the bipolar mode. Eur Radiol 15, 2163–2170 (2005). https://doi.org/10.1007/s00330-005-2713-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-005-2713-1