Abstract
Powellite ceramic represents a waste form matrix material to immobilize minor actinides and Mo from reprocessed UMo nuclear fuel. In this paper, the Ca1−xLix/2Gdx/2MoO4 (0 ≤ x ≤ 1) series is prepared by solid-state reaction using Gd3+ as trivalent minor actinide (Cm3+) surrogate, and the structure/microstructure is characterized by XRD, HRTEM, Raman spectroscopy and SEM. Rietveld refinements show that the couple (Gd3+ and Li+) enters into the eightfold coordinated Ca site of the powellite structure. With the increase in the contents of Gd and Li, Raman bands broaden due to the distortion of MoO4 tetrahedra and disordered arrangements of Gd3+ and Li+. The chemical durability analyzed by the PCT-B indicates that the leaching behaviors of Gd and Mo are related to the interfacial dissolution–reprecipitation mechanism. For the Ca0.5Li0.25Gd0.25MoO4 ceramic, 7-day NLGd and NLMo are shown in the order of ~ 10−4 and ~ 10−4 g m−2, respectively. Thus, our initial results of the structure and chemical durability will provide insights to design new single- or multiphase waste forms for the Mo-rich HLW conditioning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The high-level wastes (HLWs) stored in large underground steel tanks have been accumulating from reprocessed nuclear fuel [1]. Due to the long-term radiotoxic of radioisotopes existing in the HLWs, such storage is acceptable for few years and exerts an ever-increasing threat to the biosphere. Thus, these wastes must be immobilized in non-dispersible and leach-resistant matrix, stored and disposed off within suitable geological repositories [1,2,3]. For UMo HLWs containing a high concentration of molybdenum, borosilicate glassy matrices have been developed to immobilize molybdenum, other fission products and actinides. Researches on the solubility of Mo within borosilicate glass melts indicate that is relatively low (~ 1 wt% MoO3), which limits the amount of waste loading (15–20 wt%) [4, 5]. Moreover, borosilicate glass waste form accompanies the shortcoming of phase separation on melting and can crystallize simultaneously with poorly durable Na2MoO4 and more durable CaMoO4 (powellite) phases [6,7,8]. In this regard, significant efforts are being made to incorporate Mo, other fission products and actinides in a highly stable and durable ceramic matrix. The main ceramic phases considered for immobilization of theses nuclides are sodium zirconium phosphate (NZP) [9,10,11], monazite [12, 13], powellite [14, 15], etc.
Molybdenum is a multivalent element and can exist as + 4 or + 6 oxidation state in the compounds [16]. Tetravalent and hexavalent Mo can enter either octahedral (Oh) position L or tetrahedral (Td) position T of the NZP framework, likely NaMo2(PO4)3, AMo2P3O12 (A = K, Rb, Ti) and AMo2(PO4)3 (A = Ba, Sr, Ca) compounds [17,18,19]. However, the NZP structure for the immobilization of Mo (IV) is not stable under air atmosphere because tetravalent Mo can be easily oxidized [10]. An attempt has been made to introduce Mo as +6 oxidation state in monazite waste forms with La1−xCaxP1−yMoO4 (x = y = 0.1–0.9) compositions, which can crystallize in either monazite or scheelite structure depending on the level of Mo (VI) substitution [12]. The chemical durability of monazite waste form La0.4Nd0.1Y0.1Gd0.1Sm0.1Ce0.1Ca0.1P0.9Mo0.1O4 has been tested through dynamic MCC-5 method, and the leach rate of molybdenum is found to be in the order of 10−3–10−4 g m−2 d−1 [13].
