Abstract
Background
For pancreatic ductal adenocarcinoma (PDAC) surgery, extended dissection of the nerve plexus (pl) around the superior mesenteric artery (SMA) or celiac artery (CA) is sometimes necessary. This consequently results in postoperative refractory diarrhea. This study aimed to evaluate the clinical impact of extended nerve plexus dissection around major arteries on postoperative diarrhea.
Methods
Patients who underwent pancreatoduodenectomy (PD) for PDAC between January 2013 and December 2016 were included. The frequency of diarrhea (defined as a condition requiring opioid antidiarrheal drug for at least 6 months after surgery) and its short- and long-term outcomes were reviewed.
Results
Of 200 consecutive patients who underwent PD, 78 (39.0%) developed postoperative refractory diarrhea (diarrhea group), and 73 of them (93.6%) underwent hemi-circumferential or more nerve dissection for SMA or CA; both plSMA and plCA dissection were associated with diarrhea. Borderline resectable artery (BR-A) PDAC was included more in the diarrhea group (32.0% vs. 13.1%, P = 0.001); however, the local recurrence rate in the diarrhea group was significantly lower than that in the non-diarrhea group (14.1% vs. 26.2%, P = 0.036). The completion of adjuvant chemotherapy and overall survival were comparable between the two groups. The pre-albumin level improved in 2 years, and 61.3% of patients with diarrhea could stop opioid antidiarrheal drugs within 3 years of surgery.
Conclusions
Although the frequency of diarrhea increased following nerve plexus dissection around arteries, diarrhea was controllable and resulted in a reduced local recurrence rate. Aggressive dissection of the nerve plexus may be justified for local disease control in BR-A PDAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The results of randomized controlled trials (RCTs) do not favor extended lymphadenectomy during pancreatoduodenectomy (PD) for pancreatic ductal adenocarcinoma (PDAC) and infer that extended lymphadenectomy could increase complications, such as delayed gastric emptying and pancreatic fistula[1,2,3,4,5,6]. However, the significance of dissection of the nerve plexus (pl) around major arteries (superior mesenteric artery [SMA] or celiac artery [CA]) is still unclear. Since PDAC often invades the nerve plexus around major arteries, dissection of the nerve plexus is sometimes necessary for R0 resection if it is close to those [7, 8]. Reports have suggested that circumferential dissection of the nerve plexus around the SMA (plSMA) causes more cases of diarrhea [4, 5, 9] than hemi-circumferential nerve dissection [6, 10]. However, the long-term outcomes of patients with diarrhea and the role of combined dissection of plSMA and plCA are unclear.
In our hospital, a standard lymphadenectomy [11] is routinely performed during PD for PDAC. The dissection level of the nerve plexus is tailored to the distance between the tumor and arteries, preservation of the nerve plexus (Level 2), or hemi to whole circumferential nerve plexus dissection (Level 3) [12,13,14,15]. These tailored nerve dissection techniques apply not only to plSMA but also to plCA [15].
The aim of this study was to determine diarrhea profiles by combining the dissection levels of plSMA and plCA, and to evaluate the short- and long-term outcomes of patients with diarrhea.
Methods
Ethics
Approval from the institutional ethical committee was obtained for this study (Institutional Review Board no. 2018-1179). The requirement of informed consent was waived because of the retrospective study design.
Patients
Patients who underwent subtotal stomach-preserving pancreatoduodenectomy (SSPPD) between January 2013 and December 2016 were included. Patient demographics, perioperative and postoperative details, nutritional status, and clinical course of diarrhea were collected retrospectively from charts.
Surgical technique and the degree of plexus dissection (level classification)
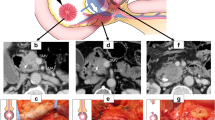
An artery-first approach in SSPPD was performed to judge resectability, such as the invasion to the SMA, maximize the resection margin to the SMA, or reduce blood loss. A standard lymphadenectomy was routinely performed, as previously described [12, 14]. The plSMA and plCA indicate a bundle of nerves that circumferentially surround the SMA and CA, respectively, and lead to the plexus of pancreatic head. The degree of plSMA and plCA dissection was categorized into three levels [12,13,14,15]. Level (LV) 1 dissection comprised simple resection of the pancreatic head, duodenum, and biliary system without dissection of LNs or the nerve plexus, and this was not applied to PDAC surgery. LV2 dissection included en bloc resection of LNs along the artery, preserving the plSMA or plCA. LV3 included en bloc dissection of the regional LNs and the nerve plexus close to cancer invasion accompanied by hemi-circumferential or more dissection of the plSMA or plCA. A combined dissection of SMA LV2-3 and CA LV2-3 in SSPPD was performed for PDAC (Fig. 1). The level of nerve plexus dissection was not an operating surgeon’s discretion, but rather determined preoperatively in the multidisciplinary conference, based on the preoperative CT scan.
