Abstract
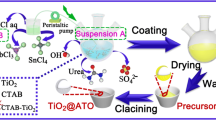
In our present work, we report the synthesis of Sb-doped SnO2-coated TiO2 (TiO2@Sb-SnO2) conductive nanocomposites by hydrothermal method. Firstly, titanate whiskers were hydrothermally synthesized at 175 °C using sodium hydroxide and metatitanic acid as starting materials with a molar ratio of 4:1. And then, the TiO2@Sb-SnO2 nanocomposites were hydrothermally synthesized with the use of the as-synthesized titanate whiskers, tin tetrachloride, and antimony trichloride as starting materials at 185 °C. In the hydrothermal reaction process, Sb-doped SnO2 nanoparticles enhanced the phase transition from titanate to rutile TiO2. The oxidation states of tin and antimony elements are Sn4+, Sb5+, and Sb3+, respectively. TEM and HRTEM analyses indicated that the Sb-doped SnO2 nanoparticles with an average particle size of around 1.5 nm were coated on the surfaces of TiO2 nanoparticulates with an average particle size of around 20 nm. The TiO2@(1%)Sb-(10%)SnO2 nanocomposites had a minimum electric resistivity of 5.97 × 103 Ω·cm. Filling of the TiO2@Sb-SnO2 nanocomposites increased the electric conductivity of waterborne polyester films, endowing the polyester films with static electron dissipativity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanocomposites with distinguished properties have been widely used as electrical conduction materials (Ref 1,2,3), sensors (Ref 4, 5), dielectric materials (Ref 6), additives in polymers (Ref 7), catalysts in thermal and photochemical reactions (Ref 8, 9), and pigments (Ref 10, 11). The synthesis and application of nanocomposites have attracted a great attention of researchers.
In the past decades, researchers found that low-cost n-type Sb-doped SnO2 semiconductors exhibit high electrical conductivity, transparency, and band gap. The Sb-doped SnO2 semiconductors can be used as functional materials in many fields, such as electrical conduction fillers for improving the conductivity of polycarbonate/acrylonitrile butadiene styrene (Ref 1), waterborne epoxy coating (Ref 2), rubber, and plastics (Ref 3); sensors for analyzing acetone, ethanol, liquefied petroleum gas (Ref 4), and formaldehyde (Ref 5); thermal insulation films for blocking ultraviolet light, reflecting near-infrared light, and maintaining visible light transparency (Ref 12,13,14,15,16,17); electrodes for degrading phenol, benzoic acid, norfloxacin, and tetracycline (Ref 18,19,20,21), inactivating E. coli (Ref 18), and oxidizing water (Ref 22). The Sb-doped SnO2 semiconductors can also be used as laser stealth materials (Ref 23) and transparent electrodes for perovskite solar cell (Ref 24).
Sb-doped SnO2 semiconductors could be synthesized by the chemical vapor deposition (Ref 25), aerosol-assisted chemical vapor deposition at 450 °C (Ref 26), oxygen-reactive DC and RF magnetron sputtering (Ref 27, 28), nonaqueous sol–gel (Ref 17, 29, 30), co-precipitation (Ref 16), and hydrothermal (Ref 31) methods using tin and antimony powders (Ref 25), butyl tin trichloride and antimony(III) ethoxide (Ref 26), Sn/Sb disk (Ref 27), Sb2O3 and SnO2 (Ref 28), and SnCl4 (SnCl2) and SbCl5 (SbCl3, antimony(III) acetate, and antimony(III) ethoxide) (Ref 16, 17, 29, 31) as starting materials.
To obtain high electric conductivity, Sb-doped SnO2 semiconductors are usually annealed at 450-550 °C (Ref 17, 26, 28,29,30,31). The electric resistivity of the annealed Sb-doped SnO2 semiconductors ranges from 4.7 × 10‒4 to 1.72 × 10‒2 Ω·cm (Ref 17, 26,27,28,29,30). Without annealing at a high temperature, the electric resistivity of the as-synthesized (4-30 at.%) Sb-doped SnO2 nanoparticles is at a relative low level, ranging from 1 × 104 to 1 × 106 Ω·cm (Ref 30).
To reduce the cost, Sb-doped SnO2 semiconductors could be coated on oxides or mineral matrixes to form conductive composites. It was reported that the electric resistivity of porous coal slag@Sb-SnO2 (Sb/Sn molar ratio of 1:6) powders prepared by calcination at 750 °C was 2.6 × 103 Ω·cm (Ref 32). The electric resistivity of TiO2@(5%)Sb-SnO2 nanocomposites prepared by calcination at 550 °C was 3.5 × 104 Ω·cm (Ref 3). The electric conductivity of rod-like TiO2@Sb(10 at.%)-SnO2 nanocomposites prepared at 75 °C was around 2 × 105 Ω·cm (Ref 33). The support type, Sb doping extent, and reaction temperature significantly affect the electric conductivity of supported Sb-SnO2 nanocomposites. Although the supported Sb-doped SnO2 nanocomposites had a larger electric resistivity than the “naked” Sb-doped SnO2 semiconductors, the large-sized supported conductive nanocomposites not only decrease the cost but also facilely construct conductive networks in polymer matrixes, which effectively improves their electric conductivity.
