Abstract
Background
Preoperative portal vein embolization (PVE) induces shrinkage of the embolized lobe and compensatory regeneration in the non-embolized lobe, but does not always induce sufficient regeneration of the future remnant liver (FRL). We previously developed preoperative sequential PVE–hepatic vein embolization (HVE), and here we present our experience of treating 42 patients with sequential PVE–HVE.
Methods
During 8-year study period, preoperative PVE–HVE was performed on 42 patients with hepatobiliary malignancies.
Results
Primary diseases were bile duct cancers [perihilar cholangiocarcinoma (n = 33) and diffuse bile duct cancer (n = 1)], hepatocellular carcinomas (n = 4), and intrahepatic tumors [intrahepatic cholangiocarcinoma (n = 3) and gallbladder cancer liver invasion (n = 1)]. These patients demonstrated insufficient FRL regeneration following PVE, thus HVE was performed to induce further regeneration. No PVE–HVE procedure-associated complications occurred. In the bile duct cancer group, FRL volume was 33.9 ± 2.2 % before PVE, 38.4 ± 1.5 % before HVE, 43.7 ± 2.1 % at surgery, and 73.6 ± 8.3 % at 2 weeks after right hepatectomy. The degree of FRL hypertrophy was 13.3 % after PVE, 28.9 % after PHV–HVE, and 117.1 % at 2 weeks after right hepatectomy. All patients except one recovered uneventfully after surgery, and the 3-year patient survival rate was 45.1 %. In the HCC group, transarterial chemoembolization was initially performed and FRL regeneration following PVE–HVE occurred very slowly. Active FRL regeneration occurred in the liver tumor group, but rapid tumor growth was observed in 1 of 4 patients.
Conclusion
The sequential application of HVE following PVE safely and effectively induces further FRL regeneration in non-cirrhotic livers. Further validation using larger patient population and multicenter studies is needed to reliably widen the indications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preoperative embolization of the portal vein (PV) induces shrinkage of the embolized lobe and compensatory regeneration in the non-embolized lobe, but does not always sufficiently induce liver regeneration [1, 2]. Its main underlying causes include liver cirrhosis that innately limits regenerative capability and incomplete blockade of PV flow due to minute residual flow or intrahepatic shunting/collateral formation [3]. Because nearly no effective method is available that accelerates liver regeneration in addition to PV embolization (PVE) [4–6], we developed the concept of sequential ipsilateral hepatic vein embolization (HVE). Our early experiences of treating the first 12 patients who underwent preoperative sequential PVE–HVE were reported in 2009 [3].
Since then, preoperative sequential PVE–HVE for major hepatectomy has been sporadically reported [7, 8], but no high-volume studies existed yet. We have gradually accumulated more experience and here we present the results of 42 patients who underwent preoperative sequential PVE–HVE for major hepatectomy.
Patients and methods
Study design
This is a retrospective review of 42 patients, which includes the initial 12 patients who were enrolled in the preliminary prospective clinical trial [3] and additional 30 patients as a part of an ongoing continuation study. These 42 patients underwent preoperative sequential PVE–HVE between February 2007 and December 2013. It occupied about 10 % of overall PVE cases during the study period. They were followed up for ≥12 months until December 2014 or until patient death.
In this study, we intended to primarily assess the regeneration of the future remnant liver (FRL) following PVE–HVE, regarding on degree of hypertrophy and kinetic growth rate (KGR). In addition, we analyzed the oncological influence of PVE–HVE on long-term patient survival and tumor recurrence. The degree of FRL hypertrophy was defined as the volume percentage difference between the FRL volume before and after PVE and HVE. The KGR was calculated as degree of hypertrophy at first post-PVE volume assessment (%) divided by time elapsed since PVE and HVE (weeks) at first post-procedure volume assessment [9].
The initiation of this study was registered to ClinicalTrials.gov (identifier: NCT00698880) and the maintenance study was approved by the institutional review board of our institution.
Patient selection
The indications for right PVE included patients waiting for right hepatectomy or more extensive liver surgery for hepatobiliary malignancy, but demonstrating a small FRL <40 % of the total liver volume (TLV) on computed tomography (CT) volumetry [3, 10–12]. Patients with large liver tumors were not usually indicated because their parenchymal hepatic resection rate (PHRR) excluding tumor volume was usually <60 % and FRL volume >40 % of TLV. PVE was performed on patients with obstructive jaundice, in general, when the total serum bilirubin level was approximately 5 mg/dL [10, 13], but the threshold was often intentionally raised to around 7 mg/dL in order to provide a longer waiting period after PVE and HVE.
