Abstract
Current protocols for surveillance after endovascular repair (EVAR) of abdominal aortic aneurysms are mostly based on costly and time-consuming imaging procedures and aim to detect adverse events such as graft migration, endoleaks or aneurysm sac enlargement. These imaging procedures are either associated with radiation exposure to the patients or may be harmful to the patient due to the use of iodine- or gadolinium-containing contrast agents. Furthermore the advantages of EVAR in the short term might be negated by the necessity for endograft surveillance over years. Thus, alternative modalities for follow-up are being investigated. One of these technologies provides pressure information directly from the aneurysm sac. This noninvasive, telemetric pressure sensing was tested in vitro as well as in first clinical trials and was able to identify successful aneurysm exclusion after EVAR. The telemetric pressure sensors showed a promising efficacy and accuracy in detecting type I and type III endoleaks and will help to clarify the clinical relevance of type II endoleaks. This article provides an overview of the in vitro sensors investigated as well as the first clinical trials and the sensors’ potential to change the current endograft surveillance regimes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For decades the gold standard for the treatment of abdominal aortic aneurysm (AAA) was an open surgical repair. As an alternative to conventional surgery, Parodi et al. first reported in 1991 the endovascular repair (EVAR) of AAA as a less invasive technique to exclude the aneurysm sac from systemic pressure [1]. After several clinical trials two devices were subsequently granted US Food and Drugs Administration (FDA) approval for clinical use in 1999, and the first encouraging results led to their uptake as an alternative for AAA repair. In recent years this endovascular treatment has become more prevalent, mostly due to its lower perioperative risks and a shorter hospitalization [2]. But the promising low rate of short-term complications might be negated by increased complications in the long term and the need for regular follow-up investigations over years. One could argue that during the initial clinical use the knowledge of stent-graft design, materials, and implantation techniques was not sufficient to provide reliable long-term durability after EVAR. Despite improvements in stent-grafts over the last decade one of the most frequent complications is still the occurrence of endoleaks. Associated with a recurrent high pressure in the aneurysm sac, type I and type III endoleaks in particular are undoubtedly considered clinically relevant, in the same way as a continuous enlargement of the aneurysm [3]. The clinical relevance of type II endoleaks is still controversial. However, even if there is no evidence of an endoleak detected by current surveillance techniques, including contrast-enhanced computed tomography (CT), duplex ultrasound, and angiography, the aneurysm sac may not be completely isolated from the circulation. This phenomenon has been termed “endotension” and might occur due to a lack of sensitivity of the techniques used [4–6]. To improve the sensitivity ultrasound contrast agents and advanced magnetic resonance imaging (MRI) were investigated but have yet to demonstrate widespread clinical feasibility [7–12]. These sophisticated techniques are either strongly dependent on the examination skills of the radiologist or require expensive imaging modalities.
Nowadays, the imaging follow-up after EVAR focuses, among other things, on safe endograft fixation without any evidence of graft migration, module disconnection, or material fatigue. Moreover, recurrent pressurization of the aneurysm sac is indirectly measured by changes in aneurysm size as well as contrast enhancement in the aneurysm sac. Since these techniques do not provide any direct pressure information from the aneurysm sac additional pressure measurements have been proposed to either identify successful exclusion or to simplify the detection of endoleaks during follow-up. A few invasive pressure measurement techniques have been investigated and showed clinical feasibility in a small group of patients [13–16], but they have not made their way into the current surveillance protocols.
The development of minimally invasive implantable telemetric pressure sensors was encouraged with the introduction of EVAR. Furthermore the discussion about the clinical relevance of endoleaks led to some in vitro and in vivo investigations as well as the first multicenter clinical trial demonstrating the feasibility and efficacy of noninvasive pressure-measuring systems. Subsequently one of the currently investigated systems recently received FDA approval for use in an acute setting after EVAR.