Powellite (nominally, CaMoO4), which crystallizes in the scheelite-type tetragonal structure (space group I41/a, Z = 4), consists of CaO8 polyhedron and MoO4 tetrahedron sharing common vertices and can be visualized as the assembly of columns made up by –CaO8–MoO4–CaO8–MoO4– along c-axis [20, 21]. The polyhedral view of the powellite structure is shown in Fig. 1. Based on the structural flexibility, the powellite structure can accommodate considerable trivalent actinides. Until now to our knowledge, previous researches, which mainly employ the formation of powellite phase during glass composite materials processing, are investigated as a component in the multiphase waste forms [7, 15, 22,23,24,25,26]. However, limited work focuses on the relation between structure and property of single-phase powellite materials [15, 21, 27]. For instance, the chemical durability of powellite CaMoO4 has been investigated using the PCT-B. The present work shows low normalized release rates for Ba, Ca and Mo, suggesting high chemical durability [15]; however, little information is known regarding the leaching behavior of actinides incorporated in the powellite structure. A systematic investigation of structure and aqueous durability of powellite materials can provide useful information for understanding current and future single- or multiphase waste forms.
To close this gap, the immobilization of actinides in single-phase powellite ceramics and chemical durability of the corresponding waste forms are investigated in this paper. According to the isomorphism theory, Gd3+ is used to mimic trivalent curium (Cm). Thus, a series of ceramics Ca1−xLix/2Gdx/2MoO4 (0 ≤ x ≤ 1) are prepared by a solid-state reaction process. The effect of the nature of minor actinide surrogate (Gd) on the structure in the Ca1−xLix/2Gdx/2MoO4 system is undertaken, with leaching behaviors of Gd and Mo analyzed by PCT-B as well.
Experimental
Preparation of samples
Ceramics with the general formula Ca1−xLix/2Gdx/2MoO4 (0 ≤ x ≤ 1) are synthesized by conventional solid-state reaction of the starting reactants, CaCO3 (Macklin and purity > 99%), Gd2O3 (Aladdin and purity > 99%), Li2CO3 (Aladdin and purity > 99%) and Mo2O3 (Aladdin and purity > 99%). The corresponding stoichiometric compositions of powders are milled on the planetary milling machine (Nanjing Machine Factory, China) at 300 rpm for 4 h using anhydrous ethanol as the milling media and calcined at 500 °C for 3 h. The obtained slurries are dried at 90 °C for 24 h, calcined in air at 500 °C for 3 h and milled for 4 h afterward. Subsequently, the homogeneous powders are dried, granulated with 5 wt% PVA and pressed into cylinders (15 mm in diameter and 4–5 mm in height) under a uniaxial pressure of 200 MPa. Finally, these cylinders are sintered from 525 to 950 °C for 3 h in the ambient atmosphere.
X-ray diffraction (XRD)
The crystalline structures of the sintered products are investigated by X-ray diffraction analysis with Cu Kα radiation (PANalytical X′pert PRO diffractometer). The XRD data are collected over the 2θ ranging from 10° to 120° with steps of 0.02°. The structural parameters are analyzed with the Rietveld refinement method using the FullProf package [28].
HRTEM, SEM and Raman spectroscopy
The high-resolution transmission electron microscopy (HRTEM) is performed using a Tecnai G2 F20 S-TWIN FEI transmission electron microscope operating at 200 kV. Microstructures of platinum coated samples are observed using a Hitachi S-3700 N scanning electron microscopy (SEM). Raman spectra at room temperature are collected with a microspectrometer DXR SmartRaman (Thermo Fisher) excited by an argon laser at 514.5 nm.
Dissolution study
The chemical durability of ceramics is examined by the ASTM C1285-14 product consistency test method B (PCT-B) [29]. In the test method, samples are crushed and sieved to isolate the size fraction of the USA (Standard ASTM (100–200 mesh)). Three grams of cleaned particles is placed in a 60-ml polytetrafluoroethylene (PTFE) vessel. An amount of deionized water equal to 30 ml is added, and the PTFE vessel is sealed. The PTFE vessel is placed in a constant temperature device at 90 °C. After different leaching times, the concentrations of the released Gd and Mo are measured with inductively coupled plasma mass spectrometry (ICP-MS) using XSENIES apparatus. From ICP-MS results, normalized elemental mass losses of gadolinium and molybdenum are determined by the equation derived from PCT-B [29]. To analyze the differences between microstructures at longer leaching durations, the deionized water in the testing vessel is renewed at every 28-day test.
