Abstract
A Cs-bearing polyphase aggregate with composition (in wt%): 76(1)CsAlSi5O12 + 7(1)CsAlSi2O6 + 17(1)amorphous, was obtained from a clinoptilolite-rich epiclastic rock after a beneficiation process of the starting material (aimed to increase the fraction of zeolite to 90 wt%), cation exchange and then thermal treatment. CsAlSi5O12 is an open-framework compound with CAS topology; CsAlSi2O6 is a pollucite-like material with ANA topology. The thermal stability of this polyphase material was investigated by in situ high-T X-ray powder diffraction, the combined P–T effects by a series of runs with a single-stage piston cylinder apparatus, and its chemical stability following the “availability test” (“AVA test”) protocol. A series of additional investigations were performed by WDS–electron microprobe analysis in order to describe the P–T-induced modification of the material texture, and to chemically characterize the starting material and the run products. The “AVA tests” of the polyphase aggregate show an extremely modest release of Cs+: 0.05 mg/g. In response to applied temperature and at room P, CsAlSi5O12 experiences an unquenchable and displacive Ama2-to-Amam phase transition at about 770 K, and the Amam polymorph is stable in its crystalline form up to 1600 K; a crystalline-to-amorphous phase transition occurs between 1600 and 1650 K. In response to the applied P = 0.5 GPa, the crystalline-to-amorphous transition of CsAlSi5O12 occurs between 1670 and 1770 K. This leads to a positive Clapeyron slope (i.e., dP/dT > 0) of the crystalline-to-amorphous transition. When the polyphase aggregate is subjected at P = 0.5 GPa and T > 1770 K, CsAlSi5O12 melts and only CsAlSi2O6 (pollucite-like; dominant) and Cs-rich glass (subordinate) are observed in the quenched sample. Based on its thermo-elastic behavior, P–T phase stability fields, and Cs+ retention capacity, CsAlSi5O12 is a possible candidate for use in the immobilization of radioactive isotopes of Cs, or as potential solid hosts for 137Cs γ-radiation source in sterilization applications. More in general, even the CsAlSi5O12-rich aggregate obtained by a clinoptilolite-rich epiclastic rock appears to be suitable for this type of utilizations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cs-bearing minerals are rare in nature. Likely, the most common ones are represented by pollucite [(Cs, Na)AlSi2O6·nH2O, e.g., Gatta et al. 2009a, b; Bellatreccia et al. 2012], pezzottaite [Cs(Be2Li)Al2Si6O18, e.g., Gatta et al. 2012a; Lambruschi et al. 2014], and londonite (Cs, K, Rb)Al4(Be,B)4(B,Be)12O28, e.g., Gatta et al. 2010, 2011]. On the other hand, a number of synthetic Cs-aluminosilicates have been synthesized in the last decades in search for crystalline compounds potentially usable for immobilization of radioactive isotopes of Cs, or as potential solid hosts for 137Cs γ-radiation source in sterilization applications (e.g., Vance and Seff 1975; Firor and Seff 1977; Gallagher et al. 1977; Araki 1980; Adl and Vance 1982; Komarneni and Roy 1983; Vance et al. 1984; Taylor et al. 1989; Mellini et al. 1996; Drábek et al. 1998; Klika et al. 2006; Bubnova et al. 2007; Sanchez-Valle et al. 2010; Gatta et al. 2008a, 2012b; Cappelletti et al. 2011; Hwang et al. 2015). The synthesis conditions and the crystal structure of CsAlSi5O12 were first reported by Ito (1976) and Araki (1980). This material is an open-framework aluminosilicate, with CAS-type topology (Bearlocher et al. 2001). Its crystal structure was solved in the space group Ama2. The structure is built by the secondary building unit (SBU) 5-1 (Bearlocher et al. 2001) and contains one system of 8-membered ring (8mR) channels, running along [001], where the extra-framework Cs sites lie (Fig. 1). Several additional studies have been devoted to the crystal chemistry of this compound (e.g., Annehed and Fälth 1984; Hughes and Weller 2002; Fisch 2007; Fisch et al. 2008, 2010; Gatta et al. 2008b; Brundu and Cerri 