Monitoring the pressure within the aneurysm sac with an implantable telemetric pressure sensor will be an easy and convenient method in the surveillance of EVAR. A telemetric pressure sensor, integrated into the EVAR procedure or the endograft, will help to detect endoleaks and may obviate the necessity for further surveillance investigations. In this way a telemetric pressure sensor may help to minimize peri- and postinterventional complications and reduce the need for costly and time-consuming imaging procedures as well as the radiation exposure of the patients.
Implantable Pressure Sensors
To date three different types of pressure sensors have been investigated. All three sensors use different technologies of transmitting the pressure from inside the body to an external antenna. In 2003 the ImPressure AAA Sac Pressure Sensor (Remon Medical Technologies, Caesarea, Israel) was the first implanted pressure sensor used in animal models and a small clinical trial. The EndoSure Wireless AAA Pressure Sensor (CardioMems, Atlanta, GA, USA) is the only pressure sensor with FDA approval for acute implantation and initial confirmation of AAA exclusion. The TPS Telemetric Pressure Sensor (Helmholtz Institute for Biomedical Engineering and the Institute of Materials in Electrical Engineering, RWTH Aachen, Germany) is based on a completely digital data-processing and transmitting unit.
EndoSure Wireless AAA Pressure Sensor
The EndoSure sensor consists of a resonant circuit composed of flexible plates representing the capacity as well as inductor windings. These components are embedded within a fused silica matrix and surrounded by a nitinol basket (Fig. 1). Changes in the surrounding pressure change the capacitance and therefore the resonant frequency. This system, like the others, does not contain any internal energy source or battery. An external antenna activates the sensor over a radiofrequency impulse and receives the pressure-dependent change in the resonant frequency of the sensor. This resonant frequency change is converted into a real-time pressure measurement of the sensor-surrounding cavity. The relatively simple structure of the sensor itself is robust but does not provide any error correction systems for interference from other external radiofrequency fields. Due to the sensor’s resonant coil and its surrounding nitinol basket it is radiopaque enough to be clearly seen under fluoroscopy during the intervention [17–20].
EndoSure pressure sensor manufactured by CardioMems, Inc.. Reprinted from [27] with permission from Elsevier
ImPressure AAA Sac Pressure Sensor
The ImPressure sensor is activated by surface acoustic waves from a handheld probe which charge a capacitor by means of a piezoelectric element (Fig. 2). The incorporated sensor measures the surrounding pressure and transmits the data via acoustic waves back to the handheld probe [21–24]. These acoustic waves are translated into a pressure curve which can be monitored in real time. As the smallest of the three investigated sensors the ImPressure sensor is less radiopaque but is still visible, as well as being permanently fixed to the outer wall of the endograft. This AAA sensor has been further developed to be used in other circumstances, and Remon Medical Technologies has recently announced the first implantation of a pressure sensor as an implantable hemodynamic monitor analogous to the ImPressure sensor. The first implantation in four patients is part of a trial to fulfill the requirements of CE mark regulations and will develop the surface acoustic waves technology.
ImPressure sensor manufactured by Remon Medical Technologies, Inc. Reprinted from [27] with permission from Elsevier
TPS Telemetric Pressure Sensor
The Helmholtz-Institute for Biomedical Engineering, RWTH Aachen in cooperation with the Institute of Materials in Electrical Engineering, RWTH Aachen developed a TPS Telemetric Pressure Sensor with a completely digital in-capsule data processing unit [25, 26]. Together with the digital transmission of the data stream over an inductive link this system allows minimization of external interference and the detection of errors during transmission (Fig. 3). The sensor capsule comprises a radiopaque coil and can easily be seen under fluoroscopy. Although this system has only been tested in an in vitro model it represents another promising technology among the implantable wireless pressure sensors. In further clinical studies the durability and accuracy of the TPS Telemetric Pressure Sensor need to be evaluated.
