Abstract
Objectives
To assess the effect of salvage hepatic vein embolization (HVE) on the volume of the future liver remnant (FLR) for patients with metastatic colorectal cancer (mCRC) and inadequate hypertrophy following initial portal vein embolization (PVE).
Methods
From April 2011 to October 2018, 9 patients with mCRC underwent HVE following PVE. The right or middle hepatic vein was embolized with coils and/or vascular plugs. Liver volumes were calculated at baseline, following PVE, and following HVE, in order to assess the hypertrophic effect of PVE and HVE on the FLR.
Results
Nine patients underwent HVE (n = 3, right HVE; n = 6, middle HVE) because of inadequate FLR hypertrophy following PVE. The standardized FLR increased from 0.16 (median, range 0.08–0.24) at baseline to 0.22 (median, range 0.13–0.29) following PVE (p = 0.0005) to 0.26 (median, range 0.19–0.37) following HVE (p = 0.0050). HVE was performed 40 days (median, range 19–128 days) following PVE, and assessment of FLR hypertrophy was performed 41 days (median, range 19–92 days) following HVE. Four of nine patients underwent hepatectomy; 5 patients failed to undergo hepatectomy (n = 3, inadequate hypertrophy; n = 1, disease progression; n = 1, portal hypertension). One patient required repeat HVE due to a patent accessory vein.
Conclusions
Salvage HVE is an effective technique to induce additional FLR hypertrophy in patients with mCRC and inadequate FLR after initial PVE.
Key Points
• Hepatic vein embolization is effective to induce additional liver hypertrophy in surgical patients with metastatic colorectal carcinoma and inadequate hypertrophy after portal vein embolization.
• Increases in future liver remnant volume are feasible in patients who receive hepatotoxic neoadjuvant systemic therapy for metastatic colorectal carcinoma.
• Sequential portal vein embolization and hepatic vein embolization can be a viable technique to induce liver hypertrophy in patients with small baseline future liver remnant volumes (< 20%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgical resection of primary and secondary liver cancers is a means to achieve long-term patient survival. Risks associated with surgery include liver insufficiency, liver failure, cholestasis, and insufficient synthetic function [1]. To minimize liver insufficiency and liver failure, preoperative portal vein embolization (PVE) can be performed to induce hyperplasia of the future liver remnant (FLR) [2]. PVE has been shown to increase the FLR volume in patients with normal liver as well as in patients with liver affected by steatosis, with advanced fibrosis/cirrhosis, and following high-dose chemotherapy [3,4,5]. While the vast majority of patients who undergo PVE proceed to definitive liver resection, 3.2–17.1% of patients fail to achieve sufficient FLR hypertrophy following PVE [6,7,8]. To address insufficient FLR hypertrophy following PVE, techniques utilizing adjunctive hepatic vein embolization (HVE) have been described. Hwang et al [9, 10] performed preoperative sequential PVE and right hepatic vein embolization (RHVE); in a more contemporary case series, Guiu et al [11] performed simultaneous PVE and HVE. In these limited retrospective case series, adjunctive HVE, whether performed sequentially or simultaneously with respect to PVE, appears to increase the size of the FLR. The value of HVE is to decrease the number of patients who are precluded from surgery based on a small FLR. The impact of surgical resection for metastatic colorectal cancer (mCRC) to the liver is pronounced. Shindoh et al [12] reported a median survival of 67.4 months in patients who underwent staged PVE followed by liver resection compared to a median survival of 24 months in patients who underwent PVE but failed to undergo liver resection.
