Abstract
Endoleak, also called leakage, leak and Perigraft leak, is a major complication and its persistence represents a failure of endovascular aortic aneurysm repair. Its detection and treatment is therefore of primary importance, since endoleak can be associated with pressurization (increase in pressure) of the sac, resulting in expansion and rupture of the aneurysm. The aim of this paper is to discuss the definition, significance, diagnosis and different options to treat endoleak.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endoleak is defined as the persistent perfusion of the aneurysmal sac after endovascular aortic aneurysm repair (EVAR). A leak can appear during the first 30 days after implantation. This type of leak is called “primary endoleak”. Secondary endoleak is one that occurs after 30 days [1]. Leaks have also been classified as graft-related or nongraft-related. The incidence of endoleak varies from 10 to 50% [1, 2]. In a report from the EUROSTAR registry, the incidence of early endoleak was 18% [1]. Sixty–nine percent of these leaks were graft related. Seventy percent sealed spontaneously during the first 6 months without a difference between graft-related and non-graft-related endoleaks. There is not always a rational explanation of the cause of spontaneous resolution of some endoleaks and persistence or late occurrence of some others. The presence of outflow vessels (mainly lumbar arteries and inferior mesenteric artery) partially explains this phenomenon [3]. A leak communicating with these outflow vessels seldom disappears spontaneously [4]. Thus, these vessels should be identified. Whatever the cause of a persistent leak, it should be identified, monitored and eventually treated.
In this paper, we review endoleak classification and significance, diagnosis and treatment options for different types of endoleak.
Classification and significance
A generally accepted anatomic classification for endoleak has been developed over the years [5]. In this system, leaks are defined by their inflow source, regardless of the number and type of other vessels involved in the outflow (Table 1).
Type I endoleak
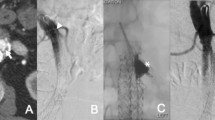
Type I endoleak is caused by failure to achieve a circumferential seal at either the proximal (Type IA) or distal end (Type IB) of the stent-graft. Type IC endoleak is due to non-occluded iliac artery in patients with aorto-mono-iliac stent and femoral-femoral bypass. With Type I endoleak, the aneurysm is perfused directly from the aorta or the iliac arteries (inflows). The leak usually communicates through a channel (sometimes multiple channels) with the aneurysmal sac (Fig. 1). There are several outflow vessels, mainly lumbar arteries and inferior mesenteric artery (IMA) that communicate with the channel and or the sac. The pressure within a Type I leak is systemic. The tension on the aortic wall remains high.
Type I endoleak. a Enhanced CT demonstrates an endoleak (white arrow). b Aortogram from right groin access demonstrates a Type IA endoleak with a good proximal position of the stentgraft. The aneurysmal sac (white arrow) is well demonstrated. c Selective catheterization of the channel using a Side Winder 2 catheter shows the aneurysmal sac and the channel (white arrow) communicating between collaterals (black arrow) and the sac. d Control aortogram after coil embolization of the channel (white arrow), the sac and the origin of outflow vessels
Causes of primary Type I endoleak include inappropriate anatomy, with a significantly angulated neck, significant calcification/plaque at the proximal or distal landing zone, a non-circular landing zone, malpositioning of the stent-graft, type of endograft and under-dilation of the stent-graft. Secondary Type I endoleak can be due to aneurysm re-modeling, resulting in stent-graft migration, progressive dilatation of the proximal neck, design and dimensions of stent-grafts or unfavorable infrarenal necks, including the conically shaped neck and neck shorter than 15 mm. Grafts whose fixation relies on radial force have been reported to be more prone to caudal migration and Type I endoleak than grafts with hooks [6]. Endothelialization of bare stents at the landing zones may contribute to a certain fixation, but endothelialization of the fabric itself does not happen. Proximal bare stent separation and hook fractures are also causes of delayed Type I endoleak. Oversizing the graft by 20% is recommended to prevent a delayed endoleak. At the iliac level, Type IB endoleak occurs when the limb of the graft is too short or migrates upward due to the sac’s retraction pressure and aortic distortion.