If FRL volume is expected to be <40 % of TLV at 2–3 weeks following PVE, these patients were primarily indicated for ipsilateral HVE because delayed liver regeneration after 2 weeks usually occurs very slowly in such patients (post-2 week KGR was <2 % per week). Hepatocellular carcinoma (HCC) developed in cirrhotic livers was not usually indicated for HVE because the short-term FRL regeneration following HVE was negligibly low (usually KGR <1 % per week during the first month).
At first, we had waited for 2 weeks after PVE to perform additional HVE. A preliminary study was performed to assess the degree of FRL hypertrophy in 20 patients of perihilar cholangiocarcinoma who underwent right PVE. On weekly CT follow-up, the KGR values during the first, second, and third weeks were 5.6 ± 2.1, 2.8 ± 1.3, and 1.9 ± 1.1 %, respectively. This result indicates that 54 % of FRL regeneration occurs during the first week in the waiting period of 3 weeks. Thereafter, post-PVE waiting period was shortened to 1 week because we were able to reliably predict the liver regeneration rate at 2 and 3 weeks. Our primary intention was to shorten the overall preoperative waiting period from the viewpoint of surgical oncology. Briefly, the indication for HVE was FRL <40 % of TLV with relatively low KGR after PVE. Patients who had small FRL despite marked PV stenosis from tumor invasion were indicated for HVE alone without precedent PVE.
Surgery was scheduled just after the 2-week post-HVE CT scan was performed. The overall waiting period from PVE to surgery was set to be ≤4 weeks in order to minimize the risk of potential tumor progression. During this waiting period, we performed triphasic dynamic liver CT scans every week to evaluate the changes in FRL volume and tumor progression.
PVE and HVE procedures
The PVE procedure was previously described, in which a gamut of embolic materials, including coils, gelfoams, liquid agents (e.g., polyvinyl acetate), and Amplatzer vascular plugs, were used alone or in combination [14–16]. The HVE procedure was also previously presented in detail [3, 14, 17], in which an Amplatzer vascular plug was placed at the proximal portion of the right hepatic vein (RHV) to prevent accidental migration of the embolization coils. Intrahepatic hemodynamic changes following PVE and HVE are illustrated in Fig. 1, in which the right hepatic arterial blood flow was drained into the RHV after PVE, into the left portal vein after HVE alone, and into the arterial collaterals after PVE–HVE.
Hemodynamic changes according to vascular embolizations. a Normal hepatic sinusoidal flow. b Hemodynamic changes after occluding only the portal vein: hepatic arterial flow increases due to buffering effects. c Hemodynamic changes after occluding the hepatic vein alone: the portal vein drains the hepatic arterial flow. d Simultaneous occlusion of the portal vein and hepatic vein: there is nearly no portal vein flow due to low perfusion pressure, and hepatic artery flow also decreased but drained into the intrahepatic arterial collaterals. HV hepatic vein, PV portal vein, HA hepatic artery, HVE hepatic vein embolization, PVE portal vein embolization
Statistical analysis
Numeric data are reported as the mean with standard deviation, or as the median with range. Continuous variables were compared using unpaired and paired t tests. Survival curves were estimated using the Kaplan–Meier method. A p < 0.05 is considered statistically significant. Statistical analyses were performed using SPSS (version 20; IBM) and Statistica (version 6.0; StatSoft, OK).
Results
Indication of HVE and patient grouping according to primary diagnoses
During the 8-year study period, 42 patients underwent HVE after PVE. The patient population consisted of 28 men and 16 women with a mean age of 65.2 ± 8.5 years (range 43–76). Their primary diagnoses were perihilar cholangiocarcinoma (n = 33), HCC (n = 4), intrahepatic cholangiocarcinoma (n = 3), gallbladder cancer (n = 1), and diffuse bile duct cancer (n = 1). The primary reasons for additional HVE were insufficient FRL volume after PVE (FRL <40 % of TLV) in 39 patients and marginal FRL volume (FRL around 40 % of TLV) with anticipated additional risk from hepatopancreatoduodenectomy (n = 2) and major comorbidities due to traffic accident-associated paraplegia (n = 1). After performing a preliminary prospective clinical trial with 12 patients [3], we recognized that the clinical sequences following preoperative sequential PVE–HVE differed depending on the background liver status and primary diseases. Thus, we divided our patients into 3 groups: bile duct cancer group (n = 34), HCC group (n = 4), or intrahepatic tumor group (n = 4). Detailed results are presented according to these groups (Fig. 2).