2015; Rampf et al. 2015). Fisch et al. (2008) described the high-temperature behavior of CsAlSi5O12 by in situ X-ray powder and single-crystal diffraction experiments, showing a T-induced displacive phase transition at 770 K (at room P) from the acentric low-temperature space group Ama2 to the centrosymmetric Amam. Data were collected up to 970 K. The volume thermal expansion coefficient of the low-T polymorph is α ~ 6.5 × 10−5 K−1, with a decrease down to α ~ 2.2 × 10−5 K−1 for the high-T polymorph. An additional in situ Raman experiment was conducted by Fisch et al. (2008) up to 1270 K, showing the stability of CsAlSi5O12, in its high-T crystalline polymorph, under such conditions. Later, Fisch et al. (2010) reported the high Cs retention of CsAlSi5O12 by treating the powdered compound in boiling 1 M NaCl solution, showing that only 16 mol% of Cs in CsAlSi5O12 was exchanged by Na after 35 days and that the zeolite remained anhydrous even after partial Na-exchange in aqueous solution. Gatta et al. (2008b) described the elastic behavior and the P-induced structural evolution (at the atomic level) of CsAlSi5O12 by in situ single-crystal X-ray diffraction with a diamond anvil cell. CsAlSi5O12 was found to be stable at least up to 8 GPa (i.e., the maximum pressure achieved in the experiment), showing a compressional behavior described with a bulk modulus (obtained by a third-order Birch–Murnaghan equation of state fit): K T0 = 20(1) GPa and ∂K T0/∂P = 6.5(7), with an anisotropic compressional scheme: K T0a:K T0b:K T0c = 1:1.50:2.36. The stability at high T (at room P) and at high P (at room T) is surprising, if we consider the microporous nature of this material, with a ceramic-like character that deserves further investigations.
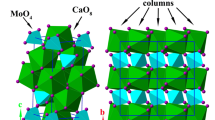
The crystal structure of CsAlSi5O12 viewed down [001], based on the structure model of Gatta et al. (2008b)
The aim of this study is: (1) to extend the previous knowledge on the T stability of CsAlSi5O12 at T > 1270 K (by in situ X-ray powder diffraction), (2) to investigate the combined effect of P and T (via single-stage piston cylinder experiments) on the stability field of this technological material, (3) to check the chemical stability of CsAlSi5O12, in order to evaluate the effective immobilization of Cs. The experiments of this study have been performed using a natural starting material, following the protocol recently reported by Brundu and Cerri (2015).
Experimental
CsAlSi5O12-rich material and its amorphous counterpart (a Cs-aluminosilicate glass) were prepared at the University of Sassari, Italy, following the protocol reported by Brundu and Cerri (2015). Briefly, a clinoptilolite-rich rock from Logudoro (northern Sardinia, Italy; sample labelled as “LacBen” in Cerri et al. 2001), was subjected to a beneficiation process as described by Cerri et al. (2016). The resulting powder (grain size: < 80 μm), containing about 90 wt% of clinoptilolite (along with residual fractions of glass, feldspars, quartz, biotite, and opal-CT) was initially Na-exchanged, and then Cs-exchanged to the final chemical composition (by ICP-AES/MS): SiO2 55.05 wt%, TiO2 0.16 wt%, P2O5 0.03 wt%, Al2O3 10.29 wt%, Fe2O3 0.61 wt%, MnO 0.01 wt%, MgO 0.33 wt%, CaO 0.26 wt%, Na2O 0.13 wt%, K2O 0.29 wt%, Cs2O 23.85 wt%, LoI 9.09 wt% (Brundu and Cerri 2015). Cs-aluminosilicate glass and CsAlSi5O12-rich material were then obtained by a heating process of the Cs-exchanged clinoptilolite in high-alumina crucibles (Coors™) for 2 h at 1320 and 1470 K, respectively. About 500 mg of glass and 2 g of CsAlSi5O12-rich material (hereafter CAS-RM) were produced. The transformation of Cs-clinoptilolite to CAS-RM leads to an increase in density of 10.9 %, being 2.57(1) g/cm3 of the unheated powder and 2.85(2) g/cm3 after the thermal treatment (density measured with a helium pycnometer AccuPyc 1340). The white color of the Cs-clinoptilolite powder changed to reddish-brown in the CAS-RM.
