Abstract
In this article, we reported the synthesis of novel Sb–SnO2/kaolinite (SK) nanocomposites by assembling antimony-doped tin oxide (ATO) nanoparticles on the surface of kaolinite rods without addition of dispersant. The samples were characterized by X-ray diffraction, transmission electron microscopy (TEM), high-resolution TEM, X-ray photoelectron spectroscopy (XPS), and N2 adsorption–desorption techniques. The crystal size and loading density of ATO nanoparticles onto kaolinite rods could be controlled through the synthetic conditions. The color and resistivity of the composites varied with the loading density of ATO nanoparticles. Investigations of the interfacial binding between ATO layer and rod surface indicated that surface characteristics could facilitate the deposition of various metal oxides nanoparticles. XPS analysis demonstrated that the entrance of Sb5+ into SnO2 crystallite led to the improvement of conductivity and the color change of the composites. The formation mechanism for SK composites was also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The compounding of the functional matrix and the silicate minerals can produce the advanced composites, and the main routes included the surface loading (Zhang et al. 2006; Aranda et al. 2008; Cao et al. 2008), porous assembly (Corma 1997; Stein et al. 2000; Gutiérrez et al. 2010), and intercalation technology (Pinnavaia 1983; Giannelis 1996; Krishnamoorti et al. 1996, Frost et al. 1998). Research achievements about the functional mineral-based composites have been successfully obtained in the fields of ecological environment (Ohashi et al. 2009), catalysis (Corma et al. 2010), optical (Wan et al. 2009; Bujdk et al. 2010), wave-absorbing materials (Mamedov et al. 2000; Blanco-Gutirrez et al. 2010), and energy storage (Chen et al. 2007). Antimony-doped tin oxide (ATO) is a well-known transparent semiconductor with a large band gap (>3.6 eV), providing transparency in the visible range and high n-type electric conductivity, which makes it a valuable alternative to the expensive indium tin oxide (ITO) (Mishra et al. 1995; Wang et al. 2009). The preparation of ATO nanoparticles with different shape, size, resistivity and degree of agglomeration has been addressed by different techniques (Zhang and Gao 2004; Posthumus et al. 2006; Li et al. 2010). Generally speaking, chemical methods provided much better controls over the particle size and their stability in solution than the physical methods. It is easy for the nanoparticles to form micron-sized secondary particles adhering to each other during the wet chemical synthesis. If that was the case, the properties of the as-synthesized samples would be affected by the size of the secondary particles. The sol–gel, polymeric precursor and co-precipitation techniques mostly provided either the large particles or the nanoparticle agglomerates, while the hydrothermal and solvothermal techniques were able to conveniently control the growth of ATO particles (Mller et al. 2009). The co-precipitation techniques highlighted the advantages in easily adjusting the preparing conditions as well as possessing the mass production capability among these methods, which makes it desirable for the large-scale production of ATO nanoparticles. Especially, uniform assembly of the ATO particles on the surface of special morphological minerals can effectively reduce the agglomeration degree of the nanoparticles and the preparation costs, and simultaneously improve its application performance. Many reports about this research focused on the silicate minerals such as mica (Tan et al. 2004; Sadeh et al. 2007), barite (Guffond et al. 2001; Hu et al. 2008), and montmorillonite (Boukerma et al. 2006).
Kaolinite (Al2Si2O5(OH)4), a 1:1 dioctahedral aluminosilicate clay mineral, has two different basal cleavages faces, namely tetrahedral siloxane (Si–O–Si) surface and octahedral gibbsite (Al2O3) sheet. At the edges of kaolinite particles, octahedral alumina and silica tetrahedral sheets exposed reactive functional moieties, including aluminol (Al–OH) and silanol (Si–OH) groups (Murray 2000; Johnston et al. 2008; Turhan et al. 2010). For example, for one thing, coated with TiO2, flake-like kaolinite potentially improved the performance of air treatment technologies via enhancing adsorption and reactivity of target volatile organic compounds (VOCs) (Chong et al. 2009; Kibanova et al. 2009; Kocí et al. 2011); for another, TiO2-coated kaolin composite was used as a white pigment in view of enhancing pigmentary performance and reducing cost (Ninness et al. 2003; Lu et al. 2009). For the rod-like kaolinite, its physicochemical properties can be dramatically changed by surface modification with selected organic, inorganic, and biological species. Besides, this kind of functionalization will lead to the advanced properties.