In this work, we firstly synthesized sodium titanate whiskers by the hydrothermal method starting from metatitanic acid and sodium hydroxide. And then, the TiO2@Sb-doped SnO2 nanocomposites were hydrothermally synthesized using the as-synthesized titanate whiskers as TiO2 precursors and SnCl4/SbCl3 as tin and antimony precursors. The as-synthesized TiO2@Sb-doped SnO2 nanocomposites exhibited good electric conductivity.
2 Experimental
2.1 Materials
Hydrated metatitanic acid (H2TiO3·nH2O, 36.5 wt.% TiO2) was purchased from the Jiangsu Taibai Group Co., Ltd. China. Sodium hydroxide, tin tetrachloride pentahydrate (SnCl4·5H2O), antimony trichloride (SbCl3), ammonia (28%), and hydrochloric acid (38%) were guaranteed reagents and purchased from the Chemical Reagent Co., Ltd. Shanghai, China.
2.2 Synthesis of Titanate Whiskers
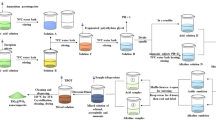
Titanate whiskers were synthesized according to the procedures as follows. 43.5 g of hydrated metatitanic acid was dispersed in 100 mL of deionized water at room temperature (25 °C). After stirring for 30 min, the pH value of the aforementioned suspension was adjusted to ca. 2 by adding a sodium hydroxide (5 wt.%) aqueous solution. And then, a given amount of sodium hydroxide (20 wt.%) aqueous solution was added into the suspension under stirring at 300 rpm. The molar ratios of NaOH to metatitanic acid (H2TiO3) were set at 1:1, 4:1, and 6:1 by changing the amount of sodium hydroxide aqueous solution. After the reaction mixture was stirred at 300 rpm for 2 h, it was poured into Teflon-lined stainless-steel autoclaves and hydrothermally reacted in an electric oven at 175 °C for 24, 48, 72, and 96 h, respectively. When the reaction suspension was cooled to room temperature, the as-synthesized titanate whiskers were filtered, washed with deionized water to neutral, and dried at 110 °C for 12 h.
2.3 Synthesis of TiO2@SnO2 and TiO2 @Sb-SnO2 Nanocomposites
10 g of sodium titanate whiskers synthesized with an NaOH/H2TiO3 molar ratio of 4:1 at 175 °C for 96 h was dispersed in 50 mL of deionized water at 50 °C under stirring. Prescribed amounts of SnCl4·5H2O and SbCl3 were dissolved in 50 mL of hydrochloric acid (2 mol·L‒1) aqueous solution. The SnCl4/SbCl3 mixed aqueous solution was pumped into the sodium titanate whisker aqueous suspension with a peristaltic pump at a feeding rate of 1 mL·min‒1 under stirring. At the same time, an ammonia aqueous solution (5 mol·L‒1) was pumped into the aqueous suspension to control the pH value of reaction solution at ca. 7. After the SnCl4/SbCl3 mixed solution was added, the reaction solution was stirred at 50 °C for 2 h. The reaction solution was transferred into three Teflon-lined stainless-steel autoclaves each with a capacity of 100 mL. The hydrothermal reaction was conducted at 185 °C for 16 h in an electric oven. After the hydrothermal reaction, the as-synthesized TiO2@SnO2 and TiO2@Sb-SnO2 nanocomposite samples were filtered and washed with deionized water until there were no Cl− anions detected in filtrate by dropping several drops of AgNO3 aqueous solution. And then, the washed samples were dried in an electric oven at 110 °C for 12 h.
The weight percentages of Sn in the TiO2@SnO2 nanocomposite samples were 5-20%. For the TiO2@Sb-(10%)SnO2 nanocomposite samples, the weight percentages of Sb were set at 1-5%. After the hydrothermal reaction, the contents of Sn and Sb in filtrates were at a negligible extent by ICP analysis, which indicates that Sb and Sn components were completely deposited in the as-synthesized TiO2@SnO2 and TiO2@Sb-SnO2 nanocomposites.
2.4 Preparation of TiO 2 @(1%)Sb-(10%)SnO2 Nanocomposite-Filled Antistatic Polyester Films
TiO2@(1%)Sb-(10%)SnO2 nanocomposites were mixed with an environmentally friendly waterborne polyester paint. The contents of nanocomposites in the paint were set at 10-25 wt.%. The nanocomposite-filled polyester paint was coated on a smooth glass surface of 40 × 40 mm by a doctor blading coating method and dried naturally at room temperature for 24 h. The thicknesses of films were ca. 1 mm.