Results and discussion
XRD and HRTEM analyses
To investigate the optimal condition for the preparation of powellite ceramics with Ca1-xLix/2Gdx/2MoO4 (0 ≤ x ≤ 1) compositions, the typical composition Ca0.5Li0.25Gd0.25MoO4 is heated at several temperatures ranging from 525 to 950 °C. Analytic results of the corresponding XRD patterns are shown in Fig. 2. At 525 °C, the crystalline phases of the sample are CaMoO4 (JCPDS file no. 85-0546), Gd2O3 (JCPDS file no. 88-2165) and Li2Mo4O13 (JCPDS file no. 25-0494). Between 525 and 550 °C, the intensities of the peaks of Gd2O3 and Li2Mo4O13 phases decrease, while those of CaMoO4 phase increase. This indicates that the isomorphism of powellite forms during the increase in the sintering temperature. At 600 °C, the XRD patterns are indexed to the single-phase powellite with Ca0.5Li0.25Gd0.25MoO4 composition, due to the disappearance of the XRD lines from CaMoO4, Gd2O3 and Li2Mo4O13 phases. Above 600 °C, the crystallinity of the powellite ceramic Ca0.5Li0.25Gd0.25MoO4 is widely improved, which is determined by the average full width at the half maximum (FWHM) of the main XRD lines, corresponding to the 011, 112, 004, 020, 211, 114, 024, 020 and 116 peaks. The average FWHM of these peaks, which are obtained at 600, 650, 700, 750, 800, 850 and 900 °C, are found to be 0.251, 0.212, 0.203, 0.193, 0143, 0.145 and 0.140 °, respectively (Fig. 3). From the FWHM, it can be deduced that the optimized temperature for the synthesis of the powellite-type Ca1−xLix/2Gdx/2MoO4 (0 ≤ x ≤ 1) solid solution is over 800 °C.
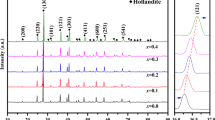
To examine the effect of the minor actinide surrogate (Gd) content on the structure in the Ca1−xLix/2Gdx/2MoO4 system, the Ca1−xLix/2Gdx/2MoO4 (0 ≤ x ≤ 1) compositions are sintered at 850 °C and corresponding powder XRD patterns are shown in Fig. 4. The XRD investigation shows that Ca1−xLix/2Gdx/2MoO4 (0 ≤ x ≤ 1) compositions crystallize in the scheelite-type structure, and intense reflections of powellite phases observed in the range from 10° to 65° are indexed to (011), (112), (004), (020), (211), (114), (024), (200), (116), (312) and (224), respectively. Meanwhile, Rietveld refinement is performed on all of these compounds. Since Li element has a very low atomic number relative to the other elements in the powellite phases and a conventional Cu anode is used to collect the diffraction patterns, structural model including or without Li is used to evaluate the confidence of the refinement result. The agreement factors for the refined model including Li with representative composition Ca0.6Li0.2Gd0.2MoO4 are: Rp = 14.4%, Rwp = 9.52%, RB = 2.38% and RF = 2.49, S = 1.77; those for the initial model without Li are Rp = 14.9%, Rwp = 10.02%, RB = 2.67%, RF = 2.74% and S = 1.89. Compared with these values, it is found that the refinement of the structural model with Li atoms is credible due to its smaller agreement factors. As a result, final refinement plots of Ca0.6Li0.2Gd0.2MoO4 composition are presented in Fig. 5, and the corresponding atomic parameters are shown in Table 1. It is clear that the couple (Gd3+, Li+) enters the eightfold coordinated Ca site of the scheelite-type structure (Table 1). The analysis of the unit cell parameters given in Table 2 indicates that a noticeable decrease occurs in the compositions 0 ≤ x ≤ 1. The result can be attributed to the increase in x with the decrease in average cationic radii in the eightfold coordination of Ca site. According to the Shannon’s data [30], the equivalent radii of Ca site for the Ca1−xLix/2Gdx/2MoO4 ceramics are \( r = 1.12 - 0.1335x \) (Ion radius of Gd3+, Li+ and Ca2+ is 1.053 Å, 0.92 Å and 1.12 Å, respectively). In addition, the value of a/c increases with the increase in the x value.