The cation retention capacity of the CAS-RM and its amorphous counterpart (the aforementioned “Cs-aluminosilicate glass”) were evaluated by the so-called availability test (“AVA test”, Van Der Sloot and Kosson 1995) at the Department of Chemical, Materials Engineering and Industrial Production, University of Napoli Federico II. This test is a measure of the fraction of an element present in the material that is not tied up in poorly soluble mineral phases and can potentially be released into environment. Accordingly, powder samples of CAS-RM and its amorphous counterpart were contacted with distilled water (solid-to-liquid weight ratio equal to 1:50). In a first stage, lasted 3 h, the pH of the contact solution was kept constant at 7.0 by adding suitable amounts of a 1 M HNO3 solution. The treatment was then replicated on the solid recovered from the mother solution, at the same conditions as above, except pH, which was fixed to 4.0. Moreover, the samples were also subjected to back-exchange with NaCl solution, in order to check the possible presence of exchangeable cations in the two phases. Accordingly, 100 mg of each powdered sample was treated for 24 h with 50 ml of a 1 M NaCl solution, under continuous stirring and at 298 K. Cation content in the final solution was analyzed, after liquid separation from solid phase, by inductively coupled plasma optical emission spectrometry (ICP–OES).
Synchrotron X-ray powder diffraction data of CAS-RM were collected at the ID09 beamline of the European Synchrotron Radiation Facility (ESRF), Grenoble (France), in transmitting geometry. Data have been collected with a MAR555 flat panel detector, using a monochromatic beam of 0.4140 Å. Data reduction was performed with the FIT2D software (Hammersley et al. 1996) and the Rietveld full-profile fit was carried out using the GSAS package (Larson and Von Dreele 1994). The whole diffraction pattern was fitted using the pseudo-Voigt profile function of Thomson et al. (1987), and the background curves were refined with a Chebyshev polynomial (with 16 coefficients). In order to estimate the amorphous content (by Rietveld/RIR method, e.g., Gualtieri 2000), a fraction of 20 wt% of standard corundum was added to the polycrystalline sample (Fig. 2). The employed structure models were: CsAlSi5O12 reported by Gatta et al. (2008b), CsAlSi2O6 by Gatta et al. (2009a), corundum by Ishizawa et al. (1980).
In situ high-temperature X-ray powder diffraction experiments were conducted with a θ–θ geometry Philips X’Pert diffractometer (CuKα radiation), equipped with an Anton Paar heating chamber (HTK 16 MSW) and a Pt heating strip, at the Earth Sciences Dept.—University of Milan (DST-MI). Data were collected in the 2θ-range 5–80° (with a step-size of 0.03° 2θ and a counting time of 1 s/step) at 298, 400, 500, 600, 700, 800, 900, 1000, 1100, 1200, 1250, 1300, 1350, 1400, 1450, 1500, 1550, 1600, 1650, and 1700 K (Fig. 3).
In order to evaluate the stability of CsAlSi5O12 under the combined effect of pressure and temperature, additional experiments were performed in a single-stage piston cylinder at the DST-MI. The treatment at non-ambient conditions was carried out at 0.5 GPa and at 1170, 1570, 1670, and 1770 K, respectively, using salt–Pyrex–MgO assemblies up to 1570 K, and talc–silica glass–MgO assemblies for the higher temperatures (Fig. 4). Straight graphite heaters were used and temperature was measured with S-type thermocouple and considered accurate to ± 5 K (see Fumagalli et al. 2009 for further details). An initial pressure of about 0.1–0.2 GPa was first applied, then the sample was heated to 670 K to soften the Pyrex, compressed to the final pressure and then heated at 100 K/min up to the experimental temperature. Run durations ranged from 1 to 24 h. The starting CAS-RM was loaded in a ~4-mm-long Pt capsule, with 3 mm of outer diameter and cold-welded. It is worth to report that a first attempt to use an Au capsule resulted in the contamination of the material and the formation of different type of Cs-aurides. The untreated CAS-RM and the P–T-treated samples were investigated by X-ray powder diffraction and with a JEOL JXA-8200 Superprobe at the DST-MI, in order to collect BSE images (Fig. 5) and to perform analyses in wavelength-dispersive mode (EPMA–WDS). EPMA–WDS data were collected operating at 15 kV and 15 nA; natural and synthetic materials were used as standards, following the same protocol adopted by Gatta et al. (2009a). All standards were calibrated within 0.5 % at 1σ. Raw data were corrected using a Phi–Rho–Z routine of the Jeol suite of programs.