In this article, we demonstrate a synthetic method to composite conductive materials with ATO nanoparticles uniformly assembled on the surface of the rod-like kaolinite without addition of dispersant. We also investigate the controllability of surface layer and the effects of assembled Sb–Sn compounds on the structure and property of the samples. The samples were characterized in detail by XRD, TEM, HRTEM, and XPS. In addition, a general synthesis mechanism is proposed.
Experimental
All of the chemicals, including stannic chloride hydrate (SnCl4·5H2O), antimony terchloride (SbCl3), hydrochloride acid (HCl), and ammonia liquor (NH3·H2O) were analytically pure and used without further treatment. A mixture acidic solution with initial Sb3+/Sn4+ salts molar ratio of 0.16 dissolved in HCl (2.0 M) was prepared. In each experiment, a known quantity of rod-like kaolin (kaolinite content >95%) was dispersed in distilled water (total solids percent mass in volume, 10 wt%) at 60°C with constant stirring. The synthesis steps were carried out under simultaneously adding above mixture acidic solution and NH3·H2O (30%), while the pH value of slurry was kept at 1.5 ± 0.1. The composites with different Sn/Sb loading were obtained by adjusting the addition of mixture acidic solution. Then, stirring was continued for another 20 min and then maintained at a certain temperature for 1 h. The resultant precipitate was collected via filtering the solution, washed several times with distilled water to remove Cl−, dried at 80°C for 4 h to produce a precursor. The precursor was calcined at 700°C for 1.5 h in air to finally form the conductive composites (SK). The Sn/Sb loading was designed as 2, 4, 8, 13, and 20%, and the corresponding conductive composites were marked as SK2, SK4, SK8, SK13, and SK20, respectively.
A powder X-ray diffractometer (RIGAKU D/max-2550 PC, Cu kα radiation, 40 kV, 300 mA) was used to identify the crystalline phase of the samples at a scanning rate of 2°/min over a range of 10–80° (2θ). The morphology and composite structure were carried out on a JEOL JEM 3010 transmission electron microscope (TEM), and energy-dispersive spectrometry (EDS) was performed with an EDAX X-ray energy-dispersive analysis system attached to the JEOL JEM 3010 TEM. The electrical structure and composition of the samples were analyzed by X-ray photoelectron spectroscopy (XPS) (Thermo ESCALAB 250, USA) measurements at room temperature. XPS spectra were recorded by using monochromatized Al Kα radiation (1,486.6 eV) and the power was set to 150 W. The binding energy values were calibrated with reference to the C 1s peak (284.6 eV). The specific surface area (S BET) of productions was calculated by the Brunauer–Emmet–Teller (BET) method. N2 adsorption–desorption isotherms were obtained at 77 K using an ASAP 2020 unit. Prior to the adsorption experiment, the materials were vacuum-dried at 150°C for 6 h. About 1.5 g powder sample was putted into Φ13 mm model of stainless steel, pressed into slice with 10 MPa pressure. The universal meter DT890 was used for testing the ohmic resistance (Ω) of slice sample. The resistivity was calculated as: ρ = RA/L, where ρ was the resistivity of the sample (Ω cm), R the measured resistance (Ω), A the area of the sample pole (cm2) and L the height of the sample pole (cm).
Results and discussion
The following reaction is suggested for the formation of conductive composites:
The theoretical mass of Sb–SnO2/kaolinite composites was calculated according to above chemical equations. The initial quality of kaolin was 5 g, the mass ratio of SnCl4 to kaolinite was 3:100, 6:100, 12:100, 20:100, and 30:100, respectively. The experimental mass of each composite was also well consistent with the calculated mass in terms of different Sn/Sb loading (Fig. 1), indicating the possibly complete coating of the initial Sn/Sb compounds on the rod surface. The color of the composites also became gradually darker with the increase of Sn/Sb loading (Fig. 1, inset).