To further understand the resulting phases of the Ca1−xLix/2Gdx/2MoO4 compositions, the samples are characterized by HRTEM. For the sake of briefness, only HRTEM result of powellite ceramic with Ca0.6Li0.2Gd0.2MoO4 composition is presented, as shown in Fig. 6. The observed zone of Ca0.6Li0.2Gd0.2MoO4 particles displays clear lattice fringes. Analyses of interplanar spacing of (022) and (020) planes along [400] axis are found to be 0.237 and 0.261 nm, respectively, which are in good agreement with Rietveld refinement results.
Raman spectra study
Previous group theory calculations have suggested that powellite (CaMoO4) crystal has 26 vibrations of species (\( \varGamma = 3A_{g} + 5A_{u} + 5B_{g} + 3B_{u} + 5E_{g} + 5E_{u} \)), where 3Ag, 5Bg and 5Eg are 13 Raman active vibrations, 4Au and 4Eu are eight infrared active vibrations and 3Bu are three silent vibrations [31,32,33]. Raman active modes in the structure also can be divided into internal and external vibrational modes. The internal modes are associated with the oscillations inside the [MoO4]2− molecular group with a stationary mass center. The external modes, which are composed of rotation and translation modes, are related to the motion of A2+ cation and the rigid molecular units [32].
The spectroscopic characterizations of some representative Ca1-xLix/2Gdx/2MoO4 (0 ≤ x ≤ 0.9) compositions through Raman experiments in the frequency range of 100–1200 cm−1 are shown in Fig. 7. The internal mode frequencies range from 305 to 900 cm−1, whereas external mode frequencies are below 305 cm−1. The high-frequency vibration Raman line around 879 cm−1 corresponds to the symmetric stretching vibration v1(Ag). Peaks observed at 795 and 848 cm−1 are associate with the stretching motions which can be assigned as v3(Eg) and v3(Bg), respectively. Raman lines centered at 392 [v4(BgEg)] and 407 [v4(BgEg)] cm−1 belong to symmetric bending vibration. The Raman frequency located at 325 cm−1 presents the asymmetric bending mode v2(AgBg). Further, the Raman frequency located at 114 and 206 cm−1 is associated with the rotational and translational modes. For all the internal vibrational modes, Raman bands broaden with the increase in the level of Gd and Li substitution, while the change in the intensity reverses. These phenomena may result from the distortion of the MoO4 tetrahedra [33, 34]. When the proportion of Gd3+ and Li1+ ions on the Ca site increases, the MoO4 may appear more distorted on the local scale. As a result, the regular change in the internal vibration modes can be observed. Compared with the internal modes, the evolution of the external modes versus x values follows the same trend. This interesting result is related to the distortion of MoO4 tetrahedra and disordered arrangements of Gd3+ and Li+ [20, 33, 35].