HVE procedure and complications
Preoperative sequential PVE–HVE was performed on the right liver in 41 patients and the left liver in 1 patient. One patient underwent repeat PVE procedures due to PV recanalization. During embolization of the RHV, the inferior RHV branch was concurrently embolized in 22 patients (53.7 %). As previously reported [3], the middle hepatic vein (MHV) trunk was erroneously embolized in 1 patient, and surgery was abandoned due to small FRL and rapid tumor progression. Erroneous MHV embolization also happened later in another patient, but we overcame this situation by performing extended right hepatectomy after additional RHV embolization. Thereafter, we have used portable angiographic CT during angiographic intervention, by which we could select the RHV trunk securely.
No significant complications that required blood transfusion or intervention occurred after PVE or HVE procedure. Following HVE, accidental migration of the embolization coil into the heart or lung did not occur in any patient. Seventeen patients (40.5 %) developed transient symptoms and signs after HVE, including mild abdominal pain, low-grade fever, and nausea. All of these symptoms are very similar to those observed after PVE.
The peak blood levels of the liver enzymes increased more following HVE than PVE [88.2 ± 33.7 IU/L vs. 52.3 ± 22.3 IU/L, respectively, for aspartate aminotransferase (p = 0.000); 93.5 ± 49.1 IU/L vs. 62.1 ± 29.8 IU/L, respectively, for alanine aminotransferase (p = 0.001)], indicating that HVE induced further tissue damage of the right liver after PVE. Immunohistochemistry showed that apoptosis occurred much more in the liver area affected by both PVE and HVE than in that affected by PVE alone [3]. No patient demonstrated any unusual deterioration in liver function after preplanned major hepatectomy.
The hepatic parenchyma territory involved in both PVE and HVE was meticulously examined through weekly dynamic liver CT scans in afraid of risk of hepatic necrosis. We measured the diameter of the right hepatic artery before and 1 week after PVE and HVE in 31 patients of perihilar cholangiocarcinoma. The mean diameter increase was by 64 ± 15 % at 1 week after PVE. Simple calculation of post-PVE right hepatic artery blood flow resulted in 269 % of the pre-PVE blood flow, thus arterial blood flow to the right liver was 2.7 times increased. At 1 week after HVE, the mean diameter increase was by 71 ± 18 % of pre-PVE size and blood flow was 2.9 times increased comparing with the pre-PVE state.
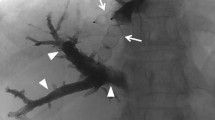
In all patients, intrahepatic arterial collateral perfusion was definitely identified at the right anterior section and noticeable hepatic necrosis was barely observed (Fig. 3). This development of intrahepatic arterial collaterals might contribute to prevention of extensive hepatic necrosis.
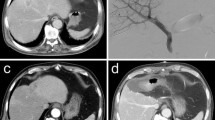
Computed tomography (CT) findings of intrahepatic hemodynamic changes following right portal vein embolization (PVE) and right hepatic vein embolization (HVE) in a patient with perihilar cholangiocarcinoma. a Before PVE, no abnormal arterial perfusion was identified; b At 7 days after PVE, enlargement of the right hepatic artery was identified on the arterial-phase CT and delayed increase of arterial perfusion was also observed in the portal-phase CT. Peripheral portal vein branches of the right liver was completely filled with gelfoams and the proximal portion was plugged with multiple coils; and c At 12 days after HVE, a wedge-shaped area of arterial hyperperfusion (arrow) was identified at the right anterior section on the arterial-phase CT and prominent increase of arterial perfusion (arrow) was also observed in the portal-phase CT. These findings support that the right hepatic arterial blood flow is drained through the intrahepatic arterial collaterals in the adjacent hepatic segments
In the overall 42 patients of this study, there was only one case of mortality within first 3 months after surgery.
Outcomes in the bile duct cancer group
Bile duct cancer without evidence of liver cirrhosis was the main indication for PVE–HVE, and thus 34 patients with perihilar cholangiocarcinoma (n = 33) or diffuse bile duct cancer (n = 1) received preoperative PVE–HVE. Preoperative biliary decompression was performed on 28 patients (82.4 %). The total serum bilirubin level was 5.6 ± 2.3 mg/dL (range 1.2–8.8) at PVE, 3.4 ± 1.8 mg/dL (range 1.0–5.8) at HVE, and 1.9 ± 1.3 mg/dL (range 0.9–3.4) at surgery. Of these 34 patients, 3 patients (8.8 %) did not undergo PVE because the right PV was severely stenotic due to tumor invasion at the time of diagnosis; thus, PVE was unnecessary or infeasible and only HVE was performed.