Degree of nerve plexus dissection (level classification). LV2: preserving of the nerve plexus. LV3: hemi-circumferential or more extended dissection of the nerve plexus. a: SMA LV2 and CA LV2. b: SMA LV3 (hemi-circumferential dissection) and CA LV2. c: SMA LV2 and CA LV3 (hemi-circumferential dissection). d: SMA LV3 (whole circumferential dissection) and CA LV3 (whole circumferential dissection). The green area around the artery signifies the nerve plexus. GDA, gastroduodenal artery; CHA, common hepatic artery; CA, celiac artery; LGA, left gastric artery; SpA, splenic artery; RHA, right hepatic artery; SMA, superior mesenteric artery; IPDA, inferior pancreaticoduodenal artery; J1A, the first jejunal artery; IPA, inferior phrenic artery; PV, portal vein; SMV, superior mesenteric vein; SpV, splenic vein; LRV, left renal vein
Since neoadjuvant radiotherapy is not a standard treatment in Japan, it was not performed in our hospital.
Definitions
The extent of diarrhea was estimated based on the frequency of defecation and fecal status. If a patient experienced watery defecation more than five times a day without any evidence of bacterial enteritis and if diarrhea occurred immediately after eating, despite pancreatic enzyme administration, postoperative refractory diarrhea was suspected. Non-opioid antidiarrheal drugs (albumin tannate, 3–6 g/day; and natural aluminum silicate, 3–6 g/day) were first administered. An opioid antidiarrheal drug (Opium tincture; Daiichi Sankyo, Japan) was used if the non-opioid version could not relieve the symptoms. An initial dose of 0.3 ml of opium tinctures was administered before each meal, and the dose was modified based on the symptom to achieve solid or soft stool three or fewer times a day. Since most of the patients received adjuvant chemotherapy for 6 months after surgery and it took time to stabilize the diarrhea, we defined postoperative refractory diarrhea as a condition requiring opioid antidiarrheal drugs 6 months after surgery. All the patients had the same symptoms (watery diarrhea within 2 h after feeding), and diarrhea could not be controlled with non-opioid antidiarrheal drugs.
Resectability (resectable [R] or borderline resectable [BR]) was defined according to the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer TNM staging system and National Comprehensive Cancer Network guidelines [16, 17]. R0 resection was defined as no exposure of cancer cells in the dissection plane by pathological findings (0 mm rule). For the accurate pathological assessment, three-dimensional fixation, which we reported previously, was used for specimen staining and the evaluation of resection margin [18]. Postoperative complications were defined according to the Clavien-Dindo classification [19, 20]. For BR cases, upfront surgery was performed until 2014, and four courses of nab-paclitaxel combined with gemcitabine (GnP) were administered as neoadjuvant chemotherapy (NAC) since 2015. For adjuvant chemotherapy, S-1 has been administered as a standard therapy based on the JASPAC 01 study [21]. No radiation therapy was used in this study. Recurrence was diagnosed by contrast-enhanced computed tomography, fluorodeoxyglucose-avidity on positron emission tomography scan or elevation of CA 19-9 during follow-up. “Local recurrence" was defined as an increase of low-density area or lymph node around the vascular or remnant pancreas regardless of distant metastasis, and “distant metastasis” was defined as distant metastasis without any local recurrence.
Statistical analyses
Chi-square and Mann–Whitney U tests or Wilcoxon rank-sum tests were used. Survival curves were constructed using the Kaplan–Meier method. Differences in survival were analyzed using log-rank tests. A P-value of < 0.05 denoted statistical significance. All statistical analyses were performed using SPSS (version 21.0, SPSS Inc., Chicago, IL, USA).
Results
Frequency of postoperative refractory diarrhea by dissection levels of plSMA or plCA (Table 1, Fig. 2)
Of 200 consecutive patients who underwent SSPPD, 83 and 117 underwent SMA LV2 and SMA LV3, and 141 and 59 CA LV2 and CA LV3 dissection, respectively. As shown in Table 1, diarrhea developed in 78 patients; diarrhea was significantly higher in those who underwent SMA LV3 than those who underwent SMA LV2 (n = 68 [87.2%] vs. n = 10 [12.9%], P < 0.001). Likewise, more patients who underwent CA LV3 developed diarrhea than those who underwent CA LV 2 (n = 35 [59.3%] vs. n = 43 [30.4%], P < 0.001).