AAA pressure sensor manufactured by the Helmholtz Institute for Biomedical Engineering, RWTH Aachen in cooperation with the Institute of Materials in Electrical Engineering, RWTH Aachen, Germany. Reprinted from [26] with permission from Springer
Sensor Implantation
The above-mentioned pressure sensors were implanted into the aneurysm sac using two different techniques. The ImPressure sensor was hand-sewn to the outside of the endograft before the EVAR procedure. Aorto-uniiliac endografts did not need an upsized delivery sheath but could be repackaged into the original one. Bifurcated endografts needed only a slight upsizing of their delivery sheath from 18 Fr to 20 Fr [23, 24]. Moreover, the ImPressure sensor should be attached to the main body of the endograft in such a way that the sensor will measure the pressure inside the excluded aneurysm sac without being pushed against either the aneurysm wall or the iliac limbs. Positioning the sensor between the two limbs of the endograft has resulted in less reliable pressure measurements and should be avoided [23].
In contrast, the EndoSure sensor is deployed through its own delivery catheter over a superstiff guidewire in the contralateral iliac artery during the EVAR procedure [18]. The sensor with its surrounding wire basket is positioned inside the aneurysm sac and fixed to its tether. Subsequently the tether is removed at the end of the EVAR procedure and releases the pressure sensor into the aneurysm sac. The sensor will then be kept in place by means of its surrounding wire basket, which does not have any electrical function (Fig. 4).
Computed tomography scan demonstrating the EndoSure sensor. Black arrow highlights the sensor residing in the mural thrombus separately from the endograft which is clearly visualized within the aneurysm sac. The sensor is surrounded by the nitinol cage. Reprinted from [27] with permission from Elsevier
The TPS telemetric pressure sensor can easily be inserted over a regular 11 Fr introducer sheath and pushed with the dilator tip to the aneurysm sac [26]. Because of its small dimensions it might also be possible to suture the sensor to the outside of the endograft and deploy the complete system during EVAR. The feasibility of this method has already been demonstrated with the EndoSure sensor, which is of comparable size [18]. Since the TPS sensor is a relatively new one the deployment and fixation technique will be improved in future studies.
If one pressure sensor can accurately be positioned inside the aneurysm sac and measure the surrounding pressure it raises the question whether more than one sensor may increase the sensitivity in detecting endoleaks in cases of possible compartmentalization and unequal pressure distribution. However, as this issue is still controversial and not every aneurysm sac provides enough space to accommodate more than one pressure sensor, the question will have to be addressed in future studies.
In Vitro Experience and Clinical Trials
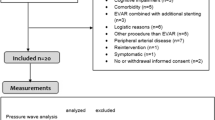
In one of the first studies the ImPressure sensor was implanted in a porcine AAA model demonstrating the feasibility and efficacy of wireless pressure measurements in excluded aneurysm sacs [22]. In a further study the sensor also showed its durability and validity over an 8 week follow-up period even if the sensor was deeply embedded in organized thrombus. Under those circumstances the sensor did not show any calibration errors or drift of pressure measurement [21]. In 2004 Ellozy et al. published the results of the first clinical trial of a noninvasive pressure measurement device integrated in an endovascular repair of an AAA [24]. In this study the ImPressure sensor was hand-sewn to the outside of the endograft before the procedure. Because of this sensor’s small dimensions the delivery sheath did not have to be upsized in the case of an aorto-uniiliac device, but in the case of a bifurcated device a small upsizing from 18 Fr to 20 Fr was necessary to repack the endograft in the delivery sheath. The results in the 14 patients enrolled in this clinical trial were promising, as in 13 of the 14 the EVAR procedure was successful at the initial implantation and intrasac pressure measurements could be obtained postoperatively in all patients. The use of the ImPressure sensor was further investigated and the results of the enlarged trial were then published in 2006 [23]. The implantation of the endograft was technically successful in 20 of 21 patients with no evidence of endoleaks. Nevertheless, four sensors were placed between the two iliac limbs and measured extraordinarily high pressures, probably because of compression artifacts. Two sensors were implanted and never functioned throughout the whole study. Both after 12 months and at the final follow-up the median mean pressure index of the aneurysm sac was significantly lower in patients with shrinking aneurysms than in patients with stable aneurysms. In the case of a type II endoleak the mean pressure index did not show a significant correlation with the aneurysm sac diameter, most likely due to the great variety in the pressure-transmitting nature of type II endoleaks.