HVE was performed 11.5 ± 6.7 days (median 9 days; range 6–34) after PVE. Right and left HVE were performed in 33 and 1 patient, respectively. As already mentioned, 2 patients erroneously received MHV embolization, and one of them also additionally underwent RHV embolization (and thus received RHV-MHV embolization). Left HVE consisted of concurrent embolization of the left hepatic vein and MHV. While waiting to undergo surgery after HVE, 2 patients abandoned surgery due to low FRL regeneration, low KGR (mean KGR per week <2 % for first 3 weeks), and rapid tumor progression. Finally, 32 patients underwent surgery 16.7 ± 8.4 days after HVE (median, 15; range 8–44), which corresponds to 27.7 ± 9.7 days after initial PVE (median, 26; range 14–52). Three patients (9.4 %) had to wait >4 weeks after HVE due to the insufficient resolution of obstructive jaundice or repeated cholangitis.
Pre-planned hemihepatectomy was performed on 28 patients (right liver resection in 27 patients and left liver resection in 1 patient), including hepatopancreatoduodenectomy in 2 patients. The caudate lobe with paracaval portion was completely removed in all cases. In contrast, 2 patients underwent palliative bile duct resection due to far advanced tumors, and another 2 patients did not undergo resection due to far advanced tumor (n = 1) and peritoneal seeding (n = 1). Thus, 28 of 34 patients (82.4 %) successfully underwent pre-planned surgery after the preparatory waiting period of mean 28 days following PVE–HVE in order to accommodate FRL regeneration and/or further biliary decompression.
The liver volume changes following PVE, HVE, and right hepatectomy in the 24 patients who underwent right hepatectomy are depicted in Fig. 4, with exclusion of 3 patients who had shown PV stenosis by tumor invasion. The proportion of FRL volume to pre-PVE TLV was 33.9 ± 2.2 % before PVE, which increased to 38.4 ± 1.5 % before HVE, 43.7 ± 2.1 % at the time of surgery, and 73.6 ± 8.3 % at 2 weeks after right hepatectomy, indicating that a combination of PVE and HVE reduced PHRR by 9.8 % (4.5 % from PVE and 5.3 % from HVE). The degree of FRL hypertrophy was estimated to be 13.3 % after PVE, 28.9 % after PHV-HVE, and 117.1 % 2 weeks after right hepatectomy. Considering that TLV also decreased along with right liver atrophy despite concurrent FRL regeneration (TLV proportion to pre-PVE baseline TLV was 97.3 ± 4.1 % before HVE and 94.3 ± 5.3 % before surgery), the actual PHRR at the time of surgery further decreased to 53.7 ± 2.8 %.
Comparative increase in future remnant liver (FRL) volume to total liver volume (TLV) following right portal vein embolization (PVE), right hepatic vein embolization (HVE), and right liver resection in 24 patients with perihilar cholangiocarcinoma. FRL volume before PVE was used as the baseline denominator volume
The 27 patients who survived after right or left hepatectomy were classified as stage I in 3 patients, stage II in 8 patients, stage IIIA in 3 patients, stage IIIB in 10 patients, and stage IVA in 3 patients according to the 7th AJCC TNM staging system. R1 resection was performed in 4 patients. Survival analysis showed that their 1-, 3-, and 5-year survival rates were 96.2, 45.1, and 30.0 %, respectively, with the exclusion of 1 patient due to perioperative mortality who died of uncontrolled pneumonia on postoperative day 28 [3] (Fig. 5).
Outcomes in the HCC group
The HCC group demonstrates two distinguishing features from other groups, which are additional requirement for performing transarterial chemoembolization (TACE) before PVE in order to prevent HCC growth and longer waiting period after PVE or HVE due to low KGR [18]. Only 1 of 4 patients underwent surgery at 31 days after initial PVE. Another 1 patient did not undergo surgery due to near-absent FRL regeneration at 2 months after PVE–HVE (KGR <1 % per week). The other 2 patients had post-PVE waiting periods that were more than 6 months, and right hepatectomy was performed at 43 days and 17 months after HVE, respectively. During the long waiting period following PVE and HVE, they received TACE to control recurrent HCC lesions. After observing 2 patients for more than 1 year after HVE, the FRL regeneration rate was very low and barely noticeable, but the estimated PHRR was progressively reduced due to the marked shrinkage of the embolized right liver (actual PHRR was 51 % in the patient who underwent right hepatectomy after 17 months) [3]. All 3 patients who underwent right hepatectomy postoperatively survived for >5 years with post-hepatectomy tumor recurrence only in 1 patient.