Results
Reverse-exchange tests showed that the Cs-aluminosilicate glass releases 1.10 mg/g of Cs+, whereas CAS-RM does not release any Cs+ at significant level (i.e., below L.O.Q). The “AVA tests” showed that the amorphous Cs-aluminosilicate glass releases 1.94 mg/g of Cs+, whereas the CAS-RM releases 0.05 mg/g of Cs+.
The high-quality Rietveld full-profile fit (Rp 4.12 %, wRp 5.89 %) of the synchrotron powder diffraction pattern of CAS-RM, modelled with the co-presence of three crystalline phases (i.e., CsAlSi5O12, pollucite-like CsAlSi2O6, and corundum added as internal standard, Fig. 2), leads to the following wt% fractions:
This result is consistent with the experimental findings based on the BSE images (see below).
The in situ X-ray powder diffraction patterns collected at high T (at DST-MI) show that CsAlSi5O12 maintains its crystalline form up to 1600 K (at room P). At 1650 K, the material appears being completely amorphous, as shown in Fig. 3. Diffraction data were collected up to 1700 K. Diffraction patterns collected decreasing temperature show that the T-induced crystalline-to-amorphous transformation is completely irreversible.
The average composition of CsAlSi5O12, pollucite-like compound, and Cs-rich glass obtained by EPMA–WDS are reported in Table 1. The BSE images obtained from the (untreated) CAS-RM and from the material run at 1570 K and 0.5 GPa (Fig. 5; Table 2), in a piston cylinder, show the occurrence of CsAlSi5O12, pollucite-type compound (ideally CsAlSi2O6), and a small fraction of Cs-rich glass. CsAlSi5O12 crystals are elongated and euhedral, with an average size of ~10 μm. Some CsAlSi5O12 crystals are poikilitic and contain several inclusions of few μm-sized pollucite. Glass often forms pools, and CsAlSi5O12 crystals that coexist with glass show well developed crystal faces toward the liquid (Fig. 5). Comparing the untreated CAS-RM with the P–T-treated samples, which do not encompass melting, no relevant textural and chemical changes have been detected (Fig. 5). This scenario is preserved up to 1660 K and 0.5 GPa, as deduced on the basis of the ex situ X-ray diffraction data (Table 2). The run conducted at 1770 K and 0.5 GPa shows that CsAlSi5O12 experiences a crystalline-to-amorphous state transformation between 1670 and 1770 K (at 0.5 GPa), and at 1770 K and 0.5 GPa only a pollucite-like compound as dominant phase, along with a subordinate amorphous phase, occurs (Table 2).
Discussion and conclusions
This study shows, for the first time, how a Cs-bearing ceramic-like material, able to preserve its crystallinity in response to extreme P–T conditions and its chemical integrity even under acid attack, can be obtained starting from a natural material, following the protocol of Brundu and Cerri (2015), here briefly described:
-
1.
Raw material: clinoptilolite-rich epiclastic rock from Sardinia (60–70 wt% of zeolite);
-
2.
Beneficiation process toward a 90 wt% of clinoptilolite;
-
3.
Cation-exchange processes: first to a Na form and then to a Cs form, obtaining a material rich in Cs6Al6Si30O72·nH2O (i.e., Cs-clinoptilolite);
-
4.
Transformation process by thermal treatment at 1423 K for 2 h:
$$ {\text{Cs}}_{6} {\text{Al}}_{6} {\text{Si}}_{30} {\text{O}}_{72} \cdot n{\text{H}}_{2} {\text{O}} \to 6{\text{CsAlSi}}_{5} {\text{O}}_{12} + n{\text{H}}_{2} {\text{O}} \uparrow $$
This protocol shows how a natural clinoptilolite can be used to capture Cs dispersed in a solution, on the basis of the high affinity of this zeolite for Cs (e.g., Pabalan and Bertetti 2001 and references therein). This is the typical scenario occurring when Cs, in different isotopic configurations and its ionic form, is concentrated in heavy water of the primary circuit of pressurized water reactor, or dispersed in soils or hydrologic systems after nuclear testing or nuclear accidents, as the Chernobyl disaster (in 1986) or, more recently, the Fukushima Daiichi nuclear disaster (in 2011). There have been dozens of accidents at nuclear power plants documented worldwide (more than 90 are listed by Sovacool 2010), and further details about the recent accidents are reported in the webpage of the International Atomic Energy Agency (IAEA) (http://www-pub.iaea.org/books/IAEABooks/Publications_on_Accident_Response).
