Abstract
The aim of this study was to investigate the efficacy of bipolar radiofrequency ablation (RFA) with the open-perfused electrode and cooled-wet electrode. Bipolar RF was applied for 20 min to the ex vivo bovine liver using either the Berchtold system with two 16-gauge open-perfused electrodes (group A, n=15) or the Radionics system with two 15-gauge cooled-wet electrodes (group B, n=15). In both groups, two electrodes were placed 3 cm apart. The ablation zone was created by the RF energy delivered together with the infusion of 5% hypertonic saline (2 ml/min). The dimension of the ablation zone, its shape and the changes in the impedance and W s of two groups during the RFA were examined and documented. The vertical diameter (Dv) along the probe, the long-axis diameter (Dl) perpendicular to the Dv in the longitudinal plane and the short-axis diameter of the ablation zone (Ds) in the transverse plane through the midpoint between the tips of two probes were measured. The mean accumulated energy output in the Radionics system was higher than in the Berchtold system (159,887.0±36,423 W s vs. 87,555.1±86,787 W s). The difference was statistically significant (P<0.05). In group A, the impedance intermittently rose to above 700 Ω during the RFA in all sessions, which led to a gradual decrease of the power output to lower than 30 W. In group B, on the other hand, the impedance did not change markedly. The mean Dv value of the coagulation necrosis in group B was significantly longer than in group A (5.0±0.4 cm vs. 4.3±0.6 cm, P<0.05). The mean Dl and Ds were 6.7±0.5 cm and 5.0±0.8 cm in group A, and 6.5±0.8 cm and 5.5±0.7 cm in group B, respectively (P>0.05). The data demonstrate that the cooled-wet electrode generates the more spherical ablation zone than the open-perfused electrode. With approximately doubled power output, the bipolar RFA with the cooled-wet electrodes induces a larger volume of tissue coagulation than with the open-perfused electrodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The image-guided percutaneous tumor ablation using radiofrequency (RF) energy has become increasingly popular in recent years [1]. Currently, in the treatment of primary and secondary hepatic malignancy, radiofrequency ablation (RFA) is the most widely used technique among several thermal ablative therapies [2–4]. However, a major limitation of the RFA is the small size of the necrosis that can be achieved by a single RF application: it is too small to encompass the entire tumor, and the required safety margin of 0.5–1.0 cm results in a relatively high rate (34–55%) of recurrence after the RFA therapy [5–7]. To improve the efficacy of the RFA therapy, therefore, strategies must be developed to increase the size of the ablated region.
To increase RF-induced coagulation necrosis, several investigators proposed bipolar RFA that uses two electrodes [8–11]. In fact, bipolar RFA has been shown to create tissue coagulation more quickly than monopolar RFA [10, 11]. However, the study using two hypertonic saline-mediated wet electrodes showed that the impedance was rapidly increased to over 700 Ω during bipolar RFA [11]. Goldberg et al. [12] demonstrated that large volume tissue ablation could be achieved by a clustered, internally cooled electrode technique. Ni et al. [13] and Miao et al. [14] reported that the cooled-wet electrode that allows the internal cooling perfusion and the interstitial saline infusion simultaneously was more efficient in creating the larger dimension ablation zone than other monopolar electrodes. Based on these studies [12–14], we developed the prototype cool-wet electrode that is able to perform the saline interstitial infusion as well as the intra-electrode cooling by modifying the 17-gauge internally cooling electrode (Radionics, Burlington, MA). We postulate that bipolar RFA using two cooled-wet electrodes may be more effective in creating a large area of coagulation necrosis by delivering a higher current without rapid rising of the tissue impedance than using open-perfused electrodes. Here, we evaluated these two RF systems, especially the dimensions of the ablation zone in the liver tissue and the temperature at the midpoint between two electrodes.
Materials and methods
The RFA setting
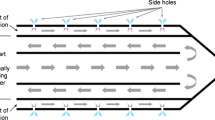
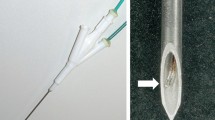
As the RFA experiments using the ex vivo tissues have been reported to generate consistent results [11, 13, 14], we performed the RFA experiments using ten ex vivo bovine livers. The bovine liver, weighing approximately 10 kg, was cut into two or three 10×10×10-cm3 blocks. The block was immersed in the 50×20×25-cm saline-filled bath at room temperature. Based on the previous studies [11], two 16-gauge open-perfused electrodes (Berchtold Medizinelektronik Tuttlingen, Germany, Fig. 1a) or two 15-gauge cooled-wet electrodes (Fig. 1b) were placed on the liver 3 cm apart through the acryl plate containing multiple holes at the 5-mm interval. The tip of the electrode penetrated the target tissue at least 4 cm.