The EndoSure sensor for EVAR was investigated in a canine model designed for the evaluation of the sensor’s accuracy in the presence of organized thrombus [19]. Moreover, the influence of intentionally created type II endoleaks on aneurysm sac pressure was investigated [20]. The intra-aneurysmal pressure was significantly lower than the systemic pressure in the case of a patent type II endoleak. The patency of the endoleak was confirmed with angiography and cine magnetic resonance angiography throughout the whole study. The endoleaks produced pulsatile pressurization of the aneurysm sac which could be accurately monitored even if surrounded by dense thrombus. To confirm aneurysm sac exclusion by means of the EndoSure sensor in a clinical setting a total of 90 patients were enrolled in the APEX Trial (Acute Pressure Measurement to Confirm Aneurysm Sac Exclusion), a prospective, multicenter and international study [18]. In this ongoing trial, more than 100 EndoSure pressure sensors have been implanted and are still in included in a continuous follow-up regime. Of the first 90 patients 76 were eligible for evaluation according to the study protocol. The EndoSure sensor was able to detect all type I equivalent endoleaks before the insertion of the contralateral limb and pressure measurements showed an excellent correlation with the simultaneously obtained angiographic catheter-based pressure measurements. According to the protocol, a reduction in sac pressure pulsatility of less than 30% was by definition treated as a type I or type III endoleak. Thus, the EndoSure wireless pressure sensor detected type I and type III endoleaks after completion of the initial EVAR procedure with a sensitivity of 0.939 and a specificity of 0.800. At the 30 day evaluation the sensor signal could be detected in 97% of the study population.
To date the TPS telemetric pressure sensor has been investigated only in an in vitro model with several settings mimicking different clinical situations [26] and has not yet been implanted in patients after EVAR. In the in vitro model the TPS sensor was able to detect every simulated endoleak as well as the complete exclusion of the aneurysm sac. Even if embedded completely into thrombus the sensor transmitted the pressure within the aneurysm sac accurately to the external antenna. The feasibility and efficacy of this sensor need to be further evaluated in clinical trials to enlarge the experience with this promising technology in a clinical setting.
Endoleak Detection
The efficacy of implantable wireless pressure sensors in detecting endoleaks has been evaluated in animal models and clinical trials. The noninvasive pressure measurement has proven its accuracy in detecting especially type I and III endoleaks, as well as in identifying successful exclusion of the aneurysm sac in the acute setting after EVAR. The clinical relevance in detecting type II endoleaks is not yet clarified since these endoleaks are associated with different sac pressures. Elevated and diminished sac pressures in cases of patent type II endoleaks strongly rely on specific configurations of in- and outflow channels through aortic side branches and cannot predict their clinical relevance. In future studies endotension as well as the clinical relevance of type II endoleaks need to be further evaluated over a longer time period and correlated with aneurysm sac growth diameter and other adverse events.
Type I Endoleaks
In an in vitro model the TPS telemetric pressure sensor was able to detect type I, II, and III endoleaks in terms of accurately measuring and transmitting an increased intrasac pressure [26].
In accordance with these in vitro results the ImPressure sensor was able to clearly diagnose a distal type I endoleak at 1 month follow-up in 1 of 21 patients. This endoleak was associated with a high intrasac pressure which diminished after the endoleak was sealed with an iliac extension cuff [23]. Furthermore, in the APEX Trial a large distal type I endoleak equivalent was defined as an endoleak after deployment of the endograft’s main body but before deploying the contralateral limb. In this case the accuracy of the EndoSure sensor proved to be 100% during EVAR. Overall, the sensitivity was 93.9% and specificity was 80% for detecting true type I or III endoleaks during follow-up [18].