Outcomes of the intrahepatic tumor group
Patients in the intrahepatic tumor group—including intrahepatic cholangiocarcinoma in 3 patients and gallbladder cancer in 1 patient—differed from other groups, in which the tumor can grow up along FRL regeneration because some triggering factors of liver regeneration may provide stimulatory effects on tumor growth. Primarily due to hypovascular tumors, TACE was not performed in the patients of this group unlike HCC group. All patients in this group demonstrated rapid FRL regeneration (KGR >4 % per week), which was similar with that observed in the bile duct cancer group. In a patient with intrahepatic cholangiocarcinoma, overt tumor growth within the shrunken right liver was observed while the contralateral FRL was rapidly regenerating after PVE and HVE. This patient had multiple tumors and lymph node metastasis, thus survived 11 months after right hepatectomy. In contrast, the other 2 patients with intrahepatic cholangiocarcinoma did not demonstrate noticeable tumor progression after PVE–HVE: one patient died after 16 months due to lymph node metastasis, and the other patient survives without tumor recurrence for 2 years. Another patient with advanced gallbladder and liver invasion and lymph node metastasis also did not demonstrate tumor growth after PVE–HVE and survived 28 months after extended right hepatectomy. Therefore, 3 of 4 patients with intrahepatic tumor did not demonstrate noticeable tumor growth during active regeneration of the contralateral liver. No patient demonstrated tumor recurrence in the remnant left liver within 6 months after hepatectomy, implicating the absence of intrahepatic metastasis at the FRL.
Discussion
The FRL regeneration rate following right PVE is approximately 10 % of TLV after 2–4 weeks [3], and thus the FRL significantly enlarges. We demonstrated that the KGR value was peak at the first week, and then decreased along time passage. This unique phenomenon can be called “interlobar volume shifting” depending on the changes in PV blood flow [1, 2, 19–21]. However, such interlobar volume shifting does not uniformly occur in all human livers, probably due to different intrahepatic hemodynamics (e.g., intrahepatic PV shunting and collateral formation) and concurrent liver diseases (e.g., liver cirrhosis and unresolved obstructive jaundice with cholangitis) [3, 21]. Impaired post-PVE liver regeneration or low KGR is associated with a higher risk for post-hepatectomy liver failure or dysfunction, especially in patients with liver cirrhosis [9, 18]. The underlying mechanisms of PVE-associated effects are complex and associated with various mediators, including inflammatory cytokines, vasoregulators, growth factors, eicosanoids, and several hormones [1, 2]. We previously reported that persistence of minute PV flow, concurrent liver cirrhosis, prominent arterial hyperperfusion, and incomplete biliary drainage clinically impede contralateral liver regeneration following PVE [3, 10, 22].
However, in practice, except for liver cirrhosis, the most common cause of insufficient FRL regeneration may be the incomplete interruption of PV blood flow. Even if the first-order PV branch is completely occluded, the distal minute PV branches can be refilled by arteriovenous shunting. Such retrograde PV filling is often noticeable, especially when the distal PV branches are not completely occluded with embolic materials. Because absolute alcohol, which is also used for sclerotherapy, can extensively destroy the endothelia of all peripheral PV branches and thereby cause adverse local tissue effects, it can more effectively induce PV flow occlusion than other less irritating embolic materials [23, 24]. Local preferences appear to dictate the choice of embolic material, as there are no clear data that support a superior choice among the gamut of embolic materials available for treating PVE [25].
Hemiliver damage due to sequential PVE–HVE was well tolerated in all patients in our present series. Deteriorated hepatic function following HVE was not evident, similar to what is seen after PVE alone. The main mechanism to induce parenchymal atrophy in the embolized liver area is apoptosis [3]. In our 7 patients who did not undergo liver resection, no noticeable adverse effects, such as liver abscess, cholangitis, or necrosis, occurred during the >1-year follow-up period or until patient death. Such PVE–HVE-associated safety is ensured probably due to the persistence of arterial perfusion at the territory of PVE–HVE. This is the first study to demonstrate overt development of intrahepatic arterial collaterals in the situation of PVE–HVE. No accidental migration of embolization coils into the cardiopulmonary system occurred, most likely probably due to the application of preventive measures such as the prior placement of a large vascular plug.
We experienced 2 patients who erroneously received embolization of the MHV instead of RHV. Such wrong HVE can happen in any institution. Surgery was performed on 1 patient who had undergone additional RHV embolization. Currently, in our institution, we can obtain angiographic 3-dimensional CT images during angiographic intervention, and thus RHV can be securely discerned from MHV. Therefore, we believe that preoperative sequential PVE–HVE is as safe as PVE alone.