As shown in Fig. 2, SMA LV3 increased the incidence of diarrhea compared to SMA LV2 regardless of CA LV dissection level. Moreover, there was no difference in the incidence of diarrhea between CA LV2 and CA LV3 dissection in the SMA LV2 group (8.0% vs. 23.8%, P = 0.113). In contrast, in the SMA LV3 group, there was significant difference in the incidence of diarrhea between CA LV2 and CA LV3 dissection (48.1% vs. 78.9%, P = 0.002).
In the SMA LV3 group, three patients underwent whole circumferential dissection of the plSMA and all developed diarrhea. In the CA LV3 group, six patients underwent circumferential dissection of the plCA, and five (83.3%) developed diarrhea. The patient who did not develop diarrhea following circumferential CA LV3 had undergone SMA LV2.
Overall, both SMA and CA LV3 dissections were associated with the incidence of diarrhea, although SMA LV3 dissection had a greater impact on diarrhea compared to CA LV3 dissection.
Short- and long-term outcomes according to the development of diarrhea (Table 2)
There were more patients with BR-A (artery) PDAC in the diarrhea group
Among the preoperative factors between the diarrhea and non-diarrhea groups, there were no significant differences in gender, body mass index, tumor size, CA 19–9, and the rate of NAC. GnP was administered as NAC to 33 patients. The age of the diarrhea group was less than that of the non-diarrhea group (66 vs. 69 years, P = 0.028). The rate of R was higher in the non-diarrhea group (P = 0.007). Although there was no significant difference in the rate of BR-PV (portal vein) (P = 0.132), the rate of BR-A was higher in the diarrhea group (P = 0.001).
Postoperative courses were comparable between the diarrhea and non-diarrhea groups
Among the diarrhea and non-diarrhea groups, there were no significant differences in the operation time, blood loss, the number of dissected LN (38 vs. 35, P = 0.084), number of LN metastases (2 vs. 2, P = 0.204), and R0 resection rate (85.8% vs. 78.6%, P = 0.200). Adjuvant chemotherapy was administered to 165 patients; 149 patients received S-1, eight received GEM, and eight received GnP. The induction (91.7% vs. 85.2%, P = 0.181) and completion (89.0% vs. 86.3%, P = 0.632) rates of adjuvant chemotherapy were comparable between the two groups. Furthermore, there were no significant differences in complications (higher than Clavien-Dindo grade 3a) (15.3% vs. 14.7%, P = 0.903) and postoperative stay (28 days vs. 25 days, P = 0.272) between the two groups.
The local recurrence rate was lower in the diarrhea group, and the overall survival was comparable between the two groups
Comparing postoperative factors, the induction and completion rates of adjuvant chemotherapy and the recurrence rates were similar in each group. The local recurrence rate was significantly lower in the diarrhea group than in the non-diarrhea group (14.1% vs. 26.2%, P = 0.036). There was no significant difference in the overall survival (median survival time 30.0 months vs. 33.4 months, P = 0.918) and recurrence-free survival (16.1 months vs. 19.6 months, P = 0.496) (Fig. 3a, b) rates between the diarrhea and non-diarrhea groups. In the sub-analysis comparing either the upfront group/neoadjuvant chemotherapy group, there was a trend toward fewer local recurrences in the diarrhea group in both the upfront surgery and the neoadjuvant chemotherapy group, but the difference was no longer significant due to the decrease in number (Supplementary Table 1, 2).
Survival curve of the two groups. No significant difference was observed between the two groups in terms of either the overall survival (a, P = 0.918) or recurrence-free survival (b, P = 0.496). Numbers below the X-axis indicate the number of patients at risk. OS, overall survival; MST, median survival time; RFS, recurrence-free survival
Long-term outcome for patients with diarrhea
There were no significant differences in terms of the body weight and albumin level between the diarrhea and non-diarrhea groups at discharge and 2 years after surgery (Table 3). The pre-albumin level was lower at discharge (11.9 g/dL vs. 16.5 g/dL, P = 0.006), but after 2 years of operation, it was significantly elevated in the diarrhea group compared to the non-diarrhea group (+ 8 g/dL vs. + 3.4 g/dL, P = 0.001). Thereafter, the pre-albumin level in the diarrhea group recovered to almost normal levels 2 years after surgery.