Durability assessment
To assess the leaching behaviors of minor actinide surrogate (Gd) and Mo in powellite ceramics, PCT-B is performed on the selected composition Ca0.5Li0.25Gd0.25MoO4. The normalized releases of Gd and Mo for different leaching times are presented in Table 3, and corresponding plots are shown in Fig. 8. As shown in Fig. 8, the initial rapid releases of Gd and Mo occur during the leaching test. For 3-h leaching, the NLGd and NLMo reach the maximum values of 5.28 × 10−2 and 2.87 × 10−2 g m−2, respectively (Table 3). With the increase in leaching time, the normalized releases decrease and attain a near-equilibrium condition after 7 days (Fig. 8). For leaching times ranging from 7 to 28 days, the NLGd and NLMo are shown in the order of ~ 10−4 and ~ 10−4 g m−2, respectively (Table 3). These results indicate that powellite ceramics exhibit the great retention of Gd and Mo.
Based on the above description, the leaching behaviors can be explained by the interfacial dissolution–reprecipitation mechanism [36,37,38,39]. In this situation, the whole leaching process can be divided into three stages. The first stage is from 0 to 3 h, in which Gd(III) and Mo (VI) from superficial surfaces are quickly dissolved in water and form hydrolyzed species on the reaction interfaces subsequently. The second stage is from 3 h to 7 days. In this leaching period, the hydrolyzed species consistently form precipitation phases and cover on the surface of the Ca0.5Li0.25Gd0.25MoO4 particles which reduce the NLGd and NLMo. For the final stage with longer leaching times ranging from 7 to 28 days, the inner immobilized Gd and Mo elements hardly escape from the matrix due to the long pathways. Meanwhile, the formation and reprecipitation of hydrolyzed species reach the dynamic equilibrium leading to the invariant magnitude of NLGd and NLMo. These results are further verified by the microstructure of the Ca0.5Li0.25Gd0.25MoO4 ceramic after leaching 90 days, as shown in Fig. 9. The clear surface of the sample without leaching test is observed, whereas small particles observed on the surface after leaching 90 days suggest that the formation of precipitate phases occurs during the dissolution test. The observations of SEM are consistent with the leaching behaviors of minor actinide surrogate (Gd) and Mo. Unfortunately, such precipitates cannot be determined by EDS due to the contents below the instrumental detection limits. All the results indicate that powellite ceramics can be used as a promising candidate waste form for the conditioning of minor actinide and Mo.
Conclusions
The aim of this work was to investigate the structure and leaching behavior in the Ca1−xLix/2Gdx/2MoO4 (0 ≤ x ≤ 1) system. The main conclusions drawn from this paper are the following:
- (i)
The preparation of the powellite-type Ca1−xLix/2Gdx/2MoO4 (0 ≤ x ≤ 1) solid solution requires sintering temperature over 800 °C. For all the Ca1−xLix/2Gdx/2MoO4 ceramics, the couple (Gd3+, Li+) is incorporated in the eightfold coordinated Ca site of the scheelite-type structure. The evolution of cell parameters decreases with the increase in x. The change in the peaks of Raman spectra is relevant to the distortion of MoO4 tetrahedra and the disordered arrangements of Gd3+ and Li+.
- (ii)
The ceramic with Ca0.5Li0.25Gd0.25MoO4 composition exhibits the great retention of Gd and Mo; 7-day NLGd and NLMo are found to be in the order of ~ 10−4 and ~ 10−4 g m−2, respectively. The leaching behaviors of minor actinide surrogate (Gd) and Mo in the powellite ceramics are attributed to the interfacial dissolution–reprecipitation mechanism.