The RFA needle was perfused continuously with hypertonic saline solution (5% NaCl solution) using a syringe pump (Pilot C; Fresenius Medical Care, Alzenau, Germany). To measure the local tissue temperature during the procedure continuously, a thermocouple was inserted to the midway between two electrodes. The tissue impedance was monitored by the circuitry incorporated generator. For the bipolar RFA, two electrodes were used: one active and one passive. To compare the two different RFA systems, the Berchtold system with open-perfused electrodes (group A) and the Radionics system with cooled-wet electrodes (group B) in bipolar mode, 15 ablation zones were created in each group.
The ablation protocol
The Berchtold RFA system consists of two 16-gauge open-perfused electrodes with a tip exposure of 2 cm and the 375-kHz generator with a maximum power output of 60 W (Elektrotom HiTT 106, Berchtold Medizinelektronik, Fig. 1a). Based on the previous study [11], 5% NaCl was used as the perfusion solution with a flow rate of 2 ml/min. The RF energy was applied for 20 min. If the impedance was moderate (100–350 Ω), the RF power input was stabilized by a control mechanism. If the impedance rose above 700 Ω, the power input was reduced to 5 W. During the RFA, the accumulated current (W s) and the impedance were continuously monitored on the generator panel and recorded manually at the 30 s interval.
The Radionics RFA system consists of two 15-gauge cooled-wet electrodes with a tip exposure of 2 cm and the 480 kHz generator (CC-3, Radionics). To allow the intra-electrode cooling perfusion and the interstitial saline infusion simultaneously, we modified a 17-gauge cooled-tip electrode with a 2-cm active tip (Radionics) by covering it with a 15-gauge outer sheath, except for a 2.5-cm distal portion (Fig. 1b). The outer sheath was made of metal and insulated. The space between the 15-gauge sheath and the cooled tip electrode permitted the saline infusion along the electrode. A peristaltic pump (Watson-Marlow, Medford, MA) was used to infuse cold saline solution (0°C) into the lumen of the electrodes at the rate sufficient to maintain the tip temperature at 20–25°C. The applied current, the power output and the impedance were continuously monitored during the RFA and recorded automatically using a computer program (Real Time Graphics Software V 2.0; Radionics). Based on our previous data (unpublished), 5% NaCl solution was infused at the rate of 2 ml/min through the cooled-wet electrode using an infusion pump (Pilotec IS). The RF energy was applied at 150 W for 20 min. The technical aspects of RFA, such as the impedance, the wattage change, the tissue temperature at the midpoint and the dimensions of the RF-induced coagulation area were compared in two groups.
The lesion size measurement
The liver blocks containing the RFA lesion were dissected along the longitudinal plane passing through the axes of both probes (L-plane) and then cut transversely into slices (T-plane). The white central area of the RF-induced ablation zone has been reported to correspond with the coagulation necrosis zone [16, 17]. Thus, two investigators measured the vertical diameter (Dv) along the probe, the long-axis diameter (Dl) perpendicular to the Dv in the longitudinal plane and the short-axis diameter of the ablation zone (Ds) in the T-plane through the midpoint between the tips of two probes. The volume of the ablation zone was calculated as follows. The volume = π(Dv × Dl × Ds)/6. The shape of the RF-induced ablation zone was assessed by the ratio of the Dl/Dv diameter.
Statistical analysis
The dimensions of the thermal ablation area and the technical parameters of the two groups were averaged for both groups and compared using the unpaired Student’s t-test. The data were presented as the means ± SD. The temperature at the midpoint between two electrode tips was compared using the Mann-Whitney U-test. A P-value less than 0.05 was considered statistically significant. The statistical analysis was performed using the Instat program (GraphPad Software, Inc., San Diego, CA).
Results
The electrical measurement
In the group A, using the Berchtold system with open-perfused electrodes, the mean initial impedance was less than 100 Ω. Within 6–10 min, the impedance rose intermittently to over 700 Ω, resulting in the gradual decrease of the energy delivery lower than 30 W. In group B, using the Radionics system with cooled-wet electrodes, the impedance was well controlled. It was lower than 100 Ω in 11 out of 15 experiments (73%). In the remaining four experiments, the impedance rose to 250–350 Ω and fluctuated. The mean accumulated energy output was 87,555.1±86,787 W s in group A and 159,887.0±36,423 W s in group B. The output in group A was significantly lower than in group B (P<0.05).