Type II Endoleaks
In a porcine model type II endoleaks were artificially created with patent lumbar arteries during initial exclusion of the aneurysm sac and a remaining high pressure (almost 70% of mean arterial pressure) could be detected by means of the ImPressure sensor. Furthermore a decrease in intrasac pressure suggested a spontaneous closure of two endoleaks but was not confirmed at CT or angiography in this study [22]. In contrast, a canine model of retrograde-collateral (type II) endoleaks was developed to evaluate the accuracy of the EndoSure sensor and to detect the intentionally created endoleaks [20]. This sensor was able to measure the increased aneurysm sac pressure (up to 60% of systemic pressure) in the case of confirmed patency of endoleaks.
But in contrast to the previous animal models the results were less clear in the first clinical trial with the ImPressure sensor [24]. Ellozy et al. published their small study in 2006 which showed that 5 of the 14 patients who finished the study protocol up to at least 6 month follow-up, developed a type II endoleak at some point in the study [23]. These endoleaks were associated with elevated sac pressures in 3 patients and diminished sac pressures in 2 and either sealed spontaneously or were left untreated. Nevertheless, the later thrombosis of one type II endoleak resulted in a significantly reduced intrasac pressure.
Type III Endoleaks
In an animal model the ImPressure sensor was able to detect intentionally created type III endoleaks by identifying intrasac pressure changes from a flatline trace to a high-pressure, pulsatile waveform in all of the 8 investigated pigs [22]. These endoleaks were created 2 weeks after the initial exclusion of the sac to mimic the clinical situation of developing type III endoleaks weeks after EVAR. As mentioned above, the EndoSure sensor was also able to detect type III endoleaks with good sensitivity (93.9%) and specificity (80%) in a large-scale clinical trial [18].
Endotension
As the phenomenon of endotension is defined as a remaining or developing high pressure in the excluded aneurysm sac without any evidence of endoleaks, a spontaneously sealed endoleak might cause endotension. For instance, in the small clinical trial reported by Ellozy et al. a lumbar endoleak thrombosed at 6 months but left an elevated intrasac pressure (106% of systemic pressure), and in the APEX Trial 4 patients showed a less than 30% reduction in sac pressure but without any evidence of endoleaks at angiography [18, 23]. Unfortunately there are no data on the aneurysm diameter in these cases, as they might support the hypothesis of endotension as sealed or thrombosed endoleaks transmitting systemic pressure to the “excluded” aneurysm sac.
Future Surveillance Regimes
Since the first years of successful use of EVAR techniques for AAA repair, continuous improvements in endograft design have been accompanied by new follow-up regimes. The new surveillance protocols are seeking both to reduce the radiation exposure to the patients as well as to minimize the administration of contrast-medium. Moreover because of exploding costs in health care another important aim is to find alternatives to the current costly and time-intensive imaging procedures [27, 28].