Patients with mCRC to the liver who are candidates for definitive liver resection present a unique challenge. While the optimal regimen to be used in the neoadjuvant setting for patients with initially resectable hepatic metastases is not established, systemic regimens used in clinical practice include leucovorin calcium, fluorouracil, and oxaliplatin (FOLFOX); irinotecan plus leucovorin calcium and fluorouracil (FOLFIRI); or capecitabine and oxaliplatin with or without bevacizumab. Liver toxicity has been reported with fluorouracil, irinotecan, and oxaliplatin. The range of liver toxicities includes steatosis, sinusoidal changes, steatohepatitis, and hemorrhagic central lobular necrosis which can result in increased 90-day mortality rates following liver resection [13,14,15,16]. Furthermore, given the multifocal nature of colorectal liver metastases, it is not uncommon for liver surgeons to resect large amounts of tissue or utilize two-stage hepatectomy to clear the FLR of visible tumor prior to definitive liver resection. In the mCRC cohort with liver-only disease and who have received more than 3 months of neoadjuvant systemic therapy, a 30% standardized future liver remnant (sFLR) is generally considered necessary prior to resection [17,18,19]. Our purpose was to describe our institutional experience with the use of sequential HVE as an adjunctive technique to induce liver regeneration in a cohort of heavily pretreated mCRC patients with low baseline sFLR (i.e., < 20%) and insufficient FLR hypertrophy following preoperative PVE.
Materials and methods
Our institutional review board approved this retrospective review. From October 2010 to November 2018, 211 patients underwent right PVE (RPVE) or right PVE extended to segment 4 portal veins (RPVE+4) at our institution prior to planned major liver resection. Ten patients (4.7%) with mCRC and inadequate FLR hypertrophy following PVE then underwent HVE; one patient was excluded because portal vein embolization was performed to segments 6 and 7 only. Thus, nine patients were included in this analysis. Clinical, pathological, and radiographic variables were reviewed from the electronic medical record.
During the work-up for potential liver surgery for patients with liver-only metastatic colorectal cancer, patients were screened for prior chemotherapy use and drinking history. Standard liver function tests (including aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, alkaline phosphatase, total bilirubin, indirect bilirubin, direct bilirubin, and serum albumin) were also obtained. During the study period, patients underwent PVE if the volume of the sFLR was ≤ 20% for normal liver and ≤ 30% in patients who received more than 3 months of chemotherapy prior to resection [17, 20, 21]. The sFLR was calculated as a ratio between the FLR volume and standardized total liver volume (sTLV), which was calculated using a formula for body surface area (BSA) in square meters: sTLV = − 794.41 + 1267.28 × BSA [22]. Systemic administration of chemotherapy and/or biologic agents was stopped at least 1 month prior to PVE. Re-administration of chemotherapy and/or biologic agents was not performed until the patient was no longer deemed a surgical candidate or completed definitive surgical resection of liver metastases. PVE was performed via a transhepatic ipsilateral (i.e., on the side of the liver being resected) approach utilizing a combination of tris-acryl particles and coils to occlude the branches of the right portal vein with or without segment 4 portal veins. Our approach has been previously described [23, 24].
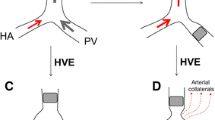
For patients with inadequate FLR hypertrophy following PVE, HVE was offered as a salvage technique to induce additional liver regeneration (n = 6, middle HVE (MHVE); n = 3, RHVE). The technique of HVE has been previously described [10, 25]. Briefly, the right internal jugular vein was accessed with an 11-French Raabe vascular sheath (Cook Medical LLC). The sheath was advanced into the hepatic vein intended for embolization, which was determined by the referring liver surgeon. Venograms were performed in at least 2 obliquities to confirm appropriate catheter placement. A 5-French catheter was used to embolize all first order branches with metallic coils. Coils were oversized by at least 20% (range 3–14 mm). The main right or left hepatic vein was embolized with serial vascular plugs (Amplatzer™ Vascular Plug II, Abbott) which were oversized by 50% (range, 10–22 mm) to within 2 cm of the hepatic vein and inferior vena cava confluence.
Enhanced CT scans were performed with a multidetector CT scanner, with 4, 16, or 64 slices (LightSpeed; GE Healthcare), using a tri-phasic liver protocol. Images were reconstructed at 2 mm to 5 mm thickness. The DICOM images were then transferred to a MIM® workstation (MIM Software, Inc.) to calculate volumes of the total liver and FLR. CT scans of the liver with intravenous contrast (iodixanol, GE Healthcare) were performed before PVE, 28 days (median, range 16–42 days) following PVE, and 42.3 days (median, range 19–92 days) following HVE.