The temperature
The temperature at the midpoint of two electrodes in group A and B is comparable. The mean temperature at the midpoint between two electrode tips is shown in Fig. 2. The temperature was 101±15°C in group A and 103±10°C in group B. The difference in the temperature between the two groups was not significant (P>0.05).
The dimension of the ablation zone
The ablation zone induced by the Radionics system is larger than by the Berchtold system (Fig. 3). The well-defined area with the central white discoloration was observed in the ablated area. The mean Dl value of the RF-induced central white zone was 6.7±0.5 cm in group A (Berchtold system) and 6.5±0.8 cm in group B (Radionics system). The difference was not significant (P>0.05; Table 1).
The mean Dv value was 4.3±0.6 cm in group A and 5.0±0.4 cm in group B. The Dv of group A was significantly smaller than in group B (P<0.05). The mean Ds was 5.0±0.8 cm in group A and 5.5±0.7 cm in group B. The difference was not significant (P=0.07). The ratio of Dl/Dv was 1.6±0.2 in group A and 1.3±0.1 in group B. The ratio in group A was significantly higher than in group B (P<0.05). The volume of the ablation zone was 76.9±22 cm3 in group A and 93.5±26 cm3 in group B. The ablation zone in group B was significantly larger than in group A (P<0.05).
Discussion
Successful tumor ablation by RFA is achieved when the area of destruction extends to the area 0.5–1 cm beyond the targeted tumor. This provides the margin equivalent to the surgery [18–24]. The desiccation and the charring of tissues at the tip of the electrode limits the dimension of coagulation necrosis created by a single application of RFA [21, 22]. Several studies demonstrated that the bipolar RFA, by increasing the current density between electrodes, creates coagulation necrosis more efficiently than the monopolar RFA [9–11]. However, the desiccation and the charring of tissues at the electrode tip of the bipolar RFA could be more intense than the monopolar RFA [10, 11]. Several approaches have been applied to overcome the problem, such as the saline infusion during the RFA or prior to the RFA, the cooling of the intra-electrode during the RFA, etc. [12–14, 25–27].
Here, we used simultaneously intra-electrode cooling and interstitial saline infusion by the cooled-wet electrodes. This allows the delivery of higher energy (150 W) to the RFA without elevating the impedance to the level that decreases the power output (mean accumulated energy: 159,887±32,423 W s). However, the bipolar RFA using 60 W of the Berchtold system and the open-perfused electrodes showed the intermittent rise of the impedance (over 700 Ω), which results in the gradual decrease of the energy delivery to less than 30 W. The decreased RF energy reduced the ablation dimension significantly, from 93.5 cm3 to 76.9 cm3 (P<0.05). The decreased impedance during the RFA with the Radionics system may be due to the combined effect of the increased electrical conductance induced by the infusion of hypertonic saline and the cooling of the tissue by intra-electrode cooling. Even if a portion of the energy delivered by the cooled-wet electrode was absorbed by the cooling solution, the total energy delivered to the tissue by the cooled electrode may be higher than the energy delivered by the open-perfused electrode [12, 22, 24].
Here, the bipolar RFA using the cooled-wet electrodes and 150 W-generator for 20 min created the ablation zone with the mean Dl 6.5±0.8 cm, the mean Dv 5.0±0.4 cm, the mean Ds 5.5±0.7 cm and the mean volume 93.5±26 cm3. Considering that the required RFA volume to create the lesion with the 3-cm diameter including the 1-cm safety margin is 65.42 cm3, the bipolar RFA consisting of the cooled-wet electrode may improve the treatment efficacy. In addition, the ratio of Dl/Dv was 1.3, which reflects its near spherical shape. Recently, Burdio et al. [29] described that the saline-enhanced bipolar RFA using the Pringle maneuver created a coagulation necrosis of 150 cm3. However, they used a single bipolar electrode with two uninsulated portions, which creates the elliptical-shaped ablation area with the long diameter along the axis of the electrode. For the induction of the complete necrosis of the tumor, the diameter of the short-axis of the ablation region is more critical than the ablation volume, because most liver tumors are spherical.