Before the experiments of Brundu and Cerri (2015), CsAlSi5O12 was reported as obtainable by a synthesis process only, under hydrothermal conditions, starting from chemicals. Several experiments aimed to describe the crystal structure, and the P–T-induced behaviors of this material were based on the use of CsAlSi5O12 obtained by hydrothermal synthesis (listed in Gatta et al. 2008b). The transformation process of an highly available mineral commodity (i.e., clinoptilolite-rich rock), which can spontaneously capture Cs+ and transforms to CsAlSi5O12 by a thermal treatment at room P, led to a change of the scenario on the potential use of CsAlSi5O12 as a host for nuclear waste. The experimental findings of this manuscript extend the previous results of Fisch et al. (2008, 2010) and Gatta et al. (2008b) toward a more complete characterization of the high pressure, high temperature, and chemical stability of CsAlSi5O12, which are mandatory for the use of a given compound in nuclear technology. However, the use of a natural raw material leads to the formation of a Cs-bearing polyphase material made by: CsAlSi5O12 (dominant), pollucite-like compound (subordinate), and Cs-rich amorphous phase (subordinate). This forced us to investigate the P–T behavior and chemical stability of the polyphase aggregate.
Average EPMA–WDS data of CsAlSi5O12 and pollucite-like compound of this study show total values of the chemical composition ranging between 97.2 and 98.6 wt% (Table 1). This deficiency might be explained by the potential low fraction of zeolitic H2O in CsAlSi5O12 and pollucite-like compound, spontaneously adsorbed by the environment as usually occurs in this class of open-framework materials. The limited size of glass pools (of few micrometers of size) can, on the other hand, explain the deficiency reported for the analyses of the glass fraction (Fig. 5; Table 1).
If we combine the experimental findings of this study (i.e., by in situ high-T X-ray powder diffraction and single-stage piston cylinder at high P–T) and those previously reported by Fisch et al. (2008) and Gatta et al. (2008b), we can have a better picture about the P–T phase stability of CsAlSi5O12:
-
In response to applied pressure and at room T, this compound is stable in its crystalline form at least up to 8.5 GPa (Gatta et al. 2008b);
-
In response to applied temperature and at room P, this compound experiences an unquenchable and displacive Ama2-to-Amam phase transition at about 770 K, and the Amam polymorph is stable in its crystalline form up to 1600 K; CsAlSi5O12 experiences a T-induced crystalline-to-amorphous phase transition between 1600 and 1650 K (Fig. 3);
-
In response to the applied P = 0.5 GPa, the crystalline-to-amorphous transition occurs between 1670 and 1770 K. This leads to a positive Clapeyron slope (i.e., dP/dT > 0), as schematically shown in Fig. 6. We can expect a positive slope even for the Ama2-to-Amam phase transition.
If we consider the microporous nature of CsAlSi5O12, which is a nominally zeolitic material, its P–T stability is surprising. Such a behavior is typical for a ceramic material, rather than for an open-framework compound. We believe that the “unusual” thermo-elastic behavior of CsAlSi5O12 and its P–T phase stability are somehow governed by the absence of extra-framework H2O molecules, which usually make zeolites highly unstable materials at high T in response to dehydration processes (zeolites can contain up to 20 wt% of H2O).