The conductive properties of SK composites varied with the content of Sn/Sb loading (Fig. 2). Kaolinite was an excellent insulating material after heat treatment. When the Sn/Sb loading was 2 wt%, the volume resistivity of composites exceeded 109 Ω cm. The volume resistivity decreased gradually with the increase in Sn/Sb loading and reached 7.8 × 103 Ω cm at 20 wt% Sn/Sb compounds, indicating that much more surfaces would be coated with Sn/Sb compounds when the exposed surface areas of kaolinite reduced. Therefore, the composites performed the better conductivity.
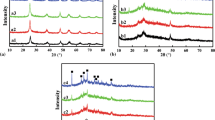
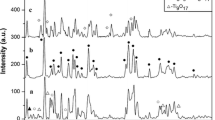
Figure 3 illustrated the XRD patterns of metakaolin, SK2, SK4, SK8, SK13, and SK20 composites. The broad peak from 15 to 30° confirmed the amorphous structure of metakaolin from the calcination of kaolin at 700°C (Fig. 3a). Furthermore, the characteristic peak of amorphous metakaolin was detected in all samples because of the lower content of SnO2 nanoparticles. No other new phases were observed.
Samples with Sn/Sb less than 4 wt% did not show any obvious diffraction peaks of SnO2, while three distinct diffraction peaks were observed at 26.5, 33.8, and 54.8° in SK samples with higher Sn/Sb loading of no less than 4 wt%, corresponding to the reflections of (110), (101), and (211) planes of SnO2 tetragonal unit cell, respectively, which indicated the cassiterite structure (JCPDS no.41-1445) of SnO2 in SK composites. On the other hand, the intensities of the above three peaks in SK20 were clearly enhanced and the peaks became more sharp than those of the other samples, mainly due to the increase in the content of Sn/Sb compounds. The increase in Sn/Sb loading (from SK2 to SK20) could make the crystalline structure more integrated and increased the density of SnO2 in the composites, but did not lead to the crystalline transformation of SnO2.
The effects of different Sn/Sb loading on the microstructure of the SK composites were further investigated. Kaolinite clay used here is a two-layered aluminosilicate with rod-like morphology in the submicrometer range, rod size of 100–200 nm in diameter and 1–5 μm in length. The rod showed an amorphous surface and obvious lengthways edge (Fig. 4a, b), possessing plenty surface hydroxyl groups. These extra hydroxyl groups are potential reaction sites for surface modification on the external surface (Yuan et al. 2008). Because surface edges and defects can bring higher specific surface energy and serve as nucleation sites for metal oxide deposition, it was demonstrated that the surface with above characteristics could facilitate the deposition of various metal oxides nanoparticles through solution processes (Fang et al. 2008).
The formation of Sn/Sb compounds on the surface of kaolinite rods in precursor was confirmed by cross-section TEM observation. Figure 4c and e showed the morphology of precursors with different Sn/Sb loading. A noticeable difference for the rods with or without loading lies in the surface roughness. Compared with original kaolinite rods (Fig. 4b), the Sn/Sb compounds on the rod surface was obvious (Fig. 4c, f). The (001) lattice fringes of kaolinite (d = 0.715 nm) and the (110) lattice fringes of SnO2 (d = 0.34 nm) were clearly observed (Fig. 4d, f). The micrographs also indicated that the surface coverage became higher with the increase of the mass ratio of SnCl4 to kaolinite. Much more nanoparticles were attached to the rod surfaces in the precursor of SK13 (Fig. 4f), we deduced that positively charged Sn(OH) n+4−n nanoparticles were first formed in the liquid and then adsorbed onto the kaolinite rods by electrostatic force. The newly formed particles were adsorbed by the exposed surface as the Sn/Sb loading increased, while previously adsorbed particles grew gradually. After the completion of the reaction and subsequent drying process, Sn(OH) n+4−n was transformed into SnO2·xH2O nanoparticles attached onto the rod surfaces steadily.