A few studies described that the saline-enhanced bipolar RFA generates coagulation necrosis more efficiently than the saline-enhanced monopolar RFA [11, 29, 30]. This may be due to several factors: first, in the bipolar mode, the current is delivered preferentially to the area between the electrodes, and therefore, a high current may be achieved. Second, heat trapping may occur between two electrodes in the bipolar mode. In the monopolar mode, the heat is diverted from the ablation site in all directions. In the bipolar mode, one electrode is thermally shielded by the opposite second electrode that also actively heats the tissue in its proximity. This traps the heat between the two electrodes, and higher temperatures are thus achieved than is the case with monopolar ablation.
However, the saline-enhanced bipolar RFA has some drawbacks. One drawback is that only two electrodes can be used at one time. Another drawback is that all the current originating from one electrode must enter the second electrode [10]. Furthermore, the heat generated in the vicinity of two electrodes cannot be controlled independently. An additional concern is the unexpected burn injury in the adjacent vital organs by the boiling saline. Since the risk of the burn injury is associated with the amount of saline used, further studies on the optimization of the amount and the concentration of NaCl solution are required. Finally, the electrodes must be placed in parallel, and the tumor must be located between them. However, in the clinical practice, the insertion of two electrodes beside the tumor in parallel is difficult.
Our experimental study has certain limitations. First, all ablations are performed in the normal liver parenchyma, not tumor tissues, in vitro. In tumor tissues, the cooling “sink” effect because of the blood flow has been detected. In tumor tissues, therefore, rapid heat exchange may occur [31]. It thus is difficult to predict accurately to what extent our results represent the real situation. However, despite such limitations, our model provides the basis for the comparison of the efficiency of different RF systems. Second, based on the procedure developed in the previous study [11], we tested only 5% NaCl solution. The procedure may not be applicable to the clinical practice due to the well-founded concern, the unexpected burn of vital tissues adjacent to the electrodes. It may be possible that the higher concentration of NaCl (10–20%) with the reduced flow rate (1 ml/min or less) may be applied without impairing the RF efficacy. Thus, to improve the efficacy of our model, the optimization of the concentration of the NaCl solution and the flow rate is required. Last, the effect of the prototype cooled-wet electrode is not optimized due to the relatively long distance between the outlet of saline and the tip of the cooled electrode (2.5 cm). Further modification that permits the saline instillation from the level of the electrode tip as the open-perfused electrode is required.
In conclusion, the saline-enhanced bipolar RFA using the cooled-wet electrode and the triple-power output generated ablation zones significantly larger than the open-perfused electrode. The increased coagulation necrosis area produced by the cooled-wet electrode may be due to the delivery of the higher energy, which was enabled by the cooling effect of the cooled-wet electrode.
References
Dodd GD, Soulen MC, Kane RA, Livraghi T, Lees WR, Yamashita Y, Gillams AR, Karahan OI, Rhim H (2000) Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics 20:9–27
Goldberg SN, Gazelle GS, Solbiati L, Livraghi T (2000) State of the art: tumor ablation with radio-frequency energy. Radiology 217:633–646
McGahan JP, Dodd GD (2001) Radiofrequency ablation of the liver: current status. Am J Roentgenol 176:3–16
Lim HK (2000) Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol 1:175–184
Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, Cova L, Halpern EF, Gazelle GS (2001) Percutaneous radiofrequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 221:159–166
Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS (2000) Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology 214:761–768
de Baere T, Elias D, Dromain C, Din MG, Kuoch V, Ducreux M, Boige V, Lassau N, Marteau V, Lasser P, Roche A (2000) Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. Am J Roentgenol 175:1619–1625
McGahan JP, Gu WZ, Brock JM, Tesluk H, Jones CD (1996) Hepatic ablation using bipolar radiofrequency electrocautery. Acad Radiol 3:418–422