To see the percentage of the patients who could discontinue opioid antidiarrheal drug, the clinical data were assessed at 2 and 3 years after operation. Patients who had died or were lost to follow-up were excluded. Consequently, 40 patients were analyzed at 2 years after operation and 31 patients at 3 years. Among them, 17 patients (42.5%) could discontinue opioid antidiarrheal drug in 2 years and 19 (61.3%) in 3 years (Fig. 4).
Discussion
We examined the frequency of diarrhea in association with the combined dissection level of plSMA and plCA and investigated the long-term outcomes of patients with diarrhea. We found that both plSMA and plCA dissection were associated with diarrhea. The local recurrence rate was significantly lower in the diarrhea group than in the non-diarrhea group. The overall survival and recurrence-free survival were similar in each group despite the high BR-A PDAC rate in the diarrhea group. Moreover, the postoperative nutritional status in the diarrhea group improved in 2 years, and more than 60% of patients with diarrhea could discontinue opioid antidiarrheal drugs within 3 years after surgery.
The results of five RCTs [1,2,3,4,5,6] do not favor extended lymphadenectomy during PD. However, the definitions of extended/standard lymphadenectomy and degrees of plSMA/CA dissection varied across these studies, and the postoperative diarrhea was not precisely defined (Table 4). Focusing on the relationship between plSMA dissection and postoperative diarrhea, Yeo et al. [2, 3] performed hemi-circumferential dissection of the plSMA in all cases, although they did not mention postoperative diarrhea. However, four other studies used extended nerve plexus dissection in patients undergoing extended lymphadenectomy; three studies used whole circumferential dissection, and one used hemi-circumferential dissection. Among the three RCTs which used circumferential dissection, Pedrazzoli [1] reported no severe diarrhea in either group, whereas Farnell [4] and Nimura [5] reported that the frequency of postoperative diarrhea increased with the degree of plSMA dissection. Farnell et al. [4] reported that 42% of patients experienced diarrhea at 4 months postoperatively in the extended lymphadenectomy group and 8% in the standard lymphadenectomy group. Nimura et al. [5] reported that diarrhea developed in 65.7% of patients at 6 months after surgery in the extended lymphadenectomy group and 31.5% in the standard lymphadenectomy group. Jang et al. [6] reported that the postoperative diarrhea could be suppressed by limiting plSMA dissection to the hemi-circumference (postoperative diarrhea at 3 months, 15.1% in the extended lymphadenectomy group, and 12.0% in the standard lymphadenectomy group). Unlike Jang's study [6], we reported that postoperative diarrhea developed in 50% of patients undergoing hemi-circumferential dissection of the plSMA and 85% of patients undergoing extended (plSMA dissection > 180º) circumferential dissection [12].
Diarrhea associated with nerve plexus dissection is an important clinical issue not only for pancreatic surgery but for colon surgery with D3 lymphadenectomy, in which nerve plexus around SMA is dissected. Denervation of plSMA with D3 extended mesenterectomy for right hemicolectomy increases the frequency of diarrhea. However, this symptom most likely improves in a half of year and does not impact gastrointestinal quality of life [22,23,24]. Comparing to colon surgery, diarrhea after PD is severer and intractable likely because broader and more proximal part of plSMA and/or plCA is dissected. Duodenum and the first part of jejunum resection may also contribute to refractory diarrhea. Although the mechanism of postoperative diarrhea can be multi-factorial, our findings for the impact of the depth of nerve plexus around major mesenteric arteries on postoperative diarrhea indicate the damage in the nerve plexus is likely at least one of the causes for this clinically important symptom. In this study, the frequency of diarrhea was evaluated in association with combined dissection of the plSMA and plCA. It was revealed that the combined dissection of plSMA and plCA (both LV3) increased the frequency of diarrhea. This result indicates that both plSMA and plCA are associated with diarrhea, although they are more strongly associated with plSMA. Circumferential dissection of the plSMA caused postoperative diarrhea in 100% of patients regardless of the plCA level, whereas 33.3% of patients with extended circumferential dissection of the plCA did not experience diarrhea if the dissection of the plSMA was LV2. This result also suggests that plSMA dissection level is more related to diarrhea than the plCA dissection level.