Medians and ranges were used to report clinical and volumetric variables. Comparisons between continuous variables were performed with a paired t test (GraphPad Prism, GraphPad Software, Inc.). P values < 0.05 were considered statistically significant.
Results
Nine patients were included in this retrospective review (n = 7 male, n = 2 female; median age 52 years, range 27–70 years). The clinical characteristics and clinical outcomes of the 9 patients are included in Table 1. Chemotherapy and/or biologic agents were administered in a neoadjuvant setting to all 9 patients included in this analysis (median number of cycles 6, range 4–24 cycles). Of the 9 patients, 3 (33.3%) patients underwent definitive liver surgery; 6 (66.7%) patients were no longer candidates for surgery because of inadequate FLR hypertrophy (n = 3), cancer progression precluding liver resection (n = 2), and worsening of comorbidities (splenomegaly and thrombocytopenia, n = 1). The clinical follow-up data for the 3 patients who underwent definitive surgical resection of their liver tumors is as follows: (a) one patient is alive with no evidence of disease at 5.5 years of follow-up, (b) one patient died from disease progression at 3.8 months following liver resection, and (c) one patient suffered an asystolic cardiac arrest on postoperative day 3 following liver resection and passed away 3 days later. Of note, for the patient who passed away within 30 days of the liver resection, the serum total bilirubin measured 1.6 mg/dL on the day before the cardiac arrest. One patient did not undergo definitive liver resection because of splenomegaly and thrombocytopenia, and an exact etiology for the worsening comorbidities was not elucidated. Nevertheless, this patient underwent proton beam radiation as salvage therapy. The patient is currently 62 months from the PVE with no evidence of disease [26]. Five of 9 (55.6%) patients underwent a first-stage liver surgery to clear the FLR prior to definitive liver resection as part of a two-stage hepatectomy approach [27, 28]. The time interval between PVE and HVE measured 40 days (median, range 19–128 days), and assessment of FLR hypertrophy was performed 41 days (median, range 19–92 days) following HVE. The median time interval between the HVE and definitive hepatectomy for the 3 patients who were candidates for surgery was 40 days, 48 days, and 96 days. For these 3 patients who underwent definitive liver surgery, there was no evidence of postoperative hepatic insufficiency as defined by a total bilirubin > 7 mg/dL within 30 days following surgery and there was no death attributable to liver insufficiency within 90 days following surgery [29].
Standardized future liver remnant was calculated on serial CT scans obtained following PVE and HVE (Fig. 1). Changes in sFLR per patient are depicted in Fig. 2. There were statistically significant increases in sFLR following PVE (p = 0.0005) and following HVE (p = 0.0050) (Table 2). In our study, MHVE was performed in 6 patients and RHVE was performed in 3 patients. The median sFLR before MHVE measured 0.20 (range, 0.13–0.22), and the median sFLR following MHVE measured 0.22 (range, 0.19–0.27); the median sFLR before RHVE measured 0.27 (range, 0.25–0.29), and the median sFLR following RHVE measured 0.34 (range 0.33–0.37) (p = 0.0870). There were no complications as a result of PVE or HVE. However, one MHVE procedure had to be repeated as a large tributary to the hepatic vein was not appreciated on the initial embolization procedure.