The surface coverage of nanoparticles on the surface of kaolinite rods was analyzed for the series of SK composites with different Sn/Sb loading. Compared with the corresponding precursor (Fig. 4), the ATO nanoparticles of SK composites contacted with each other more closely and tightly (Fig. 5). The morphology indicated that the number of ATO nanoparticles increased gradually as a consequence of Sn/Sb loading’s increase (Fig. 5a, c, e), SK4 showed the uniform coating of ATO nanoparticles on the most of rod surfaces (Fig. 5c), but partial surface was not completely coated due to the inadequate Sn/Sb loading. As for the sample SK20 (Fig. 5e), the exterior surfaces of rods were fully coated with well-dispersed nanoparticles, and EDS analysis also confirmed the existence of O, Si, Al, Sn, and Sb elements in SK composites. The interplanar spacings of 0.34 and 0.26 nm corresponded to the (110) and (101) facets of the well-crystallized SnO2, respectively (Fig. 5b, f). The crystal size of SnO2 varied with the Sn/Sb loading, but all in the range of 4–10 nm (Fig. 5b, d, f). The more the Sn/Sb compounds on the rod surface, the larger the crystal size of SnO2.
Full range XPS spectra of kaolinite, precursor, and SK20 were exhibited in Fig. 6. Compared with the original kaolinite, the peaks corresponding to Sn and Sb can be observed in both the precursor and SK20, further confirming the existence of elements Sn and Sb on the rod surface. The atomic composition, (Sn + Sb)/(Al + Si) ratio and Sb/Sn ratio were shown in Table 1. The Sb/Sn atomic ratios of precursor and SK20 were 0.17 and 0.21, respectively, near to the originally designed value of 0.16. Figure 7a and b exhibited the binding interface between Sn–Sb compounds and substrate rods for kaolinite and SK20, respectively. Clear interfaces were observed. The comparison of Al 2p and Si 2p peak positions for kaolinite, precursor and SK20 were also shown in Fig. 7c and d. There was obvious peak shift in binding energy (BE) position from original kaolinite, then precursor and finally to SK20 product, indicating the possible formation of Si–O–Sn and Al–O–Sn chemical bonds at the interfaces. Sn 3d3/2-3d5/2 spin–orbit splitting energy of 8.4 eV was in well agreement with the previously reported value (Fig. 8a), their splitting Sn 3d5/2 spectra (Fig. 8b) exhibited three peaks at 486.2, 487.1, and 488.2 eV, assigned to Sn2+, Sn4+ and Sn(–Cl) complex, respectively, the former two corresponded to SnO and SnO2, the latter may be resulted from the reaction between the Sn ions and Cl− from the reaction environment (Li et al. 2006; Yang et al. 2010). After the precursor was calcined at 700°C, the Sn2+ and Sn(–Cl) peaks almost disappeared in SK20, the Sn4+ peak accounted for the majority.
High-resolution XPS analysis of the O 1s and Sb 3d peaks was further carried out to investigate the detailed information on the chemical states (Fig. 8c, d; Table 2). XPS spectra in the range of 527–544 eV for the precursor and SK20 corresponded to the transitions of Sb and O atoms (Fig. 8c), but the Sb 3d5/2 transition was masked by the peak of O 1s transition, the Sb 3d5/2 peak was separated from the O 1s peak (Fig. 8d). The O 1s spectrum was decomposed into three contributions (Fig. 8c). The peak at BE of 531.0 eV was ascribed to the metal oxide (Sb–O) and the oxygen deficiency on the surface of SnO2−δ crystallites (Batzill et al. 2005). The peak at 532.1 eV of O 1s was attributed to the Al–O and Si–O from the substrate and the chemisorbed oxygen or hydroxyl ions such as O−, O2− or OH−on the SnO2−δ surface, the higher intensity of this peak in the precursor than that in SK20 indicated the existence of SnO2·xH2O and hydroxyl from kaolinite. The peak around 533.1 eV was directly associated with H2O on the surface (Montilla et al. 2004; Manesse et al. 2009; Yang et al. 2010).
The analysis of the surface Sb was restricted to the Sb 3d3/2 lines because of the overlap of the O 1s and Sb 3d5/2 lines, and Sb transition (3d3/2) was employed to obtain the oxidation state of antimony. The XPS spectra of Sb 3d3/2 in precursor and SK20 provided precise information on the chemical state of Sb (Fig. 8d; Table 2). The Sb 3d3/2 spectrum had two characteristic peaks at 539.6 and 540.6 eV, identified as Sb3+ and Sb5+, respectively (Marshall and Haverkamp 2010). The Sb5+ completely predominated in the SK sample, perfectly indicating the complete oxidization of Sb in precursor into valence 5+ in SK. So, most of Sb5+ could enter the SnO2 crystallite in SK, leading to the improvement of conductivity and the color change of the samples.
According to the above analysis, we suggest a possible formation mechanism for ATO/kaolinite composites. The deposition modes of ATO nanoparticles on the surface of kaolinite rods with different Sn/Sb loading were described in Fig. 9. The powdered kaolin, having the negatively charged surface, was well-dispersed in aqueous solution at pH of 1.5. After adding Sn/Sb mixed acid solution, the reaction reached a certain concentration of precipitation, and then generated a large number of positively charged sol particles, which was first adsorbed on the surfaces of kaolinite rods to form composite structure with the aid of the electrostatic attractions among them. As the amount of Sn/Sb mixed solution increased, part of the newly formed sol particles were adsorbed on the exposed surface of the substrate, while another part made the original particles grow up. The coating process was finished until the substrate was covered downright, the number, particle size and coated density of ATO nanoparticles would be increased with the increase of Sn/Sb loading. The specific surface area (S BET), pore volume of metakaolin and SK with different Sn/Sb loading were listed in Table 3, indicating that the surface area of SK4 with lower Sn/Sb loading (4 wt%) was slightly higher than that of uncovered substrate (metakaolin), while SK20 had the highest surface area of 30.4 m2/g. S BET of samples steadily increased with the increase in Sn/Sb loading, which may be attributed to the increase of surface roughness resulted from the deposition of ATO nanoparticles. No change in pore volume for all samples compared with metakaolin indicated that ATO layer onto kaolinite rods was dense without the formation of extra pores.
Conclusions
Novel conductive composites, with uniformly dispersed ATO nanoparticles on the surface of kaolinite rods, were successfully synthesized without the addition of any dispersant. The crystal size and surface coverage of ATO nanoparticles could be controlled via adjusting the reaction conditions. The surface edges and defects of rods served as nucleation sites and facilitated the deposition of various metal oxide nanoparticles via solution processes. Binding interface between ATO layer and rod surface demonstrated that surface characteristics facilitated the uniform assembly of nanosized oxides. Sb5+ entered into the SnO2 crystallites, further led to the improvement of conductivity and the color change of the composites. Thus, our method could offer a more benign route to the general synthesis of mineral-based functional materials.
References
Aranda P, Kun R, Martín-Luengo MA, Letaïef S, Dékány I, Ruiz-Hitzky E (2008) Titania-sepiolite nanocomposites prepared by a surfactant templating colloidal route. Chem Mater 20:84–91
Batzill M, Katsiev K, Burst JM, Diebold U (2005) Gas-phase-dependent properties of SnO2 (110), (100), and (101) single-crystal surfaces: structure, composition, and electronic properties. Phys Rev B 72:165414–165420
Blanco-Gutirrez V, Urones-Garrote E, Torralvo-Fernndez MJ, Sez-Puche R (2010) ZnFe2O4 nanoparticles: different magnetic behavior when they are hosted in porous structures. Chem Mater 22:6130–6137
Boukerma K, Piquemal J-Y, Chehimi MM, Mravčáková M, Omastová M, Beaunier P (2006) Synthesis and interfacial properties of montmorillonite/polypyrrole nanocomposites. Polymer 47:569–576
Bujdk J, Chorvát D Jr, Iyi N (2010) Resonance energy transfer between rhodamine molecules adsorbed on layered silicate particles. J Phys Chem C 114:1246–1252
Cao JL, Shao GS, Wang Y, Liu YP, Yuan ZY (2008) CuO catalysts supported on attapulgite clay for low-temperature CO oxidation. Catal Commun 9:2555–2559
Chen L, Singh RK, Webley P (2007) Synthesis, characterization and hydrogen storage properties of microporous carbons templated by cation exchanged forms of zeolite Y with propylene and butylene as carbon precursors. Microporous Mesoporous Mater 102:159–170
Chong MN, Vimonses V, Lei SM, Jin B, Chow C, Saint C (2009) Synthesis and characterisation of novel titania impregnated kaolinite nano-photocatalyst. Microporous Mesoporous Mater 117:233–242
Corma A (1997) From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem Rev 97:2373–2420
Corma A, Daz U, Garca T, Sastre G, Velty A (2010) Multifunctional hybrid organic-inorganic catalytic materials with a hierarchical system of well-defined micro- and mesopores. J Am Chem Soc 132:15011–15021
Fang HT, Sun X, Qian LH, Wang DW, Li F, Chu Y, Wang FP, Cheng HM (2008) Synthesis of tin (II or IV) oxide coated multiwall carbon nanotubes with controlled morphology. J Phys Chem C 112:5790–5794
Frost RL, Kloprogge JT, Tran THT, Kristof J (1998) The effect of pressure on the intercalation of an ordered kaolinite. Am Mineral 83:1182–1187
Giannelis EP (1996) Polymer layered silicate nanocomposites. Adv Mater 8:29–35
Guffond MC, Hunter RJ, Beattie JK (2001) Electroacoustic properties of barium sulfate particles coated with a conductive layer of antimony/tin(IV) oxides. Chem Mater 13:2619–2625
Gutiérrez M, Escudey M, Escrig J, Denardin JC, Altbir D, Fabris JD, Cavalcante LCD, García-González MT (2010) Preparation and characterization of magnetic composites based on a nature zeolite. Clays Clay Miner 58:589–595
Hu YH, Zhang HH, Yang HM (2008) Synthesis and electrical property of antimony-doped tin oxide powders with barite matrix. J Alloys Compd 453:292–297
Johnston CT, Kogel JE, Bish DL, Kogure T, Murray HH (2008) Low-temperature FTIR study of kaolin-group minerals. Clays Clay Miner 56:470–480
Kibanova D, Cervini-Silva J, Destaillats H (2009) Efficiency of clay-TiO2 nanocomposites on the photocatalytic elimination of a model hydrophobic airpollutant. Environ Sci Technol 43:1500–1506
Kočí K, Matějka V, Kovář P, Lacný Z, Obalová L (2011) Comparison of the pure TiO2 and kaolinite/TiO2 composite as catalyst for CO2 photocatalytic reduction. Catal Today 161:105–109
Krishnamoorti R, Vaia RA, Giannelis EP (1996) Structure and dynamics of polymer-layered silicate nanocomposites. Chem Mater 8:1728–1734
Li Y, Zhao GY, Zhang WH, Chen YQ (2006) Fine pattern fabrication on SnO2:Sb thin films formed by the sol-gel process. Surf Interface Anal 38:1291–1295
Li YQ, Wang JL, Fu SY, Mei SG, Zhang JM, Yong K (2010) Facile synthesis of antimony-doped tin oxide nanoparticles by a polymer-pyrolysis method. Mater Res Bull 45:677–681
Lu ZZ, Ren M, Yin HB, Wang AL, Ge C, Zhang YS, Yu LB, Jiang TS (2009) Preparation of nanosized anatase TiO2-coated kaolin composites and their pigmentary properties. Powder Technol 196:122–125
Mamedov A, Ostrander J, Aliev F, Kotov NA (2000) Stratified assemblies of magnetite nanoparticles and montmorillonite prepared by the layer-by-layer assembly. Langmuir 16:3941–3949
Manesse M, Sanjines R, Stambouli V, Jorel C, Pelissier B, Pisarek M, Boukherroub R, Szunerits S (2009) Preparation and characterization of silver substrates coated with antimony-doped SnO2 thin films for surface plasmon resonance studies. Langmuir 25:8036–8041