Although, Type I endoleak can seal spontaneously, risk of rupture is high and intervention is indicated [4, 7, 8].
Type II endoleak
Type II endoleak corresponds to the retrograde filling of the aneurysm mainly from lumbar arteries and/or IMA but also in rare situations from sacral, gonadal or accessory renal artery (Fig. 2).
Patient with persistent Type II endoleak and expansion of the aneurysmal sac. a CT shows an endoleak anterior (white arrow) and posterior (curved arrow) to the stentgraft. b SMA angiogram, late phase shows the endoleak (white arrow) through the arc of Riolan (black arrow). c Angiogram obtained after microcatheter catheterization of the inferior mesenteric artery (black arrow). The white arrow indicates the aneurysmal sac. The short white arrow demonstrates the channel that allows the communication between the inflow and outflow vessels. d After embolization of the channel (white arrow), the stagnation of contrast in the sac is well demonstrated (black arrow). e Angiogram obtained after embolization of the IMA (black arrow). f Aortogram shows freedom from endoleak. Note the patency of distal IMA (black arrow)
Type II endoleaks can be associated with aneurysmal expansion and rupture; however, this risk is much less than with the Type I and III endoleaks (0.5 versus 3.4 %) [9, 10]. A leak in the setting of a shrinking aneurysm can generally be followed, without immediate intervention. It is well established that up to 40% of Type II endoleaks will seal spontaneously. Some have advocated intervening in all endoleaks persisting beyond 3–6 months, while other groups recommended observing leaks in the absence of aneurysm expansion. We favor the last approach. In our experience with biphasic helical computed tomography (CT) follow-up of more than 300 patients treated by EVAR from 1994 to 1998, only three patients needed intervention for Type II endoleak.
Type III endoleak
Type III endoleaks are caused by a structural failure of the implanted device, including junctional separation of modular components, due to migration or changes in vessel morphology with aneurysm shrinkage, holes in the fabric, and fabric tears due to graft strut fracture or erosion (Fig. 3). Graft disconnections were not infrequent with the first stent-graft generation due to a short overlap between the main body and the limb [11].
Type III leaks allow direct communication between the aorta and aneurysm sac. They have systemic arterial pressure. Similar to Type I leaks, Type III endoleaks are considered to be very dangerous, since there is an acute re-pressurization of the sac and they need to be treated aggressively [10].
Type IV endoleak
Type IV leaks are caused by porosity of the graft fabric. They are seen at the time of device implantation, as a faint blush on the post-implantation angiogram, when patients are fully anti-coagulated. It is important to rule out other Types of endoleak before labeling a leak as Type IV. Type IV endoleak seal spontaneously.
Type V endoleak or endotension
Endotension (or Type V endoleak) corresponds to continued aneurysm expansion in the absence of a confirmed endoleak [12, 13]. Type V endoleak may be due to undiagnosed endoleak, presumably with very slow flow and suboptimal imaging (e.g., no delayed helical CT acquisition). Endotension has been reported in up to 18% [14] in a study evaluating the significance of endotension in 658 patients. The authors demonstrated that endotension is rare and concluded that it may represent missed endoleak rather than true aneurysm expansion in the absence of perigraft flow [15].
However, in most situations, endotension corresponds to an accumulation of yellowish fluid (seroma) [16]. Endotension is more common with ePTFE grafts due to ultra-filtration through graft pores [17].
Diagnosis
Contrast enhanced helical CT or CT angiography (CTA) is considered the imaging technique of choice for the detection of endoleak. CTA is reported to be superior to aortography for the demonstration of small leak [18]. The technique is also able to demonstrate the patency of lumbar arteries and IMA. However, selective aneurysmal angiography is superior to CTA for the detection of outflow vessels [4, 19].
The value of biphasic or triphasic CT scanning has been established for follow-up of EVAR [20, 21]. Some authors favor obtaining an unenhanced helical CT series. Rozenblit at al. [21] have demonstrated that the unenhanced series were helpful to diagnose an indeterminate endoleak in one patient. Important mimickers of endoleak include calcification, contrast within the folds of unsupported portions of the graft and residual endosac contrast from the initial procedure when early CT follow-up is obtained at 1–3 days. This “pseudo-endoleak” was seen in up to 57% of patients [22].