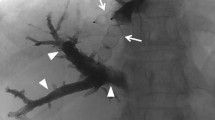
Morphologic changes involving the liver following portal vein embolization (PVE) and hepatic vein embolization (HVE). a Axial computed tomography (CT) scan of the liver demonstrates the sFLR (area enclosed by a series of white dots) measuring 0.19. Of note, patient is status post first-stage hepatectomy involving segment 2/3 resection. Because of the small sFLR, patient underwent subsequent right PVE. b Axial CT scan of the liver 4 weeks following right PVE demonstrates interval growth of the sFLR to 0.25. Because of insufficient sFLR hypertrophy, patient underwent right HVE. c Axial CT scan of the liver 7 weeks following right HVE demonstrates interval growth of the sFLR to 0.37. However, patient did not undergo definitive hepatectomy because of interval disease progression. White dots reflect boundary of the future liver remnant. sFLR standardized future liver remnant
Discussion
HVE induces increases in FLR volume for patients with mCRC and inadequate FLR hypertrophy following PVE. In our population of mCRC patients who received neoadjuvant systemic therapy with hepatotoxic chemotherapy and/or biologic agents, increases in sFLR following PVE and HVE were observed despite a small baseline sFLR (median 0.14, range 0.08–0.24; Table 2). Importantly, there were no serious adverse events in our patients; specifically, there were no reports of abdominal pain, fever, or nausea, which can be seen with post-embolization syndrome. HVE can be performed with high efficacy; only one patient in our cohort needed a follow-up procedure owing to a large tributary hepatic vein which was unrecognized at the time of the initial procedure.
The decision to embolize the right hepatic vein or middle hepatic vein was dictated by the surgeon based on the extent of the planned surgical resection (i.e., right HVE was performed prior to planned right hepatectomy, and middle HVE was performed prior to planned right trisegmentectomy). Our results indicate that there may be greater degree of hypertrophy for patients following RHVE as compared to MHVE (p = 0.0870) though additional research involving more patients will need to be performed. Prior studies have demonstrated that HVE induces centrilobular congestion and scattered areas of parenchymal hemorrhage within the embolized territory during the acute phase [30, 31]. While compensatory intrahepatic venous collateral formation can occur as early as 7 days following hepatic vein occlusion, prior work by Hwang et al [10] has shown that sequential PVE and HVE can induce additional liver regeneration by the induction of damage to the embolized liver by HVE. Tani et al [32] measured the venous drainage from three-dimensional simulations of the liver in 100 healthy donors and found that the left hepatic vein, middle hepatic vein, and right hepatic vein contributed a mean drainage of 20.7%, 32.7%, and 39.6% of the entire liver, respectively. The increased drainage territory by the right hepatic vein may be a plausible explanation for increased FLR hypertrophy following RHVE relative to MHVE. It should be noted, however, that in our study, RHVE was performed in patients who underwent RPVE alone and MHVE was performed in patients who underwent RPVE+4. The HVEs performed in this case series were performed largely before the reports on extended liver venous deprivation (eLVD), which described the safety of simultaneous RHVE and MHVE [11]. It would be interesting to evaluate whether the addition of RHVE to MHVE in patients who will undergo extended right hepatectomy may be beneficial for sFLR hypertrophy over MHVE alone.
Previous studies have described the use of adjunctive HVE for FLR hypertrophy. Hwang et al [9, 10] described the use of sequential PVE-HVE in patients with primary hepatobiliary malignancy (n = 54 patients). Guiu et al [11] described the use of simultaneous RPVE and MHVE + RHVE (i.e., eLVD) in 10 patients. The median FLR at baseline was 324 cm3 (range 241–421 cm3), and the median FLR following eLVD was 523 cm3 (range 437–670 cm3). The magnitude of the change is consistent with the findings in our study where we reported a median FLR of 224 cm3 (range 135–511 cm3) at baseline and a median FLR of 499 cm3 (range 280–776 cm3) following sequential PVE and HVE.
The optimal timing between interventions intended to provide FLR hypertrophy and definitive surgical resection is not known. In our study, four of nine patients were taken to the operating room at a median of 101 days (range 88–140 days) following PVE; three of these patients ultimately underwent definitive liver resection. Importantly, there was no evidence of postoperative liver insufficiency (total bilirubin > 7 mg/dL) in our patient cohort. For the patient who suffered a cardiac arrest on postoperative day 3, the total serum bilirubin prior to the event was 1.6 mg/dL. Using the eLVD technique, Guiu et al [11] reported that 9 of 10 patients underwent liver resection at a median of 31 days (range 22–45 days) following the procedure. However, it should be noted that longer time intervals between PVE and surgery may actually be beneficial as it may allow for selection of oncologically appropriate candidates for major hepatectomy.
Our study is a retrospective single-arm cohort study with its attendant limitations. Only three of nine (33.3%) patients in our study who underwent HVE ultimately were taken to the operating room for definitive surgical resection of liver dominant mCRC limiting evaluation of surgical outcomes. Despite significant increases in FLR volume following PVE and HVE, the decision to take patients with mCRC to liver surgery is ultimately a clinical decision based on patient factors (e.g., comorbidities), oncologic behavior of the tumor (i.e., interval disease progression), and quality of the underlying liver parenchyma and should not be taken as a singular marker for the efficacy of HVE. Also, while our study evaluated the change in sFLR volumes following PVE and HVE, additional information could be obtained from a functional assessment of the FLR [33, 34]. A recent study by Theilig et al [33] showed that FLR function is accurately predicted with gadoxetic acid–enhanced MRI before and after PVE with a reduction in FLR enhancement correlating with post-hepatectomy liver failure. These results suggest the potential importance of physiologic parameters in addition to volumetric assessment in the evaluation of patients prior to surgical. Furthermore, histopathologic evaluation of the underlying liver parenchyma evaluating for liver injury (e.g., steatosis and/or fibrosis) was not performed, limiting our assessment for the impact of PVE and HVE on FLR hypertrophy in patients with varying degrees of chemotherapy-associated liver injury. Nonetheless, the ubiquitous use of systemic agents in this study in a neoadjuvant setting is consistent with current standard of care for treatment of patients with surgically resectable liver dominant mCRC.
In conclusion, in our study of nine patients with surgically resectable mCRC who had inadequate FLR hypertrophy following PVE, sequential HVE was a viable technique to achieve sufficient FLR growth to allow for safe surgical resection of the tumor-bearing liver in a subset of our patient cohort (n = 3 of 9 patients). Factors warranting further investigation include the optimal timing of HVE relative to PVE (e.g., simultaneous PVE and HVE versus sequential PVE and HVE) as well as the effect of administration of interval chemotherapy and/or biologic agents in the periprocedural period to mitigate the risk of interval tumor growth. Our study adds to the growing literature regarding the use of HVE as a safe and effective adjunctive technique to induce additional FLR hypertrophy for patients with mCRC.
Abbreviations
- BSA:
-
Body surface area
- eLVD:
-
Extended liver venous deprivation
- FLR:
-
Future liver remnant
- FOLFIRI:
-
Irinotecan plus leucovorin calcium and fluorouracil
- FOLFOX:
-
Leucovorin calcium, fluorouracil, and oxaliplatin
- HVE:
-
Hepatic vein embolization
- mCRC:
-
Metastatic colorectal carcinoma
- MHVE:
-
Middle hepatic vein embolization
- PVE:
-
Portal vein embolization
- RHVE:
-
Right hepatic vein embolization
- RPVE+4:
-
Right PVE extended to segment 4 portal veins
- sFLR:
-
Standardized future liver remnant
- sTLV:
-
Standardized total liver volume
References
Abdalla EK, Hicks ME, Vauthey JN (2001) Portal vein embolization: rationale, technique and future prospects. Br J Surg 88:165–175
Bellentani S, Hardison WG, Manenti F (1985) Mechanisms of liver adaptation to prolonged selective biliary obstruction (SBO) in the rat. J Hepatol 1:525–535
Goere D, Farges O, Leporrier J, Sauvanet A, Vilgrain V, Belghiti J (2006) Chemotherapy does not impair hypertrophy of the left liver after right portal vein obstruction. J Gastrointest Surg 10:365–370
Covey AM, Brown KT, Jarnagin WR et al (2008) Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg 247:451–455
Farges O, Belghiti J, Kianmanesh R et al (2003) Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg 237:208–217
Abulkhir A, Limongelli P, Healey AJ et al (2008) Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg 247:49–57
Shindoh J, Tzeng CW, Aloia TA et al (2014) Safety and efficacy of portal vein embolization before planned major or extended hepatectomy: an institutional experience of 358 patients. J Gastrointest Surg 18:45–51
Yamashita S, Sakamoto Y, Yamamoto S et al (2017) Efficacy of preoperative portal vein embolization among patients with hepatocellular carcinoma, biliary tract cancer, and colorectal liver metastases: a comparative study based on single-center experience of 319 cases. Ann Surg Oncol 24:1557–1568