At this point, the underlying mechanisms of HVE should be reviewed. As we previously reported [3], sequential ipsilateral HVE following PVE further damages the embolized lobe, facilitating compensatory regeneration in the contralateral lobe. PVE induces PV flow deprivation and compensatory arterial hyperperfusion throughout the entire right lobe. Embolization of the RHV system including the inferior RHV induces outflow obstruction in most of the right posterior section and a considerable proportion of the right anterior section (or about two-thirds of the right liver volume) [26]. PV flow was nearly completely inhibited within the territory of RHV drainage, and arterial flow may also be severely inhibited by outflow pathway obstruction. Preserving minute arterial flow is beneficial to preventing ischemic necrosis and the subsequent formation of abscess [3, 4]. When only RHV is occluded does the PV serve as a drainage vein for hepatic arterial flow, but PV flow also significantly decreases [26]. When the RHV and right PV are concurrently occluded, the hepatic arterial blood flow of the right liver drains into the MHV after development of intrahepatic collaterals. Because of such changes in intrahepatic hemodynamics, the right posterior section territory is more damaged than the right anterior section territory [3]. In other words, liver damage is more severe after HVE following PVE than PVE alone; thus, additional damage results in the further regeneration of the contralateral liver.
In total, 27 patients with perihilar cholangiocarcinoma underwent right hepatectomy, and the regeneration rate of the FRL volume was 4.5 and 5.3 % of pre-PVE TLV following PVE and HVE, respectively. The regeneration impact from PVE was lower than expected due to the shorter post-PVE waiting period during the late phase of this study. PHRR decreased from 66.1 % before PVE to 56.3 % after HVE. However, TLV also decreased following PVE and HVE, and the actual PHRR at the time of surgery further decreased to 53.7 %. This value implies that PHRR can be lowered toward 50 % when patients with not so small FRL undergo sequential PVE–HVE. Previously, nearly all studies regarding on PVE have used the pre-PVE TLV as the baseline denominator TLV. In contrast, we demonstrated that TLV would be decreased along with right liver atrophy despite concurrent FRL regeneration. TLV after PVE–HVE was decreased by more than 5 % of pre-PVE TLV in this study, thus the actual PHRR would be further decreased by about 2 %. This strategy toward 50 % resection can be applied to patients with major comorbidities, such as the patient with paraplegia in this study series.
Most patients with limited FRL regeneration after PVE demonstrated further increases in FRL volume after receiving sequential PVE–HVE. However, the effect of HVE on liver volume was much smaller in all 4 patients with viral hepatitis-associated liver cirrhosis than in patients with non-cirrhotic livers. Their KGR was usually less than 1 % per week. After treating the first 3 patients, patients with definite liver cirrhosis were no longer considered proper candidates for HVE. However, very slow but progressive liver atrophy developed in 1 patient after sequential PVE–HVE, and the patient successfully underwent right hepatectomy for recurrent HCC at 17 months after HVE.
Artificially inducing FRL regeneration using PVE or PVE–HVE is effective, but increasing evidence suggests that such preoperative procedures can stimulate tumor growth both in the embolized and non-embolized parts of the liver by altering the blood supply and/or inducing a network of cytokines and growth factors [27]. Theoretically, rapid FRL regeneration may cause negative effects on tumor growth following PVE or PVE–HVE. Such stimulating effects are more obvious in patients with HCC [28], and thus TACE is usually performed before PVE [18, 29]. In contrast, no effective preventive measures are available for hypovascular tumors, such as intrahepatic cholangiocarcinoma or hepatic invasion of gallbladder cancer. We noted accelerated tumor growth in 1 of 4 patients, and thus the indications for PVE or PVE–HVE should be prudently selected according to the types of intrahepatic malignancy. In contrast, the bile duct cancer group demonstrated a 3-year survival rate of 45.1 %. Considering the high proportion of patients with advanced tumors, such survival outcomes appear comparable to our previously reported survival rate of 50.7 % at 3 years after R0 resection in 214 patients with perihilar cholangiocarcinoma [30].
Unlike PVE experience in Western countries, our experience on PVE included only a small proportion of colorectal cancer liver metastasis [9, 11]. We presented only 5 cases of metastatic liver disease in 79 cases of PVE during the year of 2006 [3]. It was probably due to its lower incidence per se as well as anxiousness regarding on PVE-induced tumor progression [27, 28]. Therefore, no patient with colorectal cancer liver metastasis was indicated for PVE–HVE yet in our series.