It has been demonstrated that delayed acquisition uncovered up to 11% of endoleaks that were missed by arterial phase alone [20, 21]. An optimal CT protocol for the monitoring of the aorta after endoluminal therapy should include a delayed acquisition (Fig. 4).
After obtaining the noncontrast images (1.25- to 5.0-mm thickness), an arterial phase will be obtained using bolus track technique. Depending on the length of the stented area, 100-150 ml of 300 mg of iodine per ml of contrast medium is injected. We usually use a flow rate of 3.5 ml of contrast medium per second. A delayed acquisition is typically acquired 70 s after the contrast medium is administered. To maximize aortic branch vessel and subtle endoleak depiction, thin section, overlapping images are suggested (1.0- to 1.5-mm detector width). However, for studies on 16–64 channel systems, acquiring isotropic datasets (submillimeter collimation) should always be considered, factoring in volume coverage, body habitus, and noise. For the delayed phase, similar parameters as the arterial phase are used.
Color Doppler ultrasound (CDUS) is a non-invasive and cost-effective imaging modality. It is highly dependent on the operator and has limitations in obese patients and those with excessive bowel gas. Patients should be evaluated after 5–6 h fasting, in the supine and lateral position. The aorta is evaluated both transversally and longitudinally. A leak is suspected when a reproducible color and Doppler signal inside the aneurysm is visualized.
Variable success is reported for the detection and localization of the source of endoleaks with ultrasound, depending on technical factors, the imaging protocol, and the image quality. Reported sensitivities for overall endoleak detection range between 12% and 100%, with specificities of 74–99% [23–27].In a series of 55 patients with CDUS compared with biphasic CTA, CT was superior in the detection of a small leak. Discrepancies between helical CT and CDUS were observed in eight patients (14.5%). In five cases, a small perigraft leak that was clearly demonstrated by helical CT was not found on CDUS. All these leaks were small and disappeared during the follow-up. For the diagnosis of endoleak, the sensitivity, the specificity, the positive and negative predictive values for CDUS compared with helical CT were, respectively, 77%, 90%, 85%, and 85% [27].
Administration of an ultrasound (US) contrast agent can increase the sensitivity for detecting endoleaks with color and power Doppler by 33–300%; however, the specificity may decrease by 17–30% [28–32]. Utilizing a US contrast agent may also enable detection of endoleaks that are not seen by CT angiography [31, 32].
MRI and MR angiography can provide all the information during EVAR follow-up for Nitinol based stent-grafts. As to detection of endoleaks, results are comparable with CT angiography for detecting Type I and Type III endoleaks. Depending on the CT section thickness and imaging protocol, MR angiography may yield a greater sensitivity to detect slow flow Type II endoleaks [33–36]. Blood pool magnetic resonance angiography has been found useful in detecting small endoleaks. A small study of six patients after EVAR using Ferumoxytol, a blood pool agent, showed four low flow endoleaks that were not detected by CT. Most importantly, these patients also demonstrated no reduction in endograft size after EVAR [37]. Contrast enhanced MRA (CEMRA) with time-resolved (TR) technique provides dynamic angiographic information—similar to conventional angiography. TR-CEMRA affords a more comprehensive evaluation than standard MR angiography. The source and flow direction of endoleaks can be depicted, improving the characterization of the inflow and potential outflow of endoleaks. As this information impacts decision making for appropriate management, with advances in parallel imaging to reduce MR scan time, TR-CEMRA may become the routine method for post-EVAR MR angiography [38]. Phase contrast imaging can be applied to demonstrate endoleak direction and quantify flow and velocity [39].
Although all these non-invasive techniques are reliable to demonstrate an endoleak, the characterization and the type of endoleak can still be difficult. Digital subtraction angiography (DSA) remains the “gold standard” for characterization of the endoleaks and their endovascular treatment [4, 19].