2.5 Characterization
The crystal structures of the titanate whisker, TiO2@SnO2, and TiO2@Sb-SnO2 samples were determined with the use of a powder XRD apparatus. The morphologies of the titanate whisker, TiO2@SnO2, and TiO2@Sb-SnO2 samples were observed on a scanning electron microscope. The microstructures of representative titanate whisker and TiO2@Sb-SnO2 samples were analyzed with the use of TEM and HRTEM techniques. The chemical states of tin and antimony components in representative TiO2@SnO2 and TiO2@Sb-SnO2 samples were analyzed with the use of XPS technique. The electric resistivity of the TiO2@SnO2, TiO2@Sb-SnO2, and TiO2@Sb-SnO2 nanocomposite-filled polyester films was measured on an ultra-high resistance microcurrent tester.
3 Results and Discussion
3.1 Evolution of Titanate Whiskers
Figure 1 shows the XRD patterns of the samples hydrothermally synthesized starting from sodium hydroxide and metatitanic acid with different NaOH/H2TiO3 molar ratios at 175 °C for prescribed time periods. When the molar ratio of NaOH to H2TiO3 was 1:1 and the hydrothermal reaction was conducted at 175 °C for 24-96 h, the XRD peaks of the as-synthesized samples were observed at (2θ) 25.3, 37.8, 48.1, 53.9, 55.1, 62.7, 68.8, 70.3, and 75.0°, respectively (Fig. 1a). These peaks belong to the characteristic ones of anatase TiO2 (JCPDS No. 21–1272). The XRD peak intensity increased upon prolonging the hydrothermal reaction time, which reveals that the prolonging of hydrothermal reaction time increases the crystallinity of resultant anatase TiO2 samples.
XRD patterns of the samples hydrothermally synthesized with the NaOH/H2TiO3 molar ratios of (a1-a4) 1:1, (b1-b4) 4:1, and (c1-c3) 6:1 at 175 °C for given time periods of (a1,b1,c1) 24, (a2,b2,c2) 48, (a3,b3,c3) 72, and (a4,b4,c4) 96 h. (●) anatase TiO2 (JCPDS No. 21–1272). (■) Na0.98H1.02Ti4O9·3.3H2O (JCPDS No. 38–0221)
When the samples were hydrothermally synthesized with the NaOH/H2TiO3 molar ratio of 4:1 at 175 °C for 24-96 h, after washing and drying, several diffraction peaks were observed at (2θ) 17.6, 23.9, 26.5, 29.6, 35.3, 35.8, 37.6, 47.3, and 48.3° (Fig. 1b), which could be ascribed to those of distorted titanate salt, Na0.98H1.02Ti4O9·3.3H2O (JCPDS No. 38–0221). The peak intensity increased with the prolonging of hydrothermal reaction time. While the hydrothermal reaction time was 72 h or longer, the appearance of a peak at (2θ) 17.6° indicates that the as-synthesized titanate salt has a layered structure.
As the molar ratio of NaOH to H2TiO3 was increased to 6:1, the XRD peaks of the distorted titanate salt (Na0.98H1.02Ti4O9·3.3H2O) were obviously observed for the washed and dried samples (Fig. 1c). The peak intensity of these samples was stronger than that of those samples synthesized with the NaOH/H2TiO3 molar ratio of 4:1. It is worthy to note that increasing the molar ratio of sodium hydroxide to metatitanic acid and prolonging the hydrothermal reaction time facilitate the formation of titanate salt.
We suggested that at a lower NaOH/H2TiO3 molar ratio of 1:1, metatitanic acid was converted to anatase TiO2. Upon increasing the NaOH/H2TiO3 molar ratio to 4:1 or 6:1, sodium hydroxide could react with metatitanic acid to form layer-structured sodium tetratitanate and water molecules could intercalate into the layers of titanate salt. During washing process, Na+ ions were substituted by H+ ions, which results in the formation of Na0.98H1.02Ti4O9·3.3H2O. The evolution procedures are illustrated by the following equations.
The SEM images of the anatase TiO2 samples show that these samples were composed of small-sized TiO2 primary nanoparticles with the particle sizes of ca. 25 nm. The primary nanoparticles aggregated to form secondary particles with the sizes of ca. 1 μm (Fig. 2a1–a4).
When the titanate salt samples were synthesized with the NaOH/H2TiO3 molar ratio of 4:1, the washed and dried samples were composed of nanosized titanate whiskers (Fig. 2b1-b4). Diameters and lengths of the as-synthesized titanate whiskers increased with the prolonging of hydrothermal reaction time periods. When the titanate whiskers were synthesized at 175 °C for 96 h, after washing and drying, their average diameter and length were 320 nm and 3.0 μm, respectively.