Hwang S, Ha TY, Ko GY et al (2015) Preoperative sequential portal and hepatic vein embolization in patients with hepatobiliary malignancy. World J Surg 39:2990–2998
Hwang S, Lee SG, Ko GY et al (2009) Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg 249:608–616
Guiu B, Quenet F, Escal L et al (2017) Extended liver venous deprivation before major hepatectomy induces marked and very rapid increase in future liver remnant function. Eur Radiol 27:3343–3352
Shindoh J, Vauthey JN, Zimmitti G et al (2013) Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg 217:126–133 discussion 124–133
Kooby DA, Fong Y, Suriawinata A et al (2003) Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg 7:1034–1044
Rubbia-Brandt L, Audard V, Sartoretti P et al (2004) Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 15:460–466
Vauthey JN, Pawlik TM, Ribero D et al (2006) Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 24:2065–2072
Tisman G, MacDonald D, Shindell N et al (2004) Oxaliplatin toxicity masquerading as recurrent colon cancer. J Clin Oncol 22:3202–3204
Azoulay D, Castaing D, Krissat J et al (2000) Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg 232:665–672
Adam R, Delvart V, Pascal G et al (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 240:644–657 discussion 648–657
Kishi Y, Zorzi D, Contreras CM et al (2010) Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol 17:2870–2876
Vauthey JN, Pawlik TM, Abdalla EK et al (2004) Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg 239:722–730 discussion 722–730
Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN (2002) Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg 137:675–680 discussion 671–680
Vauthey JN, Abdalla EK, Doherty DA et al (2002) Body surface area and body weight predict total liver volume in Western adults. Liver Transpl 8:233–240
Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN (2003) Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness—study in 26 patients. Radiology 227:251–260
Madoff DC, Abdalla EK, Gupta S et al (2005) Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol 16:215–225
Ku Y, Fukumoto T (2011) Hepaic vein embolization. In: Madoff DC, Makuuchi M, Nagino M, Vauthey J-N (eds) Venous embolization of the liver: radiologic and surgical practice. Springer, London, pp 169–175
Colbert LE, Cloyd JM, Koay EJ, Crane CH, Vauthey JN (2017) Proton beam radiation as salvage therapy for bilateral colorectal liver metastases not amenable to second-stage hepatectomy. Surgery 161:1543–1548
Brouquet A, Abdalla EK, Kopetz S et al (2011) High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 29:1083–1090
Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H (2000) Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg 232:777–785
Mullen JT, Ribero D, Reddy SK et al (2007) Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 204:854–862 discussion 854–862
Widmann WD, Hales MR, Greenspan RH (1962) The effects of hepatic vein occlusions. Am J Pathol 41:439–454
Maetani S (1965) Experimental obstruction of left hepatic vein in dogs. I. Histopathological changes and gross vascular alterations. Nihon Geka Hokan 34:216–233
Tani K, Shindoh J, Akamatsu N et al (2016) Venous drainage map of the liver for complex hepatobiliary surgery and liver transplantation. HPB (Oxford) 18:1031–1038
Theilig D, Steffen I, Malinowski M et al (2019) Predicting liver failure after extended right hepatectomy following right portal vein embolization with gadoxetic acid-enhanced MRI. Eur Radiol 29:5861–5872
Asenbaum U, Kaczirek K, Ba-Ssalamah A et al (2018) Post-hepatectomy liver failure after major hepatic surgery: not only size matters. Eur Radiol 28:4748–4756
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Steven Y. Huang.
Conflict of interest
The authors declare that they have no conflict of interest.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Methodology
• Retrospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Niekamp, A.S., Huang, S.Y., Mahvash, A. et al. Hepatic vein embolization after portal vein embolization to induce additional liver hypertrophy in patients with metastatic colorectal carcinoma. Eur Radiol 30, 3862–3868 (2020). https://doi.org/10.1007/s00330-020-06746-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06746-4