Angiographic examination should include a global pigtail injection of the aorta at the level of renal arteries and inside the stent-graft. Acquisition time must be long enough to allow the detection of Type II endoleak. In case of Type I endoleak, the origin of the sac is catheterized by placing the catheter between the stent-graft and aortic wall and intra-aneurysmal injection is performed for optimal evaluation of the outflow vessels. For Type II endoleak, the superior mesenteric artery and each internal iliac artery must be injected in order to detect retrograde filling of the aneurysm from the IMA and/or the ilio-lumbar arteries.
Treatment
Type I
Multiple modalities are available for the treatment of Type I endoleak (Table 2). The choice of the optimal treatment is based on the source of the leak. Our policy in this matter is to use the least invasive, yet the most durable, treatment.
Type I-A endoleak (proximal)
Placement of a proximal cuff or extension endograft is the most commonly used treatment in the case of a proximal endoleak associated with malpositioning, angulated neck or migration. This technique needs a new cut down and is not always feasible due to different anatomical and technical challenges.
In the case of a proximal endoleak associated with an irregular neck with no migration, simple balloon angioplasty with large balloons (25–30 mm) or large Palmaz stent placement could be sufficient to apply the stent-graft to the aortic wall. This procedure can be performed under local anesthesia using a long 12 Fr sheath that can allow the passage of a large Palmaz stent (Fig. 5). The large Palmaz stent can be expanded to a diameter of 40 mm.
a CTA demonstrates an endoleak lateral to the stent-graft (white arrow). b Aortogram obtained with a pigtail catheter confirmed a Type I endoleak (black arrows). c–e A large Palmaz stent is implanted through a 12 Fr sheath and inflated to 15 mm. d The angiogram after stent placement shows no endoleak. e CTA after stent placement confirms freedom from endoleak
Embolization and coiling of the aneurysmal sac and the outflow vessels has been proposed as an alternative treatment for Type I endoleak in selected patients [4, 40–43]. Historically, this technique was used when proximal extension cuffs were not available. With current devices, proper size cuffs are generally always available. The majority of patients treated with this technique had extensive medical co-morbidities and short or highly angulated proximal neck. Although there have been concerns about the long-term efficacy of this technique, the results seem to be encouraging. Gorich et al. [41] have successfully treated 13 patients with embolization (mean follow-up: 6.8 months). Sheehan et al. [42] have reported a high clinical success rate in nine patients with Type I endoleak treated just by coil embolization with a mean follow-up of 24 months. We have treated 32 patients with Type I endoleak from 1996 to 2003. The majority of the patients received a Corvita stentgraft (n=28), two Talent endografts and two AneuRx. All patients were considered high risk for surgery. Embolization was successful in 29 patients with the occlusion of the outflow vessels and the aortic channel and/or sac. Three patients with large neck had persistent endoleak after several procedures. Six patients were lost to follow-up. Among the remaining 26 patients, four died of cardiac disease between 7 and 90 days after the procedure. Twenty-two patients could be followed with a mean follow-up of 38.6 months. The aneurysm shrank in 15 patients and remained stable in five and increased in two patients with persistent endoleak. None of the patients with successful embolization has developed a new endoleak or an aneurysmal expansion (Fig. 1). This study confirms that upon achievement of thrombosis, embolization of the outflow vessels and the sac can be associated with long-term clinical success and freedom from endoleak.
The key to success for Type I endoleak is to disrupt the communications between the inflow and outflow vessels involved in the leak.
Type I-B endoleak (distal)
All treatment options for the Type IA endoleaks are valid for distal endoleak. In case of short landing or enlarged iliac artery, an extension endograft will be necessary. However, occasionally Type I-B endoleaks can be treated with balloon angioplasty or bare stent implantation, allowing the sealing of the stentgraft to the aortic wall. If the origin of internal iliac artery needs to be covered, it should be embolized to prevent from retrograde leak.
Embolization of the sac or the channel, although feasible, is usually not indicated in Type I-B endoleak.