At the NaOH/H2TiO3 molar ratio of 6:1, diameters and lengths of the as-synthesized titanate whiskers also increased with the prolonging of hydrothermal reaction time periods (Fig. 2c1–c4). When the titanate whiskers were hydrothermally synthesized at 175 °C for 96 h, after washing and drying, their average diameter and length were 530 nm and 4.93 μm, respectively. Higher NaOH/H2TiO3 molar ratio and longer hydrothermal reaction time are beneficial to the crystal growth of titanate whiskers.
To determine the microstructures of titanate whiskers, a representative sample hydrothermally synthesized with the NaOH/H2TiO3 molar ratio of 4:1 at 175 °C for 96 h was analyzed by HRTEM. The HRTEM image shows that the as-synthesized titanate whiskers have a layered structure (Fig. 2d1). The layer distance is 0.766 nm, which is large enough for the intercalating of water molecules with a diameter of ca. 0.4 nm.
Considering that the titanate whisker sample synthesized with the NaOH/H2TiO3 molar ratio of 4:1 at 175 °C for 96 has a well crystal structure and small diameter, it was selected as the TiO2 precursor for the synthesis of TiO2@SnO2 and TiO2@Sb-SnO2 conductive nanocomposites.
3.2 Evolution of TiO 2 @SnO 2 and TiO 2 @Sb-SnO 2 Nanocomposites
The titanate whiskers hydrothermally synthesized with the NaOH/H2TiO3 molar ratio of 4:1 at 175 °C for 96 h were used as TiO2 precursors to synthesize TiO2@SnO2 and TiO2@Sb-doped SnO2 semiconductors. When the TiO2@SnO2 samples with 5-20% Sn were hydrothermally synthesized at 185 °C for 16 h, weak XRD peaks at 26.4 (shoulder), 34.1, and 51.9° ascribed to cassiterite SnO2 phase (JCPDS No. 41–1445), peaks at 25.3, 37.8, 48.0, and 62.7° ascribed to anatase TiO2 phase (JPCDS No. 21–1272), and strong peaks at 27.3, 35.9, 39.0, 41.1, 43.9, 54.1, 56.5, 62.7, 64.0, and 68.8° ascribed to rutile TiO2 phase (JPCDS 21–1276) were observed (Fig. 3). The XRD analysis revealed that during the hydrothermal reaction process, SnO2 phase evolved. At the same time, titanate whiskers were converted to both anatase and rutile TiO2. It is worthy to indicate that at a higher SnO2 content, the peak intensity of rutile TiO2 phase became stronger, whereas the peak intensity of anatase TiO2 phase became weaker. It is reasonable to propose that SnO2 crystallites could induce the phase transition from titanate to rutile even at a mild hydrothermal reaction temperature of 185 °C.
SEM images of the TiO2@SnO2 samples with 5-20% Sn contents show that these samples were composed of TiO2 and SnO2 nanoparticles (Fig. 4). The magnified SEM images show that the particle sizes of the samples ranged from 10 to 70 nm and their average particle sizes were ca. 30 nm. The elemental mapping images show that the tin and titanium elements uniformly dispersed in these samples, which indicates that the TiO2 and SnO2 nanoparticles well dispersed in the as-synthesized TiO2@SnO2 nanocomposites.
To increase the electric conductivity of TiO2@SnO2 nanocomposites, antimony component was added into the samples. The XRD patterns showed that at lower Sb contents of 1-2%, weak peaks at (2θ)25.2 and 37.8° ascribed to anatase TiO2 phase (JPCDS No. 21–1272) and strong peaks at (2θ)27.5, 35.9, 39.0, 41.1, 43.9,54.1, 56.6, 62.6, 63.9, and 69.0° ascribed to rutile TiO2 phase (JPCDS No. 21–1276) were obviously observed (Fig. 5). The peak intensity of rutile TiO2 phase was much stronger than that of anatase TiO2 phase, indicating that rutile TiO2 phase dominantly formed. Furthermore, a weak peak at (2θ) 52.4° ascribed to cassiterite SnO2 (JPCDS No. 41–1445) was observed.
At larger Sb contents of 3-5%, rutile TiO2 phase existed, whereas anatase TiO2 phase disappeared. The addition of Sb component enhanced the phase transition from titanate to rutile TiO2. A weak peak at (2θ) 52.4° ascribed to cassiterite SnO2 phase was also observed. There were no antimony-containing compounds detected. It is worthy to note that with the addition of Sb component, the XRD peaks of cassiterite SnO2 shift to large angles by 0.5° as compared to those of the TiO2@SnO2 samples. It could be explained as that antimony ions with smaller radius can substitute the Sn4+ ions of SnO2 crystallites. With the increasing of substituting extent, the lattice constants of Sb-SnO2 crystallites decrease, leading to the increase in diffraction angle (2θ) (Ref 14).