Although none of the RCTs demonstrated any improvement in the prognosis with extended lymphadenectomy, those studies did not take into account the tumor resectability (R or BR-PDAC) [25,26,27,28]. If the tumor is in contact with the arteries, the nerve plexus should be resected to achieve R0 resection. Tailored and strict adjustments of nerve plexus dissection should be considered to avoid inadequate or excessive dissection. In this study, the level of nerve plexus dissection was adjusted according to the tumor location. Despite the higher BR-A rate in the diarrhea group, the R0 resection rate and the prognosis were comparable in both groups. The postoperative local recurrence rate was predominantly lower in the diarrhea group than in the non-diarrhea group, indicating the significance of local disease control by extending nerve plexus dissection in BR-A PDAC.
It is also important to evaluate the patients’ diarrhea and nutritional status several years after surgery. Although the pre-albumin level at discharge was significantly lower in the diarrhea group than in the non-diarrhea group, it returned to a comparable level within 2 years. More than 60% of patients could discontinue opioid antidiarrheal drugs within 3 years after surgery. Overall, even if postoperative diarrhea occurs by aggressive nerve plexus dissection for BR-A PDAC, intestinal function, including regulation of peristalsis, could recover with time. Considering that PDAC easily invades the plexus [7, 29], LV3 dissection is justified to obtain R0 resection in BR-A cases.
This study has several limitations. Firstly, only a small number of patients underwent NAC. In an era of multidisciplinary therapy for BR-PDAC, preserving the nerve plexus by combining neoadjuvant chemotherapy or chemoradiation therapy is becoming a preferred treatment modality for PDAC [30,31,32,33,34,35,36]. However, in contrast, some expert surgeons advocate that arterial LV3 dissection (arterial skeletonization) can be performed successfully after NAC in patients who have previously been subjected to either arterial resection or non-operative therapy [37, 38]. In order to perform such aggressive surgery, the clinical course of postoperative diarrhea should be well understood. Secondly, long-term outcomes for 47 patients (61.3%) at 3 years after surgery could not be evaluated due to death or loss to follow-up; only 31 patients (38.7%) could be evaluated.
Conclusion
While combined plSMA and plCA dissection increased the frequency of diarrhea, most of the diarrhea status was controllable within 2–3 years after surgery. Considering the low local recurrence rate in the diarrhea group, aggressive dissection of the nerve plexus and diarrhea may be justified for local disease control in BR-A PDAC.
References
Pedrazzoli S, DiCarlo V, Dionigi R et al (1998) Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study lymphadenectomy study group. Ann Surg 228:508–517
Yeo CJ, Cameron JL, Sohn TA et al (1999) Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg 229:613–622
Yeo CJ, Cameron JL, Lillemoe KD et al (2002) Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 236:355–366
Farnell MB, Pearson RK, Sarr MG et al (2005) A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery 138:618–628
Nimura Y, Nagino M, Takao S et al (2012) Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci 19:230–241
Jang JY, Kang MJ, Heo JS et al (2014) A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg 259:656–664
Nagakawa T, Kayahara M, Ueno K et al (1992) A clinicopathologic study on neural invasion in cancer of the pancreatic head. Cancer 69:930–935
Nagakawa T, Mori K, Nakano T et al (1993) Perineural invasion of carcinoma of the pancreas and biliary tract. Br J Surg 80:619–621
Nagakawa T, Ueno K, Ohta T et al (1990) Appraisal of extended operation for Carcinoma of the pancreas and biliary tract observated from quality of life. Jpn J Gastroenterol Surg 23:967–972
Orci LA, Meyer J, Combescure C et al (2015) A meta-analysis of extended versus standard lymphadenectomy in patients undergoing pancreatoduodenectomy for pancreatic adenocarcinoma. HPB (Oxford) 17:565–572
Tol JA, Gouma DJ, Bassi C et al (2014) Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 156:591–600
Inoue Y, Saiura A, Yoshioka R et al (2015) Pancreatoduodenectomy with systematic mesopancreas dissection using a supracolic anterior artery-first approach. Ann Surg 262:1092–1101
Inoue Y, Saiura A, Tanaka M et al (2016) Technical details of an anterior approach to the superior mesenteric artery during pancreaticoduodenectomy. J Gastrointest Surg 20:1769–1777