Marshall AT, Haverkamp RG (2010) Electrocatalytic activity of IrO2-RuO2 supported on Sb-doped SnO2 nanoparticles. Electrochim Acta 55:1978–1984
Mishra KC, Johnson KH, Schmidt PC (1995) Electronic structure of antimony-doped tin oxide. Phys Rev B 51:13972–13976
Mller V, Rasp M, Tefani G, Ba JH, Gnther S, Rathousky J, Niederberger M, Fattakhova-Rohlfing D (2009) Highly conducting nanosized monodispersed antimony-doped tin oxide particles synthesized via nonaqueous sol-gel procedure. Chemi Materr 21:5229–5236
Montilla F, Morallon E, Battisti AD, Barison S, Daolio S, Vazquez JL (2004) Preparation and characterization of antimony-doped tin dioxide electrodes. 3. XPS and SIMS characterization. J Phys Chem B 108:15976–15981
Murray HH (2000) Traditional and new applications for kaolin, smectite, and palygorskite: a general overview. Appl Clay Sci 17:207–221
Ninness BJ, Bousfield DW, Tripp CP (2003) Formation of a thin TiO2 layer on the surfaces of silica and kaolin pigments through atomic layer deposition. Colloids Surf A Physicochem Eng Asp 214:195–204
Ohashi F, Ueda S, Taguri T, Kawachi S, Abe H (2009) Antimicrobial activity and thermostability of silver 6-benzylaminopurine montmorillonite. Appl Clay Sci 46:296–299
Pinnavaia TJ (1983) Intercalated clay catalysts. Science 220:365–371
Posthumus W, Laven J, Gde With, van der Linde R (2006) Control of the electrical conductivity of composites of antimony doped tin oxide (ATO) nanoparticles and acrylate by grafting of 3-methacryloxypropyltrimethoxysilane (MPS). J Colloid Interf Sci 304:394–401
Sadeh A, Sladkevich S, Gelman F, Prikhodchenko P, Baumberg I, Berezin O, Lev O (2007) Sol-gel-derived composite antimony-doped, tin oxide-coated clay-silicate semitransparent and conductive electrodes. Anal Chem 79:5188–5195
Stein A, Melde BJ, Schroden RC (2000) Hybrid inorganic–organic mesoporous silicates-nanoscopic reactors coming of age. Adv Mater 12:1403–1419
Tan JR, Shen LZ, Fu XS, Hou WX, Chen XZ (2004) Preparation and conductive mechanism of mica titania conductive pigment. Dyes Pigment 62:107–114
Turhan Y, Dogan M, Alkan M (2010) Poly(vinyl chloride)/kaolinite nanocomposites: characterization and thermal and optical properties. Ind Eng Chem Res 49:1503–1513
Wan CY, Li M, Bai X, Zhang Y (2009) Synthesis and characterization of photoluminescent Eu(III) coordination halloysite nanotube-based nanohybrids. J Phys Chem C 113:16238–16246
Wang YD, Brezesinski T, Antonietti M, Smarsly B (2009) Ordered mesoporous Sb-, Nb-, and Ta-doped SnO2 thin films with adjustable doping levels and high electrical conductivity. ACS Nano 3:1373–1378
Yang D-J, Kamienchick I, Youn DY, Rothschild A, Kim I-D (2010) Ultrasensitive and highly selective gas sensors based on electrospun SnO2 nanofibers modifi ed by Pd loading. Adv Funct Mater 20:4258–4264
Yuan P, Southon PD, Liu ZW, Green MER, Hook JM, Antill SJ, Kepert CJ (2008) Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane. J Phys Chem C 112:15742–15751
Zhang JR, Gao L (2004) Synthesis and characterization of antimony-doped tin oxide (ATO) nanoparticles. Inorg Chem Commun 7:91–93
Zhang WD, Phang IY, Liu TX (2006) Growth of carbon nanotubes on clay: unique nanostructured filler for high-performance polymer nanocomposites. Adv Mater 18:73–77
Acknowledgments
This work was supported by the National Natural Science Foundation of China (50774095), the Scientific Research Foundation for ROCS of SEM (2011-1139), Hunan Provincial Innovation Foundation for Postgraduates (CX2010B042) and Excellent PhD Thesis Support Foundation of Central South University (2010ybfz056).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, P., Yang, H. Sb–SnO2 nanoparticles onto kaolinite rods: assembling process and interfacial investigation. Phys Chem Minerals 39, 339–349 (2012). https://doi.org/10.1007/s00269-012-0492-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-012-0492-1