The reverse-exchange and the “AVA” tests provided excellent results in terms of Cs+ retention capacity of the CAS-RM, which are typical of a ceramic compound rather than for an open-framework compounds (Liguori et al. 2013). These experimental findings corroborate the first results on the chemical stability of CsAlSi5O12 reported by Komarneni and Roy (1983) and by Fisch et al. (2010). The retention capacity for Cs+ in CsAlSi5O12 can be explained on the basis of the bonding configuration of Cs in the 8mR channels running along [001]. As discussed by Gatta et al. (2008b), the Cs-site lies off-center in the 8mR channel, with a coordination number CN = 13, and Cs–Omax ~3.609 and Cs–Omin ~3.490 Å. The oxygens belonging to the opposite part of the channel wall, with respect to the Cs atom, are not bonded to it. With this configuration, the bond strength is superior if compared with that of a cation site potentially located in the center of the 8mR channel. Similarly, the high Cs-retention capacity of pollucite is governed by the short free diameter (~2.86 Å) of the 6-membered ring channels along [111], which implies a high coordination number of Cs (CN = 12, Gatta et al. 2009a, b) and relatively short Cs–O bond distances (six Cs–O bond distances of ~3.397 Å and six of ~3.555 Å, Gatta et al. 2009a, b). Likely, the synthetic counterpart of pollucite is the best open-framework material so far known for its high-T stability and for its retention capacity of Cs+ (e.g., Komarneni and White 1981; Komarneni and Roy 1983; Kobayashi et al. 1997, 2006; Gatta et al. 2009a, b). In this light, the presence of a pollucite-like compound as dominant phase obtained at 1770 K and 0.5 GPa (i.e., at T above the stability field of CsAlSi5O12) is a positive result.
On the basis of its thermo-elastic behavior, P–T phase stability fields, and Cs+ retention capacity, CsAlSi5O12 is a possible candidate for use in the immobilization of radioactive isotopes of Cs, or as potential solid hosts for 137Cs γ-radiation source in sterilization applications. The CsAlSi5O12-rich material obtained by a clinoptilolite-rich epiclastic rock, following the protocol of Brundu and Cerri (2015), appears to be suitable for this type of utilizations.
References
Adl T, Vance ER (1982) CsAlSi5O12: a possible host for 137Cs immobilization. J Mater Sci 17:849–855
Annehed H, Fälth L (1984) The crystal structure of Cs0.35Al0.35Si2.65O6, a cesium-aluminosilicate with the bikitaite framework. Z Kristallogr 166:301–306
Araki T (1980) Crystal structure of a cesium aluminosilicate, Cs[AlSi5O12]. Z Kristallogr 152:207–213
Bearlocher Ch, Meier WM, Olson DH (2001) Atlas of zeolite framework types, fifth revised version. Elsevier, Amsterdam
Bellatreccia F, Della Ventura G, Gatta GD, Cestelli Guidi M, Harley S (2012) Carbon dioxide in pollucite, a feldspathoid with the ideal composition (Cs,Na)16Al16Si32O96·nH2O. Min Mag 76:903–911
Brundu A, Cerri G (2015) Thermal transformation of Cs-clinoptilolite to CsAlSi5O12. Micropor Mesopor Mat 208:44–49
Bubnova RS, Krzhizhanovskaya MG, Filatov SK, Ugolkov VL, Paufler P (2007) XRD and DSC study of the formation and the melting of a new zeolite-like borosilicate CsBSi5O12 and (Cs,Rb)BSi5O12 solid solutions. Z Kristallogr 222:83–88
Cappelletti P, Rapisardo G, de’ Gennaro B, Colella A, Langella A, Graziano SF, Bish DL, de’ Gennaro M (2011) Immobilization of Cs and Sr in aluminosilicate matrices derived from natural zeolites. J Nucl Mater 414:451–457
Cerri G, Cappelletti P, Langella A, de’ Gennaro M (2001) Zeolitization of Oligo-Miocene volcaniclastic rocks from Logudoro (northern Sardinia, Italy). Contrib Mineral Petrol 140:404–421