Direct pressure measurement within the excluded aneurysm sac is still one of the most promising technologies to simplify the surveillance protocols. The first clinical trials with minimally invasive implantable telemetric pressure sensors have demonstrated the feasibility and durability of the sensors [18, 23, 24]. Since the clinical trials have not yet evaluated a sufficient number of patients over the long term, i.e. several years, it is not clear how current protocols might be changed without failing to detect adverse events such as graft migration. In clinical trials minimally invasive implanted pressure sensors were able to detect type I and type III endoleaks directly after EVAR [18] as well as type II endoleaks [23, 24]. The pressure sensors mostly functioned without any problems for months, even if embedded deeply into thrombus, although a possible compartmentalization of the excluded aneurysm sac could not be investigated in detail. Thus new surveillance protocols based on additional noninvasive pressure measurements seem to be achievable but still have to include other improved imaging modalities. In a literature review the pooled estimates of sensitivity and specificity for unenhanced color duplex ultrasound (CDU) compared with CT angiography were 66% and 93%, respectively. This relatively low sensitivity was improved to 81%, with a specificity of 82%, for enhanced CDU [29]. A systematic literature review revealed that unenhanced CDU is particular insensitive in the detection of type II endoleaks [30, 31]. With the use of second-generation ultrasound contrast agents the sensitivity is increased and might even be better than the current gold standard of CT angiography [7, 29, 32, 33]. However, this examination still needs to be performed by highly skilled radiologists. Furthermore, the use of magnetic resonance imaging (MRI) for surveillance of endovascular aneurysm repair has been evaluated with good results [9–12]. In patients with nitinol endografts the sensitivity of gadolinium-enhanced MRI compared with CT for the detection of type II endoleaks was 94% and 50%, respectively [10]. Pitton et al. reported that the overall accuracy of MRI in endoleak detection and correct sizing was 95.2%, compared with only 58.3% for contrast-enhanced biphasic CT, and concluded that MRI is significantly superior [34]. These results are supported by a newer published clinical trial by van der Laan et al. [35]. Krämer and colleagues demonstrated that MRI is also superior to CT in demonstrating the anatomy of the feeding vessel and shows fewer artifacts after coil embolization of endoleaks [36].
However, the discussion about nephrogenic systemic fibrosis possibly associated with gadolinium-based contrast agents may cast a negative light on this promising technique, especially in patients with severe renal impairment [37–41]. Moreover all investigated pressure sensors either have to be fixed to the outer surface of the endograft [21–24], leading to an upsizing of the introducer sheath, or have to be deployed through their own catheter system [18–20]. To prevent this upsizing, in vitro and then animal studies have been carried out with flexible and foldable wireless passive pressure sensors, with encouraging results. But these sensors showed a significant baseline drift of the pressure measurements which needs to be improved in the future [42].
Conclusion
Since the results of the first large clinical trials with minimally invasive implantable pressure sensors are encouraging, this technology might be able to change the current surveillance protocols after EVAR. In leakage simulation models and in clinical trials the sensors demonstrated their feasibility and efficacy for detecting type I and III endoleaks. The sensitivity in detecting type II endoleaks and classifying their clinical relevance is less clear and has to be evaluated in further trials with follow-up of several years. Nevertheless, EVAR will be more widely accepted if foldable telemetric pressure sensors can be integrated into the endograft without the necessity of upsizing the introducer sheath or performing additional procedures during EVAR. The possibility of simplifying the routine follow-up regime as well as of reducing costs and adverse events of imaging procedures will then change the current surveillance protocols.
