Abstