Abstract
Background
The value of pancreatoduodenectomy (PD) with extended lymphadenectomy for pancreatic cancer has been evaluated by many retrospective studies and 3 randomized controlled trials (RCT). However, the protocols used and the results found in the 3 RCTs were diverse. Therefore, a multicenter RCT was proposed in 1998 to evaluate the primary end point of long-term survival and the secondary end points of morbidity, mortality and quality of life of patients undergoing standard versus extended lymphadenectomy in radical PD for pancreatic cancer.
Methods
From March 2000 to May 2003, 112 patients with potentially curable pancreatic head cancer were enrolled and intraoperatively randomized to a standard or extended lymphadenectomy group. No resected patients received any adjuvant treatments.
Results
A hundred and one eligible patients were analyzed. Demographic and histopathological characteristics of the two groups were similar. The mean operating time, intraoperative blood loss and number of retrieved lymph nodes were greater in the extended group, but the other operative results were comparable.
Conclusions
Although this multicenter RCT was conducted in a strict setting, extended lymphadenectomy in radical PD did not benefit long-term survival in patients with resectable pancreatic head cancer and led to levels of morbidity, mortality and quality of life comparable to those found after standard lymphadenectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As extended lymphadenectomy in radical cancer surgery had been developed among the leading hospitals in Japan since the mid-1970s, Fortner’s concept of regional pancreatectomy had a major impact on the clinical practice of Japanese surgeons [1, 2]. Since then, the benefits of extended radical pancreatectomy have been evaluated and various results of retrospective studies have been reported from not only Japan but also Western countries [3–14].
The first prospective randomized controlled trial (RCT) to compare the results of standard versus extended lymphadenectomy in radical pancreatoduodenectomy (PD) for carcinoma of the head of the pancreas was reported by Pedrazzoli [15], followed by Yeo and Farnell [16–19]. During the above period, Japanese surgeons established a study group which has discussed how to use RCT to assess the benefits of extended lymphadenectomy for resectable pancreatic cancer since 1998. The surgical results of the previous RCTs were found to vary. Also, various adjuvant treatments, which might have affected the outcomes for the resected patients, had been applied during the study period in all 3 RCTs. Therefore, the authors of this study have adopted a stricter policy to determine whether or not extended lymphadenectomy improves the survival of patients with potentially curable carcinoma of the head of the pancreas.
Methods
Recruitment of researchers
The chairman (YN) of this study group sponsored by the Ministry of Health, Labor, and Welfare of Japan recruited surgeons who had experience of more than 50 cases of extended lymphadenectomy for PD. All participating surgeons from 14 centers in Japan – 8 university hospitals, 2 cancer center hospitals and 4 national or municipal hospitals – agreed on the details and proposed surgical procedures of this RCT.
Eligibility
For this trial, we enrolled only patients younger than 80 years old with potentially curable carcinoma of the pancreatic head, excluding invasive mucinous cystoadenocarcinoma or intraductal papillary mucinous carcinoma. Additional exclusion criteria included severe cardiovascular and pulmonary diseases, gross metastases to the para-aortic nodes and/or marked portal vein stenosis with collateral circulation. This study protocol was approved by the institutional review boards of each of the 14 participating hospitals, and all patients gave written informed consent before undergoing randomization.
Statistical considerations
Retrospective studies have shown 2-year survival rates for patients undergoing regional lymphadenectomy of about 20% (Mukaiya [11], Henne-Bruns [12]) and 2-year survival rates for those with extended lymphatic and connective tissue clearance of 38% (Ishikawa [3]). Based on these observations, we assumed that the 2-year survival rate of the standard lymphadenectomy group would be 20% and initially planned to recruit 130 patients (65 in each group), an adequate number to detect a 20% increase in survival in the extended lymphadenectomy group, with a one-sided alpha level of 0.05 and a power of 80%, and with a total accrual period of 3 years and an additional 5-year follow-up.
The primary end point of this study was overall survival, defined as the time from randomization to death. The secondary end points were disease-free survival, morbidity, mortality, periods of postoperative hospital stay and postoperative quality of life. Disease-free survival was defined as the time from randomization to the first recurrence of cancer or death from any cause. Survival curves were estimated by the Kaplan–Meier method and compared by using the log-rank test. P values less than 0.05 were considered to indicate statistical significances.
Randomization and data management
After confirming the eligibility of the patient during surgery, the surgeon contacted our central office by telephone to receive a randomly generated assignment of the patient to standard lymphadenectomy or extended lymphadenectomy associated with PD if the operating surgeon considered that all macroscopic tumors could be excised. The surgeon then performed the assigned operation according to the methods described in the protocol. The data and safety monitoring committee of Kyoto University performed data management, central monitoring and statistical analysis. None of the surgeons in this study group were involved in data analysis.
Surgical procedure
Pylorus-preserving pancreatoduodenectomy (PPPD) was used as a standard surgical procedure; however classical PD with distal gastrectomy or subtotal stomach-preserving pancreatoduodenectomy (SSPPD) could be selected according to the extent of the cancer. Although a frozen section of the primary tumor at the head of the pancreas was not taken, the resection margins of the pancreatic body and the bile duct had to be confirmed as histologically negative by frozen sections to complete R0 resection. Combined portal vein resection was indicated if curative resection was possible.
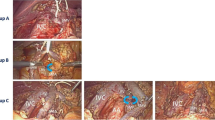
Lymphadenectomy including anterior and posterior pancreatoduodenal nodes (Nos. 13a, 13b, 17a, 17b) without nerve dissection is defined as a standard operation. For the extended operation, nodes around the common hepatic artery (CHA) (Nos. 8a, 8p), celiac artery (CA) (No. 9), superior mesenteric artery (SMA) (Nos. 14p, 14d) and abdominal aorta (AA) between the origin of the CA and the inferior mesenteric artery (IMA) (Nos. 16a2, 16b1) were uniformly dissected and the hepatoduodenal ligament was skeletonized to remove nodes of Nos. 12a, 12b and 12p. Furthermore, nerve dissection was carried out circumferentially around the CHA and SMA, and semicircumferentially on the right lateral aspect of the CA. The above lymph node station nomenclature was defined according to the General Rules for Surgical and Pathological Studies on Cancer of the Pancreas by The Japan Pancreas Society [20, 21] which was also used in the previously reported Farnell’s RCT [19]. Then, to assess adherence to the lymphadenectomy protocol, intraoperative pictures had to be taken, and the dissection status of all nodal stations and the number of retrieved lymph nodes were recorded on case report forms by the surgeons and pathologists.
Postoperative data management
Histopathological examination of the resected specimens was performed in the recruiting hospitals. The stages of the resected patients were classified according to the internationally recognized UICC-TMN Classification (6th edition). Also, at the regularly organized meetings of the study group, participating surgeons observed intraoperative photographs of both types of procedures to ensure consistency of extension of lymphadenectomy.
Mortality was defined as any death related to surgery. The patients were followed monthly, and no adjuvant treatment was allowed before recurrence of a cancer was detected. Postoperative quality of life (QOL) was assessed regularly at 3, 6, and 12 postoperative months. Questionnaires about postoperative changes in body weight, oral intake, stool and satisfaction level were filled in at the outpatient clinic.
Results
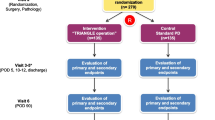
Since March 2000, 146 patients have been considered for recruitment. As shown in Fig. 1, however, a considerable number (n = 34, 23%) of enrolled patients were excluded before randomization for several reasons: 18 did not meet the inclusion criteria and 16 refused to participate. The data and safety committee reviewed the results at the formal interim analysis in May 2003 after enrolment of the first 112 patients over a period of 38 months.
Of the 112 registered patients, eleven were excluded from this study because of a different histological diagnosis of the resected specimen: 7 patients with chronic pancreatitis and 4 with cholangiocarcinoma. The remaining 101 patients were included in the interim analysis (Fig. 1). The committee did not approve continuation of the planned recruitment of patients because better survival could not be detected, and data collection could not be continued in the extended operation group for ever. Then recruitment of patients was closed, and the analyzed patients were carefully followed up until May 2008, which was 5 years after the last patient had been registered, to compare long-term survival between the 2 groups.
The demographics of the patients in the standard (N = 51) and extended (N = 50) operation groups were well balanced. The mean patient age (62.7 vs. 62.9 years old), body mass index (22.3 vs. 21.3) and preoperative serum bilirubin level (1.9 vs. 2.1 mg/dl) were similar. The distribution of gender (male/female 32/19 vs. 34/16), presence of preoperative biliary drainage (72.5% vs. 78.0%) and comorbidity (23.5% vs. 30.0%) were not different between the 2 groups. The mean operative time (426 min vs. 547 min, P < 0.0001) and intraoperative blood loss volume (1118ml vs. 1680 ml, P < 0.005) were significantly higher in the extended operation group. The mean number of retrieved lymph nodes in the standard and extended operation groups were 13.3 and 40.1, respectively (P < 0.0001). However, no significant difference was found in the rate and volume of blood transfusion (49.0% vs. 48.0%, 2.1 U vs. 2.4 U), rates of combined portal vein resection (47.1% vs. 48.0%, 24 patients each) and R0 resection (48/51, 45/50) between the 2 groups. Positive invasion was revealed histologically in the resected portal vein in 11 (45.8%) and 9 (37.5%) patients, respectively. Duration of postoperative gastric suction (7.5 vs. 7.0 days), hospital stay (43.8 vs. 42.4 days), mortality (0 vs. 2.0%), and the incidence of postoperative morbidity (19.6 vs. 22.0%) excluding severe diarrhea were not different in the 2 groups.
Although histological findings of the resected tumors were similar in the 2 groups, a difference was found between them in Stage (I/IIa/IIb/III/IV: 2/17/32/0/0 vs. 1/19/21/0/9) (P < 0.01) because positive para-aortic node metastasis was revealed postoperatively in 9 of the 50 patients who underwent extended lymphadenectomy. Details of dissected nodal status in the extended lymphadenectomy are shown in Table 1. The highest incidence and actual involvement rates of lymph node metastasis from carcinoma of the head of the pancreas were found at the anterior and posterior pancreatoduodenal nodes (Nos. 13 and 17) which are automatically removed by both standard and extended lymphadenectomy. The next higher incidence of metastasis was found in the para-aortic nodes (No. 16), which were more frequently involved than those around the SMA (Nos. 14p and 14d) or in the hepatoduodenal ligament (No. 12).
Mode of cancer recurrence was carefully investigated by clinical signs, and imaging studies at death and/or autopsy. Liver metastasis, peritoneal dissemination and/or local recurrence were similar in the 2 groups. Moreover, no difference was found in postoperative lymph node metastasis between the 2 groups (Table 2).
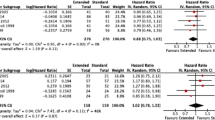
One perioperative mortality was encountered in the extended lymphadenectomy group. Although actual survival rates at 1 year show a big difference between the 2 groups (40/51 vs. 27/50), postoperative early cancer recurrence within 1 year was detected in several organs: predominantly in the: liver—9/11 patients (82%) in the standard operation group and 17/23 patients (74%) in the extended surgery group; peritoneum—5/11 (45%) versus 9/23 (39%); and local region—3/11 (27%) versus 5/23 (22%), and so on. No big difference was found in the mode of early cancer recurrence, although the local recurrence rate was higher in the extended surgery group. The 5-year overall survival rate was 15.7% for the group assigned to standard lymphadenectomy and 6.0% for the extended lymphadenectomy group. However the log-rank test showed no significant difference between the 2 groups. The number of disease-free survivors at 1 year is similar for each group (20/51 vs. 22/49), but the 5-year disease-free survival rates were 11.8% in the standard lymphadenectomy group and 6.1% in the group assigned to extended lymphadenectomy, and the log-rank test did not show any significant difference between the 2 groups (Fig. 2). Positive lymph node metastasis was similar in the standard and extended lymphadenectomy group patients (31 vs. 30). The 5-year survival rates for the negative-node patients in the standard and extended operation groups were 31.6% and 15.0%, respectively, while those for the positive-node patients in both groups were 6.3% and 0%, respectively. Stratified log-rank tests showed no significant difference between the 2 groups (Fig. 3).
Overall survival (a) for 51 patients undergoing pancreatoduodenectomy (PD) with standard lymphadenectomy (I) and 50 patients undergoing PD with extended lymphadenectomy (II). Disease-free survival (b) for 51 patients undergoing PD with standard lymphadenectomy (I) and 49 patients who survived PD with extended lymphadenectomy (II). There is no significant difference in survival rates between the 2 groups
Actuarial survival curves for node-negative (a) and node-positive (b) patients who underwent PD, comparing standard lymphadenectomy (I) with extended lymphadenectomy (II). No significant difference in survivals is observed between the 2 groups, although longer survivors with positive nodes are found in the standard lymphadenectomy group
The 5-year survival rates for patients without portal vein resection in the standard and extended operation groups were 29.6% and 3.8%, respectively, and those for patients with portal vein resection in both groups were 0% and 8.3%, respectively. The stratified log-rank tests showed no significant difference between the 2 groups (Fig. 4). In a univariate analysis of survival, age, gender, positive histological findings of retroperitoneal tissue invasion (51/101, 50.5%), serosal invasion (32/101, 31.7%), distal bile duct invasion (76/101, 75.2%) and extrapancreatic nerve plexus invasion (22/101, 21.8%) were not identified as prognostic factors, but portal vein resection and positive histological findings of lymphatic vessel invasion, venous invasion, neural invasion, lymph node metastasis and resection margin were identified as prognostic factors. A multivariate Cox regression analysis was then performed to identify the predictors of survival; portal vein resection and lymph node metastasis were shown to be independent prognostic factors (Table 3).
Actuarial survival curves for patients undergoing PD without portal vein resection (a) and those with portal vein resection (b), comparing the standard lymphadenectomy (I) to extended lymphadenectomy (II). There is no significant difference in survival rates between the 2 groups, although longer survivors are found in the standard lymphadenectomy group, and only one 5-year survivor is found in the extended lymphadenectomy group
Postoperative QOL was assessed using the postoperative changes in 4 features: body weight, oral intake, stool and satisfaction level. The postoperative changes in oral intake and stool were divided into 4 grades, and the satisfaction level into 3 grades as shown in Table 4. The assessed postoperative QOL was worse in the extended surgery group during the early postoperative period. Weight loss associated with diarrhea was more frequently encountered in the extended operation group than in the standard operation group and this continued for more than 6 months postoperatively. Oral intake was more reduced and the satisfaction level was lower in the extended operation group than in the standard operation group for more than 3 months postoperatively; however, no difference was found between the 2 groups at 6 months after surgery. The 4 items gradually improved during the postoperative observation period and reached a similar level without any significant difference between the 2 groups at 12 months after surgery.
Discussion
Although the first multicenter RCT and subsequent single institutional RCTs have not shown any survival benefit of extended lymphadenectomy, the protocols and surgical outcomes were different among the 3 previous RCTs [15–19]. Standardization of extended lymphadenectomy should be the first consideration especially in a multicenter RCT to ensure the high quality of the study. Therefore, in the present Japanese multicenter RCT, only surgeons experienced in extended pancreatic surgery participated in order to maintain the quality of the study. It took 2 years for participating Japanese surgeons to change their fixed ideas on the value of extended lymphadenectomy and to establish a strict protocol to determine whether or not an extended lymphadenectomy improves the survival of the resected patients.
RCTs on adjuvant treatments for resected patients with pancreatic cancer yielded inconsistent results [22–25]. The Italian RCT employed intraoperative radiation therapy (IORT) and both American RCTs used chemoradiation therapy in the majority of cases. However, adjuvant treatments which could confuse the real effects of surgical treatment were not used in our Japanese RCT. Uniformity of protocol and accurate evaluation of the surgical results are a major concern for RCTs on lymphadenectomy; however, dissected lymph node stations in both standard and extended lymphadenectomy groups do not resemble each other in the 4 RCTs [15–19, 26]. Also surgical procedures used for nerve plexus dissection around the CA and SMA were not the same: either right lateral aspect or circumferential dissection were used (Table 5). Dissected lymph node stations in the 4 RCTs do not resemble each other. The number of retrieved lymph nodes in the standard lymphadenectomy is comparable among the 4 RCTs, whereas that in the extended lymphadenectomy varies from 19.8 to 40.1. The range is more variable, from 3–76 to 15–81. A major concern is that the minimum number varies from 3 to 15 in extended lymphadenectomy. In fact, in the Italian multicenter RCT, the smallest number of retrieved lymph nodes was only 1 in the standard lymphadenectomy group and only 3 in the extended lymphadenectomy group. The rate of positive lymph node metastasis was not different between the 2 groups in each RCT and was comparable among the 4 RCTs. Also, significant variation in operation time was observed among the 4 RCTs. Although it was not different between the 2 groups in the Italian RCT, significant differences were found between the 2 groups in the other 3 RCTs: extended surgery took 30 min longer at Johns Hopkins, 1 h 30 min longer at Mayo Clinic, and 2 h longer in this Japanese RCT. The concomitant portal vein resection rate was higher in this than in the previously reported RCTs because participating surgeons were experienced in this technique without difficulty. Therefore, they used this procedure without hesitation for about half of the Japanese patients in both groups to achieve R0 resection. The surgical procedure was selected according to the macroscopic findings intraoperatively, and histological venous invasion is defined after surgery. A smaller number of patients underwent combined portal vein resection in the United States, and the data are not available in the Italian study. The R0 resection rate was higher in the Japanese study: 94.1% in the standard group and 90% in the extended lymphadenectomy group. These rates are higher than those in Western studies. Japanese R1 rates in the 2 groups are not significantly different (5.8% vs. 10%) and are lower than those of the other RCTs. The reason is not clear but it is speculated that definition of the surgical procedure was more strict in the Japanese RCT than in the other studies and meticulous intraoperative efforts of Japanese surgeons might have led to the lower R1 rates. There was no significant difference in postoperative hospital stay between the 2 groups except in the Johns Hopkins study, although a longer stay was observed in the Japanese study because of the different insurance system in Japan. Interestingly, differences in mortality and morbidity excluding diarrhea were not found between the 2 groups in all 4 RCTs. However, postoperative diarrhea was the major concern with extended lymph node and connective tissue dissection. This complication depends on the extent of retroperitoneal tissue dissection which involves right-half or complete circumferential nerve dissection around the SMA. Farnell and Japanese surgeons employed circumferential nerve dissection around the SMA, and postoperative bowel control and diarrhea were worse for the American patients undergoing extended lymphadenectomy at 4 months and the Japanese patients at 6 months after operation, respectively [26, 27]. However, this complication gradually improved and no difference was observed at 12 months after surgery between the 2 Japanese patients groups. It is not understood why the rate of lymph node metastasis recurrence was not different between the 2 groups and why local recurrence was more frequent in the extended surgery group. The details of mode of recurrence are shown in Table 2. There were 67 recurrence sites in 44 deceased patients with standard operation and 72 recurrence sites in 47 patients undergoing extended surgery which were observed during the postoperative period and at the time of death. No cause was found for the early recurrence in the first postoperative year in the extended lymphadenectomy group; however, it may be speculated that the greater stress of the extended surgery might have led to the resected Japanese patients being immunologically suppressed which is not beneficial for prevention of cancer recurrence. Although statistically significant differences in survival rates between the Western RCTs and Japanese RCT were not detected, the median survival time for Japanese patients with standard operation is better than that for Italian patients and similar to that for American patients. The median survival time for Japanese patients undergoing extended lymphadenectomy seems similar to that for Italian patients but worse than that for American patients. It is difficult to explain the reasons for those differences; however, it is speculated that eligibility criteria for “potentially curable carcinoma” might have been different between the surgeons in the different countries. Median survival and 1-, 3-, and 5-year survival rates were not different between the 2 groups and were comparable among the 4 RCTs (Table 6).
The study by Pedrazzoli showed improved survival for patients undergoing extended lymphadenectomy in the subset of patients with positive lymph node metastasis. However, the other 3 RCTs did not demonstrate any survival benefit for positive-node patients undergoing extended lymphadenectomy. Also, a recently published systematic review and meta-analysis including the published 3 RCTs concluded that extended lymphadenectomy does not benefit survival [28, 29].
RCT on standard versus extended lymphadenectomy must take all of the above variables into consideration, and Japanese surgeons made every effort to maintain the quality of RCT and to follow up the registered patients carefully for a long period. Nevertheless, we concluded that extended lymphadenectomy in radical PD for carcinoma of the head of the pancreas could not improve the long-term survival of the resected patients. Despite differences in the surgical outcome of the 4 RCTs, they came to a similar conclusion: extended lymphadenectomy did not benefit the long-term survival, but diarrhea associated with weight loss developed for less than 12 months postoperatively. Therefore, no further RCTs on standard versus extended lymphadenectomy associated with PD for pancreatic cancer are necessary.
References
Fortner JG. Regional resection of cancer of the pancreas: a new surgical approach. Surgery. 1973;73:307–20.
Fortner JG, Kim DK, Cubilla A, Turnbull A, Pahnke LD, Shils ME, et al. Regional pancreatectomy: en bloc pancreatic, portal vein and lymph node resection. Ann Surg. 1977;186:42–50.
Ishikawa O, Ohhigashi H, Sasaki Y, Kabuto T, Fukuda I, Furukawa H, et al. Practical usefulness of lymphatic and connective tissue clearance for the carcinoma of the pancreas head. Ann Surg. 1988;208:215–20.