SEM images of the TiO2@Sb-SnO2 samples show that these samples were composed of TiO2 and Sb-doped SnO2 nanoparticles (Fig. 6). The magnified SEM images show that the particle sizes of the TiO2@Sb-SnO2 samples ranged from 10 to 70 nm and their average particle sizes were ca. 30 nm. The elemental mapping images show that the tin, antimony, and titanium elements uniformly dispersed in these samples, which indicates that the TiO2 and Sb-SnO2 nanoparticles could well disperse in the as-synthesized TiO2@Sb-SnO2 nanocomposites.
TEM and HRTEM images of the representative TiO2@(1%,3%)Sb-(10%)SnO2 samples are shown in Fig. 7. For the TiO2@(1%)Sb-(10%)SnO2 sample, the average particle size of Sb-SnO2 nanoparticles was 1.5 nm. And the average particle size and particle size distribution of TiO2 nanoparticulates were 19 and 12-70 nm, respectively. For the TiO2@(3%)Sb-(10%)SnO2 sample, the average particle size of Sb-SnO2 nanoparticles was 1.6 nm. The average particle size and particle size distribution of TiO2 nanoparticulates were 21 and 12-33 nm.
The lattice fringes of TiO2@(1%,3%)Sb-(10%)SnO2 samples were 0.331 and 0.262 nm, which are ascribed to the lattice spacings of (1 1 0) and (1 0 1) planes of cassiterite SnO2. And the lattice fringes of 0.328 nm are ascribed to the lattice spacing of (1 1 0) plane of rutile TiO2.
The TEM and HRTEM analyses reveal that the small-sized Sb-doped SnO2 nanoparticles well anchored at the surfaces of large-sized TiO2 nanoparticulates. The TiO2@Sb-SnO2 samples had a core–shell structure.
The binding energies of Sn3d5/2 and Sn3d3/2 of the representative TiO2@(10%)SnO2, TiO2@(1%)Sb-(10%)SnO2, and TiO2@(3%)Sb-(10%)SnO2 were 486.4, 494.9; 486.6, 495; 486.6 and 495 eV, respectively (Fig. 8a). The distances between the Sn3d5/2 and Sn3d3/2 peaks were 8.5, 8.4, and 8.4 eV. The binding energy values and spin–orbit splitting of Sn3d5/2 and Sn3d3/2 indicate that the tin component in the samples is at Sn4+ oxidation state (Ref 5, 17, 32). However, the Sn3d5/2 and Sn3d3/2 peaks of the TiO2@Sb-SnO2 samples shifted to large values by 0.2 and 0.1 eV as compared to those of the TiO2@SnO2 sample. And the distances between the Sn3d5/2 and Sn3d3/2 peaks of the TiO2@Sb-SnO2 samples were slightly less than that of the TiO2@SnO2 sample. These changes could be ascribed to the substitution of Sn4+ ions in SnO2 crystallites by antimony ions.
Considering the overlap of O1s and Sb3d5/2 peaks, the Sb3d3/2 peak was used to analyze the chemical state of antimony element in the nanocomposites. According to the reports, the binding energies of Sb3d3/2 peaks for Sb3+ and Sb5+ are 539.2-539.7 and 540.1-540.6 eV, respectively (Ref 3, 5, 32). To analyze the chemical state of antimony element, the binding energies of Sb3d3/2 peaks for Sb3+ and Sb5+ were set at 539.5 and 540.1 eV, respectively. The Sb3d3/2 peak was separated to two peaks by using an XPS peak split software. The peak splitting results show that the antimony element in TiO2@(1%)Sb-(10%)SnO2 and TiO2@(3%)Sb-(10%)SnO2 samples is at both Sb5+ and Sb3+ oxidation states (Fig. 8b and c). The ratios of Sb5+/Sb3+ estimated according to their areas are 0.97:1 and 0.83:1, respectively. The XPS analysis reveals that during the hydrothermal synthesis process, part of Sb3+ was oxidized to Sb5+.
3.3 Electric Conductivity of TiO2@SnO2 and TiO2@Sb-SnO2 Nanocomposites
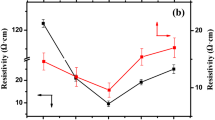
Figure 9a shows the impacts of tin contents on the electric resistivity of TiO2@SnO2 nanocomposites. It was found that when the tin contents were increased from 5 to 10%, the electric resistivity of the TiO2@SnO2 nanocomposites dramatically decreased from 2.69 × 106 to 6.62 × 105 Ω·cm. Further increasing the tin content had no obvious effect on the electric resistivity. We suggest that at a tin content of 10%, the SnO2 nanoparticles could be well coated at the surfaces of TiO2 matrixes. Therefore, 10% tin content in the TiO2/SnO2 nanocomposite is enough to obtain a good electric conductivity.
When antimony dopant contents were 1-2%, the electric resistivity of the as-synthesized TiO2@Sb-(10%)SnO2 nanocomposites reached a lower level (Fig. 9b). At the antimony content of 1%, the minimum electric resistivity of 5.97 × 103 Ω·cm was obtained. Upon further increasing the antimony content, the electric resistivity slowly increased.
It has been suggested that when Sb5+ is the doping component in SnO2 crystallite, Sb5+ introduces donor state (electron), which decreases the electric resistivity (Ref 3, 32). On the other hand, when Sb3+ and Sb5+ are the doping components in SnO2 crystallite, Sb3+ introduces acceptor state (hole) and has a compensating effect on the donor state of Sb5+, which causes an increase in the electric resistivity of Sb-doped SnO2 crystallite. Under our present hydrothermal reaction conditions, both Sb5+ and Sb3+ ions could substitute the Sn4+ ions in SnO2 crystallites. A higher Sb5+/Sb3+ ratio at a lower antimony content was favorable for obtaining higher electric conductivity. The antimony doping extent affected the Sb-SnO2 crystal structure, which influences the electric conductivity.
3.4 Electric Conductivity of TiO2@Sb-SnO2 Nanocomposites-Filled Waterborne Polyester Paint Films
The electric resistivity of TiO2@(1%)Sb-(10%)SnO2 nanocomposite-filled waterborne polyester paint films decreased upon increasing the nanocomposite contents (Fig. 10). At a lower conductive nanocomposite content of 10 wt.%, the resistivity of the nanocomposite-filled polyester film was 6.59 × 1010 Ω·cm. The filling of conductive nanocomposite endowed the polyester paint film with static electron dissipativity. It is reasonably suggested that the as-synthesized TiO2@(1%)Sb-(10%)SnO2 nanocomposites could be used as effective conduction fillers to eliminate static electron accumulation in nonconductive polymers.
4 Conclusions
Titanate whiskers were hydrothermally synthesized at 175 °C using sodium hydroxide and metatatanic acid as starting materials with a molar ratio of 4:1 or 6:1. TiO2@SnO2 nanocomposites were hydrothermally synthesized at 185 °C with the use of the titanate whiskers and tin tetrachloride as titanium and tin precursors. The presence of SnO2 phase promoted the phase transition from titanate to both anatase and rutile TiO2. At 10 wt.% tin content, the electric resistivity of the as-synthesized TiO2@(10%)SnO2 nanocomposites was 6.62 × 105 Ω·cm.
When the titanate whiskers, tin tetrachloride, and antimony trichloride were used as titanium, tin, and antimony precursors, the rutile TiO2@Sb-SnO2 nanocomposites were hydrothermally synthesized at 185 °C. The Sb-doped SnO2 nanoparticles could enhance the phase transition from titanate to rutile TiO2 when the Sb content was above 2 wt.%. The TiO2@(1%)Sb-(10%)SnO2 nanocomposites had a minimum electric resistivity of 5.97 × 103 Ω·cm.
In the TiO2@SnO2 and TiO2@Sb-SnO2 nanocomposites, the oxidation state of tin component is Sn4+. And the oxidation states of antimony component are both Sb5+ and Sb3+. TEM and HRTEM analyses show that the Sb-doped SnO2 nanoparticles with an average particle size of ca. 1.5 nm were coated on the surfaces of rutile TiO2 nanoparticulates with an average particle size of ca. 20 nm.
The filling of the as-synthesized TiO2@Sb-SnO2 nanocomposites promoted the electric conductivity of waterborne polyester paint films, which endows the polyester films with static electron dissipativity.
References
T. Chen, P. Xue, and M. Jia, The Property of Polycarbonate/Acrylonitrile Butadiene Styrene-Based Conductive Composites Filled by Nickel-Coated Carbon Fiber and Nickel-Graphite Powder, Polym. Composite, 2017, 38, p 157–163.
T. Wang, H. Ge, and K. Zhang, A Novel Core-Shell Silica@Graphene Straticulate Structured Antistatic Anticorrosion Composite Coating, J. Alloys Compd., 2018, 745, p 705–715.
X. Li, J. Qian, J. Xu, Y. Sun, and L. Liu, Synthesis and Electrical Properties of Antimony-Doped Tin Oxide-Coated TiO2 by Polymeric Precursor Method, Mat. Sci. Semicon. Proc., 2019, 98, p 70–76.
A.R. Babar, S.S. Shinde, A.V. Moholkar, C.H. Bhosale, J.H. Kim, and K.Y. Rajpure, Sensing Properties of Sprayed Antimony Doped Tin Oxide Thin Films: Solution Molarity, J. Alloys Compd., 2011, 509, p 3108–3115.
Y.D. Wang, I. Djerdj, M. Antonietti, and B. Smarsly, Polymer-Assisted Generation of Antimony-Doped SnO2 Nanoparticles with High Crystallinity for Application in Gas Sensors, Small, 2008, 4, p 1656–1660.
J. Guo, Z. Chen, Z.M. El-Bahy, H. Liu, H.M. Abo-Dief, W. Abdul, K.M. Abualnaja, A.K. Alanazi, P. Zhang, M. Huang, G. Hu, and J. Zhu, Tunable Negative Dielectric Properties of Magnetic CoFe2O4/Graphite-Polypyrrole Metacomposites, Adv. Compos. Hybrid Mater., 2022, 5, p 899–906.
T. Liu, Z. Li, T. Jiang, S. Xi, Y. Li, J. Guo, M. Huang, H. Algadi, X. Ye, and Q. Jiang, Improvement of Thermodynamic Properties of Poly(Butanediol Sebacate-Butanediol Terephthalate) (PBSeT) Composites Based on the Dispersion of PCaCO3@Tannic Acid Formed by Complexation of Tannic Acid and Ti, Adv. Compos. Hybrid Mater., 2022, 5, p 2787–2800.
A. Wang, D. Yu, H. Yin, and W. Yuan, Preparation of Bimetallic CuxAgy Nanoparticles and their Catalytic Performance in Hydrogenation of 4-Nitrophenol with H2 to 4-Aminophenol, Catal. Lett., 2022, 152, p 3691–3703.
M. Eqi, C. Shi, J. Xie, F. Kang, H. Qi, X. Tan, Z. Huang, J. Liu, and J. Guo, Synergetic Effect of Ni-Au Bimetal Nanoparticles on Urchin-Like TiO2 for Hydrogen and Arabinose Co-Production by Glucose Photoreforming, Compos. Hybrid Mater., 2023, 6, p 5.
W. Cao, A. Wang, and H. Yin, Preparation of TiO2@ZrO2@SiO2@MAA Nanocomposites and Impact of Layer Structure on Pigmentary Performance, Mater. Chem. Phys., 2021, 263, p 124403.
L. Shen, W. Cao, A. Wang, and Hengbo Yin, Preparation of TiO2@ZrO2@AlOOH@Polymethyl Acrylic Acid Nanocomposites and the Impact of Layer Structure on Color Scheme, Photocatalytic Activity, and Dispersion Stability, Ind. Eng. Chem. Res., 2020, 59, p 21811–21821.
D. Fang, H. Yu, M. Dirican, Y. Tian, J. Xie, D. Jia, C. Yan, Y. Liu, C. Li, H. Liu, J. Wang, F. Tan, G. Chen, X. Zhang, and J. Tao, Disintegrable, Transparent and Mechanically Robust High-Performance Antimony Tin Oxide/Nanocellulose/Polyvinyl Alcohol Thermal Insulation Films, Carbohyd. Polym., 2021, 266, p 118175.
J. Qu, J. Song, J. Qin, Z. Song, W. Zhang, Y. Shi, T. Zhang, H. Zhang, R. Zhang, Z. He, and X. Xue, Transparent Thermal Insulation Coatings for Energy Efficient Glass Windows and Curtain Walls, Energ. Build., 2014, 77, p 1–10.
M. Wang, Y. Xu, Y. Liu, W. Wu, and S. Xu, Synthesis of Sb-Doped SnO2 (ATO) Hollow Microspheres and Its Application in Photo-Thermal Shielding Coating, Prog. Org. Coat., 2019, 136, p 105229.
H. Li, L. Song, H. Liu, J. Li, A. Yang, C. Sun, R. Li, Y. Fu, and C. Yu, Antimony-Doped Tin Oxide Embedding Graphene-Based Aerogel for Infrared Barriering, Ceram. Int., 2019, 45, p 7894–7905.
B. Shen, Y. Wang, L. Lu, and H. Yang, pH-Dependent Doping Level and Optical Performance of Antimony-Doped Tin Oxide Nanocrystals as Nanofillers of Spectrally Selective Coating for Energy-Efficient Windows, Ceram. Int., 2021, 47, p 20335–20340.
J. Mazloom, F.E. Ghodsi, and M. Gholami, Fiber-Like Stripe ATO (SnO2:Sb) Nanostructured Thin Films Grown by Sol-Gel Method: Optical, Topographical and Electrical Properties, J. Alloys Compound, 2013, 579, p 384–393.
S.Y. Yang, D. Kim, and H. Park, Shift of the Reactive Species in the Sb−SnO2-Electrocatalyzed Inactivation of E. Coli and Degradation of Phenol: Effects of Nickel Doping and Electrolytes, Environ. Sci. Technol., 2014, 48, p 2877–2884.
X. Cui, G. Zhao, Y. Lei, H. Li, P. Li, and M. Liu, Novel Vertically Aligned TiO2 Nanotubes Embedded with Sb-Doped SnO2 Electrode with High Oxygen Evolution Potential and Long Service Time, Mater. Chem. Phys., 2009, 113, p 314–321.
S. Man, H. Bao, K. Xu, H. Yang, Q. Sun, L. Xu, W. Yang, Z. Mo, and X. Li, Preparation and Characterization of Nd-Sb co-Doped SnO2 Nanoflower Electrode by Hydrothermal Method for the Degradation of Norfloxacin, Chem. Eng. J., 2021, 417, p 129266.