Type I-C endoleak
Type I-C leaks occur in cases where an aorto-uni-iliac stent-graft has been deployed, in conjunction with a femoral-femoral bypass graft. An occluder device is then placed in the contra-lateral common iliac artery. Its function is to prevent back-filling of the aneurysm from the excluded common iliac artery. The treatment of these leaks requires completion of the intended thrombosis of the common iliac artery. Embolization is simplest way to complete this, either by passing the occluder and embolizing cranial to it, or by placing a second occluder device caudal to the original device.
The occlusion of the iliac artery is usually sufficient to treat the leak. However, in cases of long-term Type I-C endoleak, many outflow vessels have been developed and the leak may communicate with multiple lumbar arteries and the IMA. These enlarged vessels might be source of late Type II endoleak. Thus, we usually embolize both the outflow vessels and the sac before occluding the iliac artery. Another attractive technique to achieve the occlusion of the common iliac artery is to perform an endovascular internal to external iliac artery bypass using stent-graft. This technique can allow the exclusion of the common iliac preserving the internal iliac artery.
Type II endoleak
Persistent Type II endoleaks usually have a complex architecture [44]. They have been compared with an arteriovenous malformation, with the sac forming the ‘nidus’ of the lesion [45]. There are usually multiple inflow and outflow vessels. These vessels communicate through a channel. The channel is different from the endoleak sac that is generally seen during the angiogram and punctured in translumbar embolization. To achieve a successful embolization, the inflow vessels and the channel(s) need to be embolized (Fig. 2). Like in embolization of Type I endoleak, the key is to disrupt the communications between the vessels involved in the leak.
Embolization is the modality of choice. In our practice, we favor the transarterial route at the time of diagnostic angiography.
For the transarterial approach, once the diagnostic catheter is stable in the proximal SMA, a micro-catheter is advanced to the IMA via the Arc of Riolan. In some situations, the sac can be accessed from the internal iliac artery through the iliolumbar and lumbar arteries (Fig. 2).
In the translumbar approach, the aorta can be punctured under CT or fluoroscopic guidance. Ideally, the left-side access is used to avoid IVC. When performed under fluoroscopic guidance, it is useful to frequently rotate the X-ray tube from the AP to the lateral projection, and in between, to help in assessing the needle track, and to avoid puncturing the stent-graft. The access needle is angled at about 45–60° antero-medially, aimed so as to pass just anterior to the vertebral body, avoiding the adjacent transverse process. As described for traditional trans-lumbar aortography, it may be useful to actually aim for the vertebral body, then after bony contact, pull back 1 or 2 cm and aim more ventrally. Once in the sac a proper angiogram of the sac is performed. Pressure measurements should be obtained within the sac. The measurement will generally show a systemic pressure. Coil embolization is then performed as for arterial approach.
Regardless of the route chosen, the most important task is to access the channel. It is critical to disrupt the network between the involved vessels. This is more important than occluding any one vessel or even embolizing the endoleak sac. This explains the high rate of recurrence after IMA embolization compared with translumbar embolization for Type II endoleak in one report [45].
There are many choices regarding embolic agent. Permanent agents, such as coils are preferred. In most situations, if the channel between the inflow and outflow vessels is interrupted, the sac does not need to be embolized (Fig. 2). Thus, in case of complex Type II endoleak, the filling of the endoleak cavity by translumbar approach without reaching the channel might not be sufficient to treat endoleak. Some authors support in addition to coils, use of either Gelfoam slurry, or thrombin. The origin of the IMA has to be embolized with several coils adapted to its diameter.
The use of several other agents has been successfully reported with trans-lumbar treatment of Type II endoleaks, including Onyx, Ethibloc, thrombin, and Cyano-acrylate [46–50].
Surgical ligation of all relevant branches is a possible solution for Type II leaks. However experience has shown that there are often more vessels involved in these lesions than is initially suspected, and unless they are all clipped the surgical route approach risks failure or recurrence. Ligation can be accomplished by laparoscopic or open technique [51].