Cerri G, Farina M, Brundu A, Daković A, Giunchedi P, Gavini E, Rassu G (2016) Natural zeolites for pharmaceutical formulations: preparation and evaluation of a clinoptilolite-based material. Micropor Mesopor Mat 223:58–67
Drábek M, Rieder M, Viti C, Weiss Z, Frýda J (1998) Hydrothermal synthesis of a Cs ferruginous trioctahedral mica. Can Mineral 36:755–761
Firor RL, Seff K (1977) Zero-coordinate K+. Crystal structure of dehydrated cesium and potassium exchanged zeolite A, Cs7K5-A. J Am Chem Soc 99:6249–6253
Fisch M (2007) Synthesis and temperature dependent structure characterization of microporous CsAlSi5O12. MSc Thesis, Universität Bern (Switzerland), p 83
Fisch M, Armbruster Th, Kolesov B (2008) Temperature-dependent structural study of microporous CsAlSi5O12. J Solid State Chem 181:423–431
Fisch M, Armbruster Th, Libowitzky E (2010) Microporous CsAlSi5O12 at non-ambient conditions: partial Na exchange at 100 °C. In: Hadjiivanov K, Valtchev V, Mintova S, Vayssilov G (eds) Topics in chemistry and materials science: advanced micro- and mesoporous materials—09, vol 4. Heron Press, Sofia, pp 61–70
Fumagalli P, Zanchetta S, Poli S (2009) Alkali in phlogopite and amphibole and their effects on phase relations in metasomatized peridotites: a high-pressure study. Contrib Mineral Petrol 158:723–737
Gallagher SA, McCarthy GJ, Smith DK (1977) Preparation and X-ray characterization of CsAlSiO4. Mat Res Bull 12:1183–1190
Gatta GD, Rotiroti N, Zanazzi PF, Rieder M, Drabek M, Weiss Z, Klaska R (2008a) Synthesis and crystal structure of the feldspathoid CsAlSiO4: an open-framework silicate and potential nuclear waste disposal phase. Am Mineral 93:988–995
Gatta GD, Rotiroti N, Fisch M, Kadiyski M, Armbruster T (2008b) Stability at high-pressure, elastic behaviour and pressure-induced structural evolution of CsAlSi5O12, a potential nuclear waste disposal phase. Phys Chem Minerals 35:521–533
Gatta GD, Rinaldi R, McIntyre GJ, Nénert G, Bellatreccia F, Guastoni A, Della Ventura G (2009a) On the crystal structure and crystal chemistry of pollucite, (Cs,Na)16Al16Si32O96·nH2O: a natural microporous material of interest in nuclear technology. Am Mineral 94:1560–1568
Gatta GD, Rotiroti N, Boffa Ballaran T, Sanchez-Valle C, Pavese A (2009b) Elastic behavior and phase-stability of pollucite, a potential host for nuclear waste. Am Mineral 94:1137–1143
Gatta GD, Vignola P, McIntyre GJ, Diella V (2010) On the crystal chemistry of londonite [(Cs,K,Rb)Al4Be5B11O28]: a single-crystal neutron diffraction study at 300 and 20 K. Am Mineral 95:1467–1472
Gatta GD, Vignola P, Lee Y (2011) Stability of (Cs,K)Al4Be5B11O28 (londonite) at high pressure and high temperature: a potential neutron absorber material. Phys Chem Minerals 38:429–434
Gatta GD, Adamo I, Meven M, Lambruschi E (2012a) A single-crystal neutron and X-ray diffraction study of pezzottaite, Cs(Be2Li)Al2Si6O18. Phys Chem Minerals 39:829–840
Gatta GD, Merlini M, Lotti P, Lausi A, Rieder M (2012b) Phase stability and thermo-elastic behavior of CsAlSiO4 (ABW): a potential nuclear waste disposal material. Micropor Mesopor Mat 163:147–152
Gualtieri AF (2000) Accuracy of XRPD QPA using the combined Rietveld-RIR method. J Appl Cryst 33:267–278
Hammersley AP, Svensson SO, Hanfland M, Fitch AN, Häusermann D (1996) Two-dimensional detector software: from real detector to idealized image or two-theta scan. High Press Res 14:235–245
Hughes RW, Weller MT (2002) The structure of the CAS type zeolite, Cs4[Al4Si20O48] by high-resolution powder neutron diffraction and 29Si MAS NMR. Micropor Mesopor Mater 51:189–196
Hwang H, Seoung D, Gatta GD, Blom DA, Vogt T, Lee Y (2015) Topotactic and reconstructive changes at high pressures and temperatures from Cs-natrolite to Cs-hexacelsian. Am Mineral 100:1562–1567