For colorectal cancer liver metastasis, operative procedures to induce rapid liver regeneration such as associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) has been attempted in many institutions. In an European study with 9 centers of 62 cases, FRL volume was increased by 48.6 % at 7.8 days after the first stage, but the KGR decelerated beyond 7 days. Major complication occurred in 40.3 and 12.9 % of patients died [31]. Our PVE–HVE induced 28.9 % hypertrophy of FRL after PHV-HVE, which is much lower than in ALPPS. Since ALPPS are often associated with high morbidity rate, it may be possible to think of PVE–HVE instead of ALPPS, but we do not think so because they have different indications. If any tumor is present at the FRL, PVE–HVE is contraindicated on the oncological basis, thus PVE–HVE is not valid for multiple intrahepatic lesions of colorectal cancer liver metastasis. Contrarily, ALPPS is not suitable for patients with perihilar cholangiocarcinoma [32].
When assessing the risk of post-hepatectomy liver failure, the concept of standardized FRL volume was proposed to estimate PHRR objectively [9]. Such liver volumetric quantification is suitable for large intrahepatic space-occupying lesions, but not valid for perihilar cholangiocarcinoma because the native TLV is usually well preserved. We also presented our results regarding on standard liver volume-corrected FRL volume, in which a formula for standard liver volume was derived from 2155 living liver donors and FRL volume assessment with or without correction by standard liver volume was performed for 723 patients undergone right hepatectomy for HCC [33].
We have performed pre-operative PVE in more than 400 patients during the 7-year study period. Its main indication was patients with perihilar cholangiocarcinoma requiring major liver resection, in whom PVE was performed in 91 of 168 patients (54.2 %) who underwent 154 right hemihepatectomy, 9 right and 5 left trisectionectomy [30]. In contrast, patients with HCC was less frequently indicated for PVE, as shown in 116 of 723 patients (16.0 %) who underwent right hepatectomy or right trisectionectomy [33]. Based on our experience, about 10 % of patients have shown insufficient regeneration of the FRL volume following PVE, thus being indicated for subsequent HVE, especially in non-cirrhotic livers.
There were several limitations to this study. This was the first medium-volume study reported by a single center, thus multicenter studies need to be performed to validate our results. It was difficult to delineate the liver contours and hemiliver territory on CT images after PVE and HVE due to serious artifacts from multiple embolization coils. This difficulty might have negatively influenced the accuracy of CT volumetry.
In conclusion, our experience confirms that additionally applying HVE to PVE is safe and effectively induces the further regeneration of the contralateral liver in non-cirrhotic livers. Further validation in a larger patient population with multicenter studies is needed to reliably widen the indications for HVE.
Abbreviations
- FRL:
-
Future remnant liver
- HVE:
-
Hepatic vein embolization
- PV:
-
Portal vein
- PVE:
-
Portal vein embolization
- RHV:
-
Right hepatic vein
- MHV:
-
Middle hepatic vein
- TLV:
-
Total liver volume
- TACE:
-
Transarterial chemoembolization
- HCC:
-
Hepatocellular carcinoma
- PHRR:
-
Parenchymal hepatic resection rate
- KGR:
-
Kinetic growth rate
References
Yokoyama Y, Nagino M, Nimura Y (2007) Mechanisms of hepatic regeneration following portal vein embolization and partial hepatectomy: a review. World J Surg 31:367–374
Yokoyama Y, Nagino M, Nimura Y (2007) Mechanism of impaired hepatic regeneration in cholestatic liver. J Hepatobiliary Pancreat Surg 14:159–166
Hwang S, Lee SG, Ko GY et al (2009) Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg 249:608–616
Gruttadauria S, Gridelli B (2007) Sequential preoperative ipsilateral portal and arterial embolization in patients with liver tumors: is it really the best approach? World J Surg 31:2427–2428
Kyokane T, Nagino M, Oda K et al (2001) An experimental study of selective intrahepatic biliary ablation with ethanol. J Surg Res 96:188–196
am Esch JS II, Knoefel WT, Klein M et al (2005) Portal application of autologous CD133 + bone marrow cells to the liver: a novel concept to support hepatic regeneration. Stem Cells 23:463–470
Munene G, Parker RD, Larrigan J et al (2013) Sequential preoperative hepatic vein embolization after portal vein embolization for extended left hepatectomy in colorectal liver metastases. World J Surg Oncol 11:134
Hwang S (2011) Right hepatectomy in a patient with hepatocellular carcinoma after induction of hepatic parenchymal atrophy through subsequent portal and hepatic vein embolizations. Korean J Gastroenterol 58:162–165
Shindoh J, Truty MJ, Aloia TA et al (2013) Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg 216:201–209
Lee SG, Hwang S (2005) How I do it: assessment of hepatic functional reserve for indication of hepatic resection. J Hepatobiliary Pancreat Surg 12:38–43
Higuchi R, Yamamoto M (2014) Indications for portal vein embolization in perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci 21:542–549
Ribero D, Curley SA, Imamura H et al (2008) Selection for resection of hepatocellular carcinoma and surgical strategy: indications for resection, evaluation of liver function, portal vein embolization, and resection. Ann Surg Oncol 15:986–992
Nagino M, Kamiya J, Nishio H et al (2006) Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg 243:364–372
Ko GY, Hwang S, Sung KB et al (2010) Interventional oncology: new options for interstitial treatments and intravascular approaches: right hepatic vein embolization after right portal vein embolization for inducing hypertrophy of the future liver remnant. J Hepatobiliary Pancreat Sci 17:410–412
Ko GY, Sung KB, Yoon HK et al (2003) Preoperative portal vein embolization with a new liquid embolic agent. Radiology 227:407–413
Yoo H, Ko GY, Gwon DI et al (2009) Preoperative portal vein embolization using an amplatzer vascular plug. Eur Radiol 19:1054–1061
Ku Y, Fukumoto T (2011) Hepatic vein embolization. In: Madoff DC, Makuuchi M, Nagino M, Vauthey JN (eds) Venous embolization of the liver. Radiological and surgical practice. Springer, London, pp 169–175
Ogata S, Belghiti J, Farges O et al (2006) Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg 93:1091–1098
Imamura H, Shimada R, Kubota M et al (1999) Preoperative portal vein embolization: an audit of 84 patients. Hepatology 29:1099–1105
Azoulay D, Castaing D, Smail A et al (2000) Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg 231:480–486
Komori K, Nagino M, Nimura Y (2006) Hepatocyte morphology and kinetics after portal vein embolization. Br J Surg 93:745–751
Kito Y, Nagino M, Nimura Y (2001) Doppler sonography of hepatic arterial blood flow velocity after percutaneous transhepatic portal vein embolization. Am J Roentgenol 176:909–912
Sofue K, Arai Y, Shimada K et al (2014) Right portal vein embolization with absolute ethanol in major hepatic resection for hepatobiliary malignancy. Br J Surg 101:1122–1128
Igami T, Ebata T, Yokoyama Y et al (2014) Portal vein embolization using absolute ethanol: evaluation of its safety and efficacy. J Hepatobiliary Pancreat Sci 21:676–681
Thornton RH, Covey AM, Madoff DC (2011) Embolic materials used for portal vein embolization. In: Madoff DC, Makuuchi M, Nagino M, Vauthey JN (eds) Venous embolization of the liver. Radiological and surgical practice. Springer, London, pp 29–136
Hwang S, Lee SG, Park KM et al (2004) Hepatic venous congestion in living donor liver transplantation: preoperative quantitative prediction and follow-up using computed tomography. Liver Transpl 10:763–770
Aoki T, Kokudo N (2011) Tumor growth after portal vein embolization. In: Madoff DC, Makuuchi M, Nagino M, Vauthey JN (eds) Venous embolization of the liver. Radiological and surgical practice. Springer, London, pp 271–278
Hayashi S, Baba Y, Ueno K et al (2007) Acceleration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol 48:721–727
Yoo H, Kim JH, Ko GY et al (2011) Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol 18:1251–1257
Lee SG, Song GW, Hwang S et al (2010) Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci 17:476–489
Truant S, Scatton O, Dokmak S, e-HPBchir Study Group from the Association de Chirurgie Hépato-Biliaire et de Transplantation (ACHBT) et al (2015) Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): impact of the inter-stages course on morbi-mortality and implications for management. Eur J Surg Oncol 41:674–682
Li J, Girotti P, Königsrainer I et al (2013) ALPPS in right trisectionectomy: a safe procedure to avoid postoperative liver failure? J Gastrointest Surg 17:956–961
Hwang S, Ha TY, Song GW et al (2015) Quantified risk assessment for major hepatectomy via the indocyanine green clearance rate and liver volumetry combined with standard liver volume. J Gastrointest Surg 19:1305–1314
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest.
Rights and permissions
About this article
Cite this article
Hwang, S., Ha, TY., Ko, GY. et al. Preoperative Sequential Portal and Hepatic Vein Embolization in Patients with Hepatobiliary Malignancy. World J Surg 39, 2990–2998 (2015). https://doi.org/10.1007/s00268-015-3194-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3194-2