Z. Liu, M. Zhu, L. Zhao, C. Deng, J. Ma, Z. Wang, H. Liu, and H. Wang, Aqueous Tetracycline Degradation by Coal-Based Carbon Electrocatalytic Filtration Membrane: Effect of Nano Antimony-Doped Tin Dioxide Coating, Chem. Eng. J., 2017, 314, p 59–68.
Y. Sun, W.D. Chemelewski, S.P. Berglund, C. Li, H. He, G. Shi, and C.B. Mullins, Antimony-Doped Tin Oxide Nanorods as a Transparent Conducting Electrode for Enhancing Photoelectrochemical Oxidation of Water by Hematite, ACS Appl. Mater. Interfaces, 2014, 6, p 5494–5499.
Y. Zhang, Q. Shao, B. Zhao, B. Zhang, V. Murugadoss, S. Wu, T. Ding, and Z. Guo, Facile Bioactive Yeast Cell Templated Synthesis of Laser Stealth Antimony Doped Tin Oxide Hollow Microspheres, Colloid Surface A, 2019, 583, p 123965.
Y. Bai, Y. Fang, Y. Deng, Q. Wang, J. Zhao, X. Zheng, Y. Zhang, and J. Huang, Low Temperature Solution-Processed Sb:SnO2 Nanocrystals for Efficient Planar Perovskite Solar Cells, Chemsuschem, 2016, 9, p 2686–2691.
I.M. Costa, Y.N. Colmenares, P.S. Pizani, E.R. Leite, and A.J. Chiquito, Sb Doping of VLS Synthesized SnO2 Nanowires Probed by Raman and XPS Spectroscopy, Chem. Phys. Lett., 2018, 695, p 125–130.
S.D. Ponja, B.A.D. Williamson, S. Sathasivam, D.O. Scanlon, I.P. Parkin, and C.J. Carmalt, Enhanced Electrical Properties of Antimony Doped Tin Oxide Thin Films Deposited via Aerosol Assisted Chemical Vapour Deposition, J. Mater. Chem. C, 2018, 6, p 7257–7266.
J. Montero, J. Herrero, and C. Guillén, Preparation of Reactively Sputtered Sb-Doped SnO2 Thin Films: Structural, Electrical and Optical Properties, Sol. Energ. Mat. Sol. C., 2010, 94, p 612–616.
J. Ni, X. Zhao, X. Zheng, J. Zhao, and B. Liu, Electrical, Structural, Photoluminescence and Optical Properties of p-Type Conducting, Antimony-Doped SnO2 Thin Films, Acta Mater., 2009, 57, p 278–285.
S.S. Lekshmy, G.P. Daniel, and K. Joy, Microstructure and Physical Properties of Sol Gel Derived SnO2: Sb Thin Films for Optoelectronic Applications, Appl. Surf. Sci., 2013, 274, p 95–100.
V. Müller, M. Rasp, G. Štefanić, J. Ba, S. Günther, J. Rathousky, M. Niederberger, and D. Fattakhova-Rohlfïng, Highly Conducting Nanosized Monodispersed Antimony-Doped Tin Oxide Particles Synthesized via Nonaqueous Sol-Gel Procedure, Chem. Mater., 2009, 21, p 5229–5236.
M. Zhang, Y. Wang, Y. Ma, X. Wang, B. Zhao, and W. Ruan, Study of Charge Transfer Effect in Surface-Enhanced Raman Scattering (SERS) by Using Antimony-Doped Tin Oxide (ATO) Nanoparticles as Substrates with Tunable Optical Band Gaps and Free Charge Carrier Densities, Spectrochim. Acta A, 2022, 264, p 120288.
J. Zhang, J. Zuo, Y. Jiang, A. Ju, D. Zhu, J. Zhang, and C. Wei, Synthesis and Characterization of Composite Conductive Powders Prepared by Sb-SnO2-Coated Coal Gasification Fine Slag Porous Microbeads, Powder Technol., 2021, 385, p 409–417.
Z. Chen, M. Gu, F. Wang, C. Gao, P. Liu, Y. Ding, S. Zhang, and M. Yang, Conductive TiO2 Nanorods via Surface Coating by Antimony Doped Tin Dioxide, Mater. Chem. Phys., 2019, 225, p 181–186.
Acknowledgments
Our present work was financially supported by the fund from the Liaoning Science and Technology Department, China (2021JH1/10400063).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, X., Wang, A., Yin, H. et al. Hydrothermal Synthesis of TiO2@Sb-SnO2 Nanocomposites Starting from Titanate Whiskers, SnCl4, and SbCl3 and their Electric Conductivity. J. of Materi Eng and Perform 33, 1506–1517 (2024). https://doi.org/10.1007/s11665-023-08070-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-023-08070-6