Type III endoleak
Angiography can confirm Type III endoleak after placement of the pigtail catheter in the stent graft, just above the flow divider. If the cause is a separation of modular components there may be some difficulty in establishing guidewire access from one component to the next, but once this is accomplished deployment of a new extension is generally problem free. In some situations a new stent-graft needs to be implanted. When the leak is related to incomplete circumferential seal of different components, angioplasty or bare stent implantation can seal the leak. In case of fabric tear, re-implantation of a new stentgraft or open conversion can be considered.
Embolization is almost never indicated in Type III leaks.
Type V endoleak – endotension
There have been several reports of confirmed systemic pressurization within enlarging aneurysm sacs, despite the absence of visualized endoleak [12–16, 52]. Cases of sac enlargement and rupture have been recently reported even after treatment of abdominal aortic aneurysms (AAA) with open surgery. In one report, laparotomy demonstrated a seroma containing firm rubbery gelatinous materials [52]. Aortic puncture to analyze and empty the accumulated fluid is one way to treat this type of endoleak. However, the fluid often re-accumulates during follow-up. If the endotension is related to serous fluid accumulation, there is no need for a surgical treatment, even in case of rupture [52]. Other treatment options include retroperitoneal drainage of the fluid, explantation of the graft with open surgery or insertion of a new stent-graft to reduce the porosity.
Conclusion
Endoleak is an ongoing problem associated with EVAR. Imaging plays a critical role in detecting endoleak. CTA is the first line diagnostic modality allowing the detection of endoleak. Optimal CTA protocol needs to include a delayed acquisition. There are many endovascular options available for treatment of persistent endoleaks. The optimal treatment depends on the type of the endoleak.
References
Cuypers P, Buth J, Harris PL et al (1999) Realistic expectations for patients with stent-graft treatment of abdominal aortic aneurysms. Results of a European multicentre registry. Eur J Vasc Endovasc Surg 17:507–516
Parent FN, Meier GH, Godziachvili V et al (2002) The incidence and natural history of Type I and II endoleak: a 5-year follow-up assessment with color duplex ultrasound scan. J Vasc Surg 35:474–481
Fan CM, Rafferty EA, Geller EC et al (2001) Endovascular stent–graft in abdominal aortic aneurysms: the relationship between patent vessels that arise from the aneurysmal sac and early endoleak. Radiology 218:176–182
Golzarian J, Struyven J, Abada HT et al (1997) Endovascular aortic stent–grafts: transcatheter embolization of persistent perigraft leaks. Radiology 202:731–734
Veith FJ, Baum RA, Ohki T et al (2002) Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 35:1029–1035
Malina M, Lindblad B, Ivancev K et al (1998) Endovascular AAA exclusion: will stents with hooks and barbs prevent stent–graft migration? J Endovasc Surg 5:310–317
White GH, Yu W, May J et al (1997) Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, diagnosis, and management. J Endovasc Surg 4:152–168
Buth J, Harris PL, van Marrewijk C, Fransen G (2003) The significance and management of different types of endoleaks. Semin Vasc Surg 16:95–102
Zarins CK, White RA, Hodgson KJ et al (2000) Endoleak as a predictor of outcome after endovascular aneurysm repair: AneuRx multicenter clinical trial. J Vasc Surg 32:90–107
van Marrewijk C, Buth J, Harris PL et al (2002) Significance of endoleaks after endovascular repair of abdominal aortic aneurysms: the Eurostar experience. J Vasc Surg 35:461–473
Fransen GA, Vallabhaneni SR Sr, Van Marrewijk CJ et al (2003) Rupture of infrarenal aortic aneurysm after endovascular repair: a series from EUROSTAR registry. Eur J Vasc Endovasc Surg 26:487–493