Manabe T, Ohshio G, Baba N, Miyashita T, Asano N, Tamura K, et al. Radical pancreatectomy for ductal cell carcinoma of the head of the pancreas. Cancer. 1989;64:1132–7.
Ishikawa O, Ohigashi H, Imaoka S, Furukawa H, Sasaki Y, Fujita M, et al. Preoperative indications for extended pancreatectomy for locally advanced pancreas cancer involving the portal vein. Ann Surg. 1992;215:231–6.
Kayahara M, Nagakawa T, Ueno K, Ohta T, Takeda T, Miyazaki I. An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer. 1993;72:2118–23.
Sindelar WF. Clinical experience with regional pancreatectomy for adenocarcinoma of the pancreas. Arch Surg. 1989;124:127–32.
Satake K, Nishiwaki H, Yokomatsu H, Kawazoe Y, Kim K, Haku A, et al. Surgical curability and prognosis for standard versus extended resection for T1 carcinoma of the pancreas. Surg Gynecol Obstet. 1992;175:259–65.
Nagakawa T, Nagamori M, Futakami F, Tsukioka Y, Kayahara M, Ohta T, et al. Results of extensive surgery for pancreatic carcinoma. Cancer. 1996;77:640–5.
Hirata K, Sato T, Mukaiya M, Yamashiro K, Kimura M, Sasaki K, et al. Results of 1001 pancreatic resections for invasive ductal adenocarcinoma of the pancreas. Arch Surg. 1997;132:771–6.
Mukaiya M, Hirata K, Satoh T, Kimura M, Yamashiro K, Ura H, et al. Lack of survival benefit of extended lymph node dissection for ductal adenocarcinoma of the head of the pancreas: retrospective multi-institutional analysis in Japan. World J Surg. 1998;22:248–53.
Henne-Bruns D, Vogel I, Lüttges J, Klöppel G, Kremer B. Ductal adenocarcinoma of the pancreas head: survival after regional versus extended lymphadenectomy. Hepatogastroenterology. 1998;45:855–66.
Gazzaniga GM, Cappato S, Papadia F, Mori L, Filauro M. D1 versus D2 pancreatoduodenectomy in surgical therapy of pancreatic head cancer. Hepatogastroenterology. 2001;48:1471–8.
Iacono C, Accordini S, Bortolasi L, Facci E, Zamboni G, Montresor E, et al. Results of pancreaticoduodenectomy for pancreatic cancer: extended versus standard procedure. World J Surg. 2002;26:1309–14.
Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228:508–17.
Yeo CJ, Cameron JL, Sohn TA, Coleman J, Sauter PK, Hruban RH, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg. 1999;229:613–24.
Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–68.
Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Sauter PK, Coleman J, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma—part 3: update on 5-year survival. J Gastrointest Surg. 2005;9:1191–206.
Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138:618–30.
Japan Pancreas Society. Classification of pancreatic carcinoma. 1st English ed. Tokyo: Kanehara & Co. Ltd.; 1996.
Japan Pancreas Society. Classification of pancreatic carcinoma. 2nd English ed. Tokyo: Kanehara & Co. Ltd.; 2003.
Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903.
Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006–10.
Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. European Study Group for Pancreatic Cancer. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–85.
Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10.
Farnell MB, Aranha GV, Nimura Y, Michelassi F. The role of extended lymphadenectomy for adenocarcinoma of the head of the pancreas: strength of the evidence. J Gastrointest Surg. 2008;12:651–6.
Nguyen TC, Sohn TA, Cameron JL, Lillemoe KD, Campbell KA, Coleman J, et al. Standard vs. radical pancreaticoduodenectomy for periampullary adenocarcinoma: a prospective, randomized trial evaluating quality of life in pancreaticoduodenectomy survivors. J Gastrointest Surg. 2003;7:1–11.
Schmidt CM. Extended lymphadenectomy for pancreatic head cancer: less is more. Am J Oncol Rev. 2006;5:302–6.
Michalski CW, Kleeff J, Wente MN, Diener MK, Büchler MW, Friess H. Systemic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg. 2007;94:265–73.
Acknowledgments
This research was supported by grants-in-aid for cancer research (10-23, 14-7, 15S-2, 18S-5) from the Ministry of Health, Labor and Welfare of Japan.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nimura, Y., Nagino, M., Takao, S. et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas. J Hepatobiliary Pancreat Sci 19, 230–241 (2012). https://doi.org/10.1007/s00534-011-0466-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-011-0466-6