Ishizawa N, Miyata T, Minato I, Marumo F, Iwai S (1980) A structural investigation of alpha-Al2O3 at 2170 K. Acta Crystallogr B 36:228–230
Ito J (1976) Crystal synthesis of a new cesium alumosilicate, CsAlSi5O12. Am Mineral 61:170–171
Klika Z, Weiss Z, Mellini M, Drábek M (2006) Water leaching of cesium from selected cesium mineral analogues. Appl Geochem 21:405–418
Kobayashi H, Yanase I, Mitamura T (1997) A new model for the pollucite thermal expansion mechanism. J Am Ceramic Soc 80:2161–2164
Kobayashi H, Sumino S, Tamai S, Yanase I (2006) Phase transition and lattice thermal expansion of Cs-deficient pollucite, Cs1−x Al1−x Si2+x O6 (x ≤ 0.25), compounds. J Am Ceramic Soc 89:3157–3161
Komarneni S, Roy R (1983) Hydrothermal reaction and dissolution studies of CsAlSi5O12 in water and brines. J Am Ceramic Soc 66:471–474
Komarneni S, White WB (1981) Stability of pollucite in hydrothermal fluids. Sci Basis Nucl Waste Manag 3:387–396
Lambruschi E, Gatta GD, Adamo I, Bersani D, Salvioli-Mariani E, Lottici PP (2014) Raman and structural comparison between the new gemstone pezzottaite Cs(Be2Li)Al2Si6O18 and Cs-beryl. J Raman Spectrosc 45:993–999
Larson AC, Von Dreele RB (1994) General structure analysis system (GSAS). Los Alamos National Laboratory Report LAUR, pp 86–748
Liguori B, Caputo D, Iucolano F, Aprea P, de' Gennaro B (2013) Entrapping of Cs and Sr in heat-treated zeolite matrices. J Nucl Mat 435:196–201
Mellini M, Weiss Z, Rieder M, Drábek M (1996) Cs-ferriannite as a possible host for waste cesium: crystal structure and synthesis. Eur J Mineral 8:1265–1271
Pabalan RT, Bertetti FP (2001) Cation-exchange properties of natural zeolites. Rev Mineral Geochem 45:453–518
Rampf M, Fisch M, Dittmer M, Ritzberger C, Schweiger M, Höland W (2015) Tailoring the thermal expansion of glass-ceramics by controlled twofold crystallization of Li2Si2O5 and CsAlSi5O12. Inter J Appl Glass Sci (in press). doi:10.1111/ijag.12180
Sanchez-Valle V, Chi-Hong C, Gatta GD (2010) Single-crystal elastic properties of (Cs,Na)AlSi2O6·H2O pollucite: with potential use for long-term storage of Cs radioisotopes. J Appl Phys 108(1–7):093509
Sovacool BK (2010) A critical evaluation of nuclear power and renewable electricity in Asia. J Contemp Asia 40:393–400
Taylor P, DeVaal SD, Derrek G (1989) Owen Stability relationships between solid cesium aluminosilicates in aqueous solutions at 200 C. Can J Chem 67:76–81
Thomson P, Cox DE, Hastings JB (1987) Rietveld refinement of Debye-Scherrer synchrotron X-ray data from Al2O3. J Appl Crystallogr 20:79–83
Van Der Sloot HA, Kosson DS (1995) ECN-Report-94-029. Netherlands Energy Res. Foundation, Petten
Vance TB, Seff K (1975) Hydrated and dehydrated crystal structure of seven-twelfths cesium-exchanged zeolites A. J Phys Chem 79:2163–2166
Vance ER, Cartz L, Karioris FG (1984) X-ray diffraction and leaching of CsAlSi5O12 and CsZr2(PO4)3 irradiated by argon (3 MeV) ions. J Mater Sci 19:2943–2947
Acknowledgments
GDG acknowledges the Italian Ministry of Education, MIUR-Project: “Futuro in Ricerca 2012—ImPACT-RBFR12CLQD”. The authors thank Marco Merlini for the data collected at ESRF. GC acknowledges the support of Fondazione Banco di Sardegna, 2011, Project: “Synthesis of ceramic materials of environmental and industrial relevance from zeolitic precursors”. Martin Fisch (Bern) and an anonymous reviewer are thanked for the revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gatta, G.D., Brundu, A., Cappelletti, P. et al. New insights on pressure, temperature, and chemical stability of CsAlSi5O12, a potential host for nuclear waste. Phys Chem Minerals 43, 639–647 (2016). https://doi.org/10.1007/s00269-016-0823-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-016-0823-8