References
Parodi JC, Palmaz JC, Barone HD (1991) Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 5:491–499
Jordan WD, Alcocer F, Wirthlin DJ, et al. (2003) Abdominal aortic aneurysms in “high-risk” surgical patients: Comparison of open and endovascular repair. Ann Surg 237:623–629; discussion 629–630
Golzarian J, Valenti D (2006) Endoleakage after endovascular treatment of abdominal aortic aneurysms: Diagnosis, significance and treatment. Eur Radiol 16:2849–2857
Dubenec SR, White GH, Pasenau J, et al. (2003) Endotension. A review of current views on pathophysiology and treatment. J Cardiovasc Surg (Torino) 44:553–557
Gilling-Smith G, Brennan J, Harris P, et al. (1999) Endotension after endovascular aneurysm repair: Definition, classification, and strategies for surveillance and intervention. J Endovasc Surg 6:305–307
White GH, May J, Petrasek P, et al. (1999) Endotension: An explanation for continued AAA growth after successful endoluminal repair. J Endovasc Surg 6:308–315
Napoli V, Bargellini I, Sardella SG, et al. (2004) Abdominal aortic aneurysm: Contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology 233:217–225
d’Audiffret A, Desgranges P, Kobeiter DH, et al. (2001) Follow-up evaluation of endoluminally treated abdominal aortic aneurysms with duplex ultrasonography: Validation with computed tomography. J Vasc Surg 33:42–50
Cejna M, Loewe C, Schoder M, et al. (2002) MR angiography vs CT angiography in the follow-up of nitinol stent grafts in endoluminally treated aortic aneurysms. Eur Radiol 12:2443–2450
Haulon S, Lions C, McFadden EP, et al. (2001) Prospective evaluation of magnetic resonance imaging after endovascular treatment of infrarenal aortic aneurysms. Eur J Vasc Endovasc Surg 22:62–69
Schwope RB, Alper HJ, Talenfeld AD, et al. (2007) MR angiography for patient surveillance after endovascular repair of abdominal aortic aneurysms. AJR Am J Roentgenol 188:W334–340
Thurnher S, Cejna M (2002) Imaging of aortic stent-grafts and endoleaks. Radiol Clin North Am 40:799–833
Dias NV, Ivancev K, Malina M, et al. (2004) Intra-aneurysm sac pressure measurements after endovascular aneurysm repair: Differences between shrinking, unchanged, and expanding aneurysms with and without endoleaks. J Vasc Surg 39:1229–1235
Dias NV, Ivancev K, Malina M, et al. (2004) Direct intra-aneurysm sac pressure measurement using tip-pressure sensors: In vivo and in vitro evaluation. J Vasc Surg 40:711–716
Baum RA, Carpenter JP, Cope C, et al. (2001) Aneurysm sac pressure measurements after endovascular repair of abdominal aortic aneurysms. J Vasc Surg 33:32–41
Sonesson B, Dias N, Malina M, et al. (2003) Intra-aneurysm pressure measurements in successfully excluded abdominal aortic aneurysm after endovascular repair. J Vasc Surg 37:733–738
Allen MG (2005) Micromachined endovascularly-implantable wireless aneurysm pressure sensors: from concept to clinic. The 13th International Conference on Solid-State Sensors, Actuators and Microsystems, Seoul, Korea, Digest of Technical Papers, TRANSDUCERS ‘05:275–278
Ohki T, Ouriel K, Silveira PG, et al. (2007) Initial results of wireless pressure sensing for endovascular aneurysm repair: The APEX Trial—Acute Pressure Measurement to Confirm Aneurysm Sac EXclusion. J Vasc Surg 45:236–242
Ohki T, Yadav J, Gargiulo N, et al. (2003) Preliminary results of an implantable wireless aneurysm pressure sensor in a canine model: Will surveillance CT scan following EVAR become obsolete? J Endovasc Ther 10(Suppl):1–32
Chaer RA, Trocciola S, DeRubertis B, et al. (2006) Evaluation of the accuracy of a wireless pressure sensor in a canine model of retrograde-collateral (type II) endoleak and correlation with histologic analysis. J Vasc Surg 44:1306–1313
Milner R, Ruurda JP, Blankensteijn JD (2004) Durability and validity of a remote, miniaturized pressure sensor in an animal model of abdominal aortic aneurysm. J Endovasc Ther 11:372–377
Milner R, Verhagen HJ, Prinssen M, et al. (2004) Noninvasive intrasac pressure measurement and the influence of type 2 and type 3 endoleaks in an animal model of abdominal aortic aneurysm. Vascular 12:99–105