Gilling–Smith G, Brennan J, Harris P, Bakran A, Gould D, McWilliams R (1999) Endotension after endovascular aneurysm repair: definition, classification, and strategies for surveillance and intervention. J Endovasc Surg 6:305–307
White GH, May W, Petrasek P, Waugh R, Stephen M, Harris J (1999) Endotension: an explanation for continued AAA growth after successful endoluminal repair. J Endovasc Surg 6:308–315
Gilling–Smith GL, Martin J, Sudhindran S et al (2000) Freedom from endoleak after endovascular aneurysm repair does not equal treatment success. Eur J Vasc Endovasc Surg 19:421–425
Meier GH, Parker FM, Godziachvili V et al (2001) Endotension after endovascular aneurysm repair: the Ancure experience. Vasc Surg 34:421–427
Risberg B, Delle M, Lonn L, Syk I (2004) Management of aneurysm sac hygroma. J Endovasc Ther 11:191–195
Gawenda M, Jaschke G, Winter ST, Wassmer G, Brunkwall J (2003) Endotension as a result of pressure transmission through the graft following endovascular aneurysm repair—an in vitro study. Eur J Vasc Endovasc 26:501–505
Gorich J, Rilinger N, Sokiranski R et al (1999) Leakages after endovascular repair of aortic aneurysms: classification based on findings at CT, angiography, and radiography. Radiology 213:767–772
Gorich J, Rilinger N, Kramer S et al (2000) Angiography of leaks after endovascular repair of infrarenal aortic aneurysms. AJR Am J Roentgenol 174:811–814
Golzarian J, Dussaussois L, Abada HT et al (1998) Helical CT of aorta after endoluminal stent–graft therapy. AJR Am J Roentgenol 171:329–331
Rosenblit AM, Patlas M, Rosenbaum AT et al (2003) Detection of endoleaks after endovascular repair of abdominal aortic aneurysms: value of unenhanced and delayed CT acquisitions. Radiology 227:426–433
Sawhney R, Kerlen RK, Wall SD et al (2001) Analysis of initial CT findings after endovascular repair of abdominal aortic aneurysm. Radiology 220:157–160
Elkouri S, Panneton JM, Andrews JC et al (2004) Computed tomography and ultrasound in follow–up of patients after endovascular repair of abdominal aortic aneurysm. Ann Vasc Surg 18:271–279
Wolf YG, Johnson BL, Hill BB et al (2000) Duplex ultrasound scanning versus computed tomographic angiography for post–operative evaluation of endovascular abdominal aortic aneurysm repair. J Vasc Surg 32:1142–1148
Zannetti S, De Rango P, Parente B et al (2000) Role of duplex scan in endoleak detection after endoluminal abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 19:531–535
Pages S, Favre JP, Cerisier A et al (2001) Comparison of color duplex ultrasound and computed tomography scan for surveillance after aortic endografting. Ann Vasc Surg 15:155–162
Golzarian J, Murgo S, Dussaussois L et al (2002) Evaluation of abdominal aortic aneurysm after endoluminal treatment: comparison of color Doppler sonography with biphasic helical CT. AJR Am J Roentgenol 178:623–628
McWilliams RG, Martin J, White D et al (2002) Detection of endoleak with enhanced ultrasound imaging: comparison with biphasic computed tomography. J Endovasc Ther 9:170–179
McLafferty RB, McCrary BS, Mattos MA et al (2002) The use of color–flow duplex scan for the detection of endoleaks. J Vasc Surg 36:100–104
Raman KG, Missing–Carroll N, Richardson T et al (2003) Color–flow duplex ultrasound scan versus computed tomographic scan in the surveillance of endovascular aneurysm repair. J Vasc Surg 38:645–651
Bendick PJ, Bove PG, Long GW et al (2003) Efficacy of ultrasound scan contrast agents in the noninvasive follow–up of aortic stent grafts. J Vasc Surg 37:381–385
Napoli V, Bargellini I, Sardella SG et al (2004) Abdominal aortic aneurysm: contrast–enhanced US for missed endoleaks after endoluminal repair. Radiology 233:217–225
Haulon S, Lions C, McFadden EP, Koussa M et al (1998) Prospective evaluation of magnetic resonance imaging after endovascular treatment of infrarenal aortic aneurysms. Eur J Endovasc Surg 22:62–69
Ayuso JR, de Caralt TM, Pages M, Riambau V et al (2004) MRA is useful as a follow–up technique after endovascular repair of aortic aneurysms with nitinol endoprostheses J Magn Reson. Imaging 20:803–810
Cejna M, Loewe C, Schoder M et al (2002) MR angiography vs CT angiography in the follow–up of Nitinol stent grafts in the endoluminally treated aortic aneurysms. Eur Radiology 12:2443–2450
Lutz AM, Willmann JK, Pfammatter T, Lachat M (2003) Evaluation of aortoiliac aneurysm before endovascular repair: comparison of contrast–enhanced magnetic resonance angiography with multidetector row computed tomographic angiography with an auotmated analysis software tool. J Vasc Surg 37:619–627
Ersoy H, Jacobs P, Kent KK, Prince MR (2004) Blood pool MR Angiography of aortic stentgraft endoleak. AJR Am J Roentgenol 182:1181–1186
Lookstein RA, Goldman J, Pukin L, Marin ML (2004) Time–resolved magnetic resonance angiography as a noninvasive method to characterize endoleaks: initial results compared with conventional angiography. J Vasc Surg 39:27–33
Hellinger JC, Draney M, Markl M, Pelc NJ et al (2003) Application of cine phase contrast magnetic resonance imaging and SPAMM–tagging for assessment of endoleaks and aneurysm sac motion. Radiology 229:SS 573 (Abstract)
Amesur NB, Zajko AB, Orons PD, Makaroun MS (1999) Embolotherapy of persistent endoleaks after endovascular repair of abdominal aortic aneurysm with the ancure–endovascular technologies endograft system. J Vasc Interv Radiol 10:1175–1182
Gorich J, Rilinger N, Sokiranski R et al (2000) Treatment of leaks after endovascular repair of aortic aneurysms. Radiology 215:414–420
Faries PL, Cadot H, Agarwal G et al (2003) Management of endoleak after endovascular aneurysm repair: cuffs, coils, and conversion. J Vasc Surg 37:1155–1161
Sheehan M, Barbato J, Compton NC et al (2004) Effectiveness of coiling in the treatment of endoleak after endovascular repair. J Vasc Surg 40:430–434
Baum RA, Stavropoulos SW, Fairman RM, Carpenter JP (2003) Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol 14:1111–1117
Baum RA, Carpenter JP, Golden MA et al (2002) Treatment of type 2 endoleaks after endovascular repair of abdominal aortic aneurysms: comparison of transarterial and translumbar techniques. J Vasc Surg 35:23–29
Martin ML, Dolmatch BL, Fry PD, Machan LS (2001) Treatment of Type II endoleaks with Onyx. J Vasc Interv Radiol 2:629–632
Schmid R, Gurke L, Aschwanden M et al (2002) CT–guided percutaneous embolization of a lumbar artery maintaining a Type II endoleak. J Endovasc Ther 9:198–202
van den Berg JC, Nolthenius RP, Casparie JW et al (2000) CT–guided thrombin injection into aneurysm sac in a patient with endoleak after endovascular abdominal aortic aneurysm repair. AJR Am J Roentgenol 175:1649–1651
Ellis PK, Kennedy PT, Collins AJ, Blair PH (2003) The use of direct thrombin injection to treat a Type II endoleak following endovascular repair of abdominal aortic aneurysm. Cardiovasc Intervent Radiol 26:482–484
Gambaro E, Abou–Zamzam AM Jr, Teruya TH et al (2004) Ischemic colitis following translumbar thrombin injection for treatment of endoleak. Ann Vasc Surg 18:74–78
Richardson WS, Sternbergh WC, Money Sr (2003) Laparoscopic inferior mesenteric artery ligation: an alternative for the treatment of type II endoleaks. J Laparoendosc Adv Surg Tech A 13:355–358
Thoo CHC, Bourke BM, May J (2004) Symptomatic sac enlargement and rupture due to seroma after open abdominal aortic aneurysm repair with polytetrafluoroethylene graft: Implications for endovascular repair and Endotension. J Vasc Surg 40:1089–1094
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Golzarian, J., Valenti, D. Endoleakage after endovascular treatment of abdominal aortic aneurysms: diagnosis, significance and treatment. Eur Radiol 16, 2849–2857 (2006). https://doi.org/10.1007/s00330-005-0129-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-005-0129-6