References
Sengupta P (2012) A review on immobilization of phosphate containing high level nuclear wastes within glass matrix—present status and future challenges. J Hazard Mater 235:17–28
Donald IW, Metcalfe BL, Taylor RNJ (1997) The immobilization of high level radioactive wastes using ceramics and glasses. J Mater Sci 32:5851–5887. https://doi.org/10.1023/A:1018646507438
Crum JV, Turo L, Riley B et al (2012) Multi-phase glass-ceramics as a waste form for combined fission products: alkalis, alkaline earths, lanthanides, and transition metals. J Am Ceram Soc 95:1297–1303
Johnson LH, Shoesmith DW, Lutze W et al (1988) Radioactive waste forms for the future. North Holland, Amsterdam
Szumera M, Wacławska I (2012) Effect of molybdenum addition on the thermal properties of silicate-phosphate glasses. J Therm Anal Calorim 109:649–655
Asfari Z, Bressot C, Vicens J et al (1995) Doubly crowned calix[4]arens in the 1, 3-alternate conformation as cesium-selective carriers in supported liquid membranes. Anal Chem 67:3133–3139
Kossoy A, Schulze R, Tang M et al (2013) Nd–Mo-borosilicate glass-ceramic: synthesis, characterization and response to ionizing radiation. J Nucl Mater 437:216–221
Hyatt NC, Short RJ, Hand RJ et al (2005) The structural chemistry of molybdenum in model high level nuclear waste glasses, investigated by Mo K-edge X-ray absorption spectroscopy. Ceram Trans 168:179–187
Chourasia R, Shrivastava OP, Wattal PK (2009) Synthesis, characterization and structure refinement of sodium zirconium molibdo-phosphate: Na0.9Zr2Mo0.1P2.9O12 (MoNZP). J Alloys Compd 473:579–583
Pet’kov VI, Sukhanov MV, Kurazhkovskaya VS (2003) Molybdenum fixation in crystalline NZP matrices. Radiochemistry 45:620–625
Dahale ND, Keskar M, Sali SK et al (2008) Preparation and characterisation of Tl2Pu(MoO4)3 and Tl4Pu(MoO4)4 in Tl–Pu–Mo–O system by X-ray and thermal methods. J Nucl Mater 376:129–132
Kumar SP, Gopal B (2011) Immobilization of “Mo6+” in monazite lattice: synthesis and characterization of new phosphomolybdates, La1-xCa xP1-yMoyO4, where x = y = 0.1 - 0.9. J Am Ceram Soc 94:1008–1013
Pratheep Kumar S, Gopal B (2015) Simulated monazite crystalline wasteform La0.4Nd0.1Y0.1Gd0.1Sm0.1Ce0.1Ca0.1(P0.9Mo0.1O4): synthesis, phase stability and chemical durability study. J Nucl Mater 458:224–232
Stewart MWA, Vance ER (2006) Waste form strategies for Mo-rich radioactive waste. WM’ 06 conference, Tucson, AZ
Brinkman K, Fox K, Marra J et al (2013) Single phase melt processed powellite (Ba, Ca)MoO4 for the immobilization of Mo-rich nuclear waste. J Alloys Compd 551:136–142
Caurant D, Majérus O, Fadel E et al (2007) Effect of molybdenum on the structure and on the crystallization of SiO2–Na2O–CaO–B2O3 glasses. J Am Ceram Soc 90:774–783
Lii KH, Chen JJ, Wang SL (1989) NaMo2P3O12: a new phosphate of Mo(IV). J Solid State Chem 78:93–97
Leclaire A, Raveau B (1988) Small atomic displacements in the molybdenophosophates AMo2P3O12 (A = K, Rb, Ti). Acta Crystallogr Sect C: Cryst Struct Commun 44:226–229
Leclaire AB, Borel MM, Grandin A et al (1989) A novel family of mixed valence molybdenum phosphate with a nasicon structure, AMo2P3O12 (A = Ca, Sr, Ba). Eur J Solid State Inorg Chem 26:45–51
Rabuffetti FA, Culver SP, Leopoldo S et al (2014) Structural disorder in AMoO4 (A = Ca, Sr, Ba) scheelite nanocrystals. Inorg Chem 53:1056–1061
Achary SN, Patwe SJ, Mathews MD et al (2006) High temperature crystal chemistry and thermal expansion of synthetic powellite (CaMoO4): a high temperature X-ray diffraction (HT-XRD) study. J Phys Chem Solids 67:774–781