Curley SA, Davidson BS, Fleming RY, Izzo F, Stephens LC, Tinkey P, Cromeens D (1997) Laparoscopically guided bipolar radiofrequency ablation of areas of porcine liver. Surg Endosc 11:729–733
Haemmerich D, Staelin ST, Tungjitkusolmun S, Lee FT, Mahvi DM, Webster JG (2001) Hepatic bipolar radiofrequency ablation between separated multiprong electrodes. IEEE Trans BioMed Eng 48:1145–1152
Lee JM, Han JK, Kim SH, Yoon BJ, Lee KH, An SK, Han CJ, Choi BI (2003) A comparative experimental study of the in vitro efficiency of hypertonic saline-enhanced hepatic bipolar and monopolar radiofrequency ablation. Korean J Radiol 4:163–169
Goldberg SN, Solbiati L, Hahn PF, Cosman E, Conrad JE, Fogle R, Gazelle GS (1998) Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology 209:371–379
Ni Y, Miao Y, Mulier S, Yu J, Baert AL, Marchal G (2000) A novel cooled-wet electrode for radiofrequency ablation. Eur Radiol 10:852–854
Miao Y, Ni Y, Yu J, Marchal G (2000) A comparative study on validation of a novel cooled-wet electrode for radiofrequency liver ablation. Invest Radiol 35:438–444
Gangi A, Guth S, Imbert J (2003) Interest of radiofrequency liver tissue ablation with a bipolar-wet electrode. Eur Radiol 13 [Suppl 1]:477 (ECR 2003 meeting)
Lee JD, Lee JM, Kim SW, Kim CS, Mun WS (2001) MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol 2:151–158
Morimoto M, Sugimori K, Shirato K, Kokawa A, Tomita N, Saito T, Tanaka N, Nozawa A, Hara M, Sekihara H, Shimada H, Imada T, Tanaka K (2002) Treatment of hepatocellular carcinoma with radiofrequency ablation: radiologic–histologic correlation during follow-up periods. Hepatology 35:1467–1475
Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, Kohara K, Shigenobu S, Ishibashi K, Arima T (2003) Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer 97:1253–1262
Hori T, Nagata K, Hasuike S, Onaga M, Motoda M, Moriuchi A, Iwakiri H, Uto H, Kato J, Ido A, Hayashi K, Tsubouchi H (2003) Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol 38:977–981
Dodd GD, Frank MS, Aribandi M, Chopra S, Chintapalli KN (2002) Radiofrequency thermal ablation: computer analysis created by overlapping ablations. Am J Roentgenol 177:777–782
Goldberg SN, Gazelle GS, Mueller PR (2000) Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques and diagnostic imaging guidance. Am J Roentgenol 174:323–331
Goldberg SN (2001) Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound 13:129–147
Choi D, Lim HK, Kim MJ, Lee J, Kim SK, Kim EY, Kim S, Kim SH (2003) Overlapping ablation using a coaxial radiofrequency electrode and multiple cannulae system: experimental study in ex vivo bovine liver. Korean J Radiol 4:117–123
de Baere T, Denys A, Wood BJ, Lassau N, Kardache M, Vilgrain V, Menu Y, Roche A (2001) Radiofrequency liver ablation: experimental comparative study of water-cooled versus expandable system. Am J Roentgen 176:187–192
Miao Y, Ni Y, Mulier S, Wang K, Hoey MF, Mulier P, Penninckx F, Yu J, De Scheerder I, Baert AL, Marchal G (1997) Ex vivo experiment on radiofrequency. J Surg Res 71:19–24
Lee JM, Kim YK, Lee YH, Kim SW, Li CA, Kim CS (2003) Percutaneous radiofrequency thermal ablation with hypertonic saline injection: in vivo study in a rabbit liver model. Korean J Radiol 4:27–34
Goldberg SN, Ahmed M, Gazelle GS, Kruskal JB, Huertas JC, Halpern EF, Oliver BS, Lenkinski RE (2001) Radiofrequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating, and coagulation-phantom and porcine liver study. Radiology 219:157–165
Goldberg SN, Hahn PF, Halpern EF, Fogle RM, Gazelle GS (1998) Radiofrequency tissue ablation: effect of pharmacologic modulation of blood flow on coagulation diameter. Radiology 209:761–769
Burdio F, Guemes A, Burdio JM, Navarro A, Sousa R, Castiella T, Cruz I, Burzaco O, Lozano R (2003) Bipolar saline-enhanced electrode for radiofrequency ablation: results of experimental study of in vivo porcine liver. Radiology 229:447–456
Burdio F, Guemes A, Burdio JM, Castiella T, De Gregorio MA, Lozano R, Livraghi T(1999) Hepatic lesion ablation with bipolar saline-enhanced radiofrequency in the audible spectrum. Acad Radiol 6:680–686
Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK (1998) Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg 227:559–565
Acknowledgements
This study was supported by grant no. 09-2003-012-0 from the Seoul National University Hospital Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J.M., Han, J.K., Kim, S.H. et al. Bipolar radiofrequency ablation in ex vivo bovine liver with the open-perfused system versus the cooled-wet system. Eur Radiol 15, 759–764 (2005). https://doi.org/10.1007/s00330-004-2375-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-004-2375-4