Inoue Y, Saiura A, Oba A et al (2018) Optimal extent of superior mesenteric artery dissection during pancreaticoduodenectomy for pancreatic cancer: balancing surgical and oncological safety. J Gastrointest Surg 16:1373–1383
Inoue Y, Saiura A, Takahashi Y (2018) A novel classification and staged approach for dissection along the celiac and hepatic artery during pancreaticoduodenectomy. World J Surg 42:2963–2967. https://doi.org/10.1007/s00268-018-4550-9
Sobin L, Gospodarowicz M, Wittekind C (2009) Pancreas. In: TNM classification of malignant tumours, 7th edn. Wiley-Blackwell, 2010, Chichester, West Sussex, UK ; Hoboken, NJ, England, p 132–135
Tempero MA, Malafa MP, Al-Hawary M et al (2017) Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 15:1028–1061
Tanaka M, Inoue Y, Matsueda K et al (2020) Three-dimensional fixation: pathological protocol following pancreaticoduodenectomy with portal vein resection for pancreatic cancer. J Gastrointest Surg 24:619–626
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: 5-year experience. Ann Surg 250:187–196
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Uesaka K, Boku N, Fukutomi A et al (2016) Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 388:248–257
Thorsen Y, Stimec BV, Lindstrom JC et al (2021) Stool dynamics after extrinsic nerve injury during right colectomy with extended D3-mesenterectomy. Scand J Gastroenterol 56:770–776
Thorsen Y, Stimec BV, Lindstrom JC et al (2019) Bowel motility after injury to the superior mesenteric plexus during D3 extended mesenterectomy. J Surg Res 239:115–124
Thorsen Y, Stimec B, Andersen SN et al (2016) Bowel function and quality of life after superior mesenteric nerve plexus transection in right colectomy with D3 extended mesenterectomy. Tech Coloproctol 20:445–453
Varadhachary GR, Tamm EP, Abbruzzese JL et al (2006) Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 13:1035–1046
Vauthey JN, Dixon E (2009) AHPBA/SSO/SSAT consensus conference on resectable and borderline resectable pancreatic cancer: rationale and overview of the conference. Ann Surg Oncol 16:1725–1726
Tempero MA, Arnoletti JP, Behrman SW et al (2012) Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 10:703–713
Katz MH, Marsh R, Herman JM et al (2013) Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 20:2787–2795
Nagakawa T, Kayahara M, Ueno K et al (1992) Clinicopathological study on neural invasion to the extrapancreatic nerve plexus in pancreatic cancer. Hepatogastroenterology 39:51–55
Maeda A, Boku N, Fukutomi A et al (2008) Randomized phase III trial of adjuvant chemotherapy with gemcitabine versus S-1 in patients with resected pancreatic cancer: Japan adjuvant study group of pancreatic cancer (JASPAC-01). Jpn J Clin Oncol 38:227–229
Motoi F, Kosuge T, Ueno H et al (2019) Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol 49:190–194
Motoi F, Satoi S, Honda G et al (2019) A single-arm, phase II trial of neoadjuvant gemcitabine and S1 in patients with resectable and borderline resectable pancreatic adenocarcinoma: PREP-01 study. J Gastroenterol 54:194–203
Takahashi H, Ohigashi H, Gotoh K et al (2013) Preoperative gemcitabine-based chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Ann Surg 258:1040–1050
Talamonti MS, Small W Jr, Mulcahy MF et al (2006) A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol 13:150–158
Okano K, Suto H, Oshima M et al (2017) A prospective phase II trial of neoadjuvant S-1 with concurrent hypofractionated radiotherapy in patients with resectable and borderline resectable pancreatic ductal adenocarcinoma. Ann Surg Oncol 24:2777–2784
Eguchi H, Takeda Y, Takahashi H et al (2019) A prospective, open-label, multicenter phase 2 trial of neoadjuvant therapy using full-dose gemcitabine and S-1 concurrent with radiation for resectable pancreatic ductal adenocarcinoma. Ann Surg Oncol 26:4498–4505
Hackert T, Strobel O, Michalski CW et al (2017) The TRIANGLE operation–radical surgery after neoadjuvant treatment for advanced pancreatic cancer: a single arm observational study. HPB (Oxford) 19:1001–1007
Diener MK, Mihaljevic AL, Strobel O et al (2020) Periarterial divestment in pancreatic cancer surgery. Surgery 169(5):1019–1025
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kuroki, N., Ono, Y., Sato, T. et al. Long-Term Outcome of Patients with Postoperative Refractory Diarrhea After Tailored Nerve Plexus Dissection Around the Major Visceral Arteries During Pancreatoduodenectomy for Pancreatic Cancer. World J Surg 46, 1172–1182 (2022). https://doi.org/10.1007/s00268-022-06457-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-022-06457-5