Short RJ, Hand RJ, Hyatt NC et al (2005) Environment and oxidation state of molybdenum in simulated high level nuclear waste glass compositions. J Nucl Mater 340:179–186
Bosbach D, Luckscheiter B, Brendebach B et al (2009) High level nuclear waste glass corrosion in synthetic clay pore solution and retention of actinides in secondary phases. J Nucl Mater 385:456–460
Vance ER, Davis J, Olufson K et al (2014) Leaching behaviour of and Cs disposition in a UMo powellite glass-ceramic. J Nucl Mater 448:325–329
Ohkubo T, Monden R, Iwadate Y et al (2015) Structural investigation of aluminoborosilicate glasses containing Na2MoO4 crystallites by solid state NMR. Phys Chem Glasses B 56:139–144
Taurines T, Boizot B (2012) Microstructure of powellite-rich glass-ceramics: a model system for high level waste immobilization. J Am Ceram Soc 95:1105–1111
Bosbach D, Rabung T, Brandt F et al (2004) Trivalent actinide coprecipitation with powellite (CaMoO4): secondary solid solution formation during HLW borosilicate-glass dissolution. Radiochim Acta 92:639–643
Rodriguez-Carjaval J (1993) The programs for rietveld refinement. Phys B 192:55–69
ASTM (2014) Standard test methods for determining chemical durability of nuclear, hazardous, and mixed waste glasses and multiphase glass ceramics: the product consistency test (PCT)
Shannon RD (1976) Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr 32:751–767
Porto SPS, Scott JF (1967) Raman spectra of CaWO4 SrWO4 CaMoO4 and SrMoO4. Phys Rev 157:716–729
Basiev TT, Sobol AA, Voronko YK et al (2000) Spontaneous Raman spectroscopy of tungstate and molybdate crystals for Raman lasers. Opt Mater 15:205–216
Xi H, Di Z, Xie H et al (2015) Raman spectra, infrared spectra, and microwave dielectric properties of low-temperature firing [(Li0.5Ln0.5)1−xCax]MoO4 (Ln = Sm and Nd) solid solution ceramics with scheelite structure. J Am Ceram Soc 98:587–593
Hao S, Zhou D, Pang L (2019) The spectra analysis and microwave dielectric properties of [Ca0.55(Sm1-xBix)0.3]MoO4 ceramics. J Am Ceram Soc 102:3103–3109
Munirathnappa AK, Dwibedi D, Hester J et al (2019) In situ neutron diffraction studies of LiCe(WO4)2 polymorphs: phase transition and structure-property correlation. J Phys Chem C 123:1041–1049
Danelska A, Ulkowska U, Socha RP et al (2013) Surface properties of nanozirconia and their effect on its rheological behaviour and sinterability. J Eur Ceram Soc 33:1875–1883
Fillet C, Advocat T, Bart F et al (2004) Titanate-based ceramics for separated long-lived radionuclides. C R Chim 7:1165–1172
Zhang L, Lüttge A (2009) Theoretical approach to evaluating plagioclase dissolution mechanisms. Geochim Cosmochim Acta 73:2832–2849
Li W, Ding X, Meng C et al (2018) Phase structure evolution and chemical durability studies of Gd1−xYbxPO4 ceramics for immobilization of minor actinides. J Mater Sci 53:6366–6377. https://doi.org/10.1007/s10853-018-2031-z
Acknowledgements
The authors would like to thank the editor and the anonymous reviewers for their valuable comments on this manuscript. All authors contributed to the discussion of the results and preparation of the manuscript. The present work was supported by the Fundamental Research Funds for the Central Universities of Zhejiang University (CN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, C., Li, W., Ren, C. et al. Structure and chemical durability studies of powellite ceramics Ca1−xLix/2Gdx/2MoO4 (0 ≤ x ≤ 1) for radioactive waste storage. J Mater Sci 55, 2741–2749 (2020). https://doi.org/10.1007/s10853-019-04223-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-04223-y