Ellozy SH, Carroccio A, Lookstein RA, et al. (2006) Abdominal aortic aneurysm sac shrinkage after endovascular aneurysm repair: Correlation with chronic sac pressure measurement. J Vasc Surg 43:2–7
Ellozy SH, Carroccio A, Lookstein RA, et al. (2004) First experience in human beings with a permanently implantable intrasac pressure transducer for monitoring endovascular repair of abdominal aortic aneurysms. J Vasc Surg 40:405–412
Schlierf R, Görtz M, Schmitz-Rode T, et al. (2005) Pressure sensor to control the treatment of abdominal aorta aneurisms. The 13th International Conference on Solid-State Sensors, Actuators and Microsystems, Seoul, Korea, Digest of Technical Papers, TRANSDUCERS ‘05:1656–1659
Springer F, Schlierf R, Pfeffer JG, et al. (2007) Detecting endoleaks after endovascular AAA repair with a minimally invasive, implantable, telemetric pressure sensor: An in vitro study. Eur Radiol 17:2589–2597
Milner R, Kasirajan K, Chaikof EL (2006) Future of endograft surveillance. Semin Vasc Surg 19:75–82
Carnero L, Milner R (2006) Aneurysm sac pressure measurement with a pressure sensor in endovascular aortic aneurysm repair. Vascular 14:264–269
Sun Z (2006) Diagnostic value of color duplex ultrasonography in the follow-up of endovascular repair of abdominal aortic aneurysm. J Vasc Interv Radiol 17:759–764
AbuRahma AF, Welch CA, Mullins BB, et al. (2005) Computed tomography versus color duplex ultrasound for surveillance of abdominal aortic stent-grafts. J Endovasc Ther 12:568–573
Ashoke R, Brown LC, Rodway A, et al. (2005) Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: A systematic review. J Endovasc Ther 12:297–305
Henao EA, Hodge MD, Felkai DD, et al. (2006) Contrast-enhanced Duplex surveillance after endovascular abdominal aortic aneurysm repair: Improved efficacy using a continuous infusion technique. J Vasc Surg 43:259–264; discussion 264
Bendick PJ, Zelenock GB, Bove PG, et al. (2003) Duplex ultrasound imaging with an ultrasound contrast agent: The economic alternative to CT angiography for aortic stent graft surveillance. Vasc Endovascular Surg 37:165–170
Pitton MB, Schweitzer H, Herber S, et al. (2005) MRI versus helical CT for endoleak detection after endovascular aneurysm repair. AJR Am J Roentgenol 185:1275–1281
van der Laan MJ, Bartels LW, Viergever MA, et al. (2006) Computed tomography versus magnetic resonance imaging of endoleaks after EVAR. Eur J Vasc Endovasc Surg 32:361–365
Krämer SC, Gorich J, Pamler R, et al. (2002) The contribution of MRI to the detection of endovascular aneurysm repair. Rofo 174:1285–1288
Chewning RH, Murphy KJ (2007) Gadolinium-based Contrast Media and the Development of Nephrogenic Systemic Fibrosis in Patients with Renal Insufficiency. J Vasc Interv Radiol 18:331–333
High WA, Ayers RA, Chandler J, et al. (2007) Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 56:21–26
Khurana A, Runge VM, Narayanan M, et al. (2007) Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Invest Radiol 42:139–145
Pedersen M (2007) Safety update on the possible causal relationship between gadolinium-containing MRI agents and nephrogenic systemic fibrosis. J Magn Reson Imaging 25:881–883
Sadowski EA, Bennett LK, Chan MR, et al. (2007) Nephrogenic systemic fibrosis: Risk factors and incidence estimation. Radiology 243:148–157
Fonseca MA, Allen M, Kroh J, et al. (2006) Flexible wireless passive pressure sensors for biomedical applications. Solid-State Sensors, Actuators, and Microsystems Workshop, Hilton Head Island, South Carolina, June 4–8, pp 37–42
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Springer, F., Günther, R.W. & Schmitz-Rode, T. Aneurysm Sac Pressure Measurement with Minimally Invasive Implantable Pressure Sensors: An Alternative to Current Surveillance Regimes after EVAR?. Cardiovasc Intervent Radiol 31, 460–467 (2008). https://doi.org/10.1007/s00270-007-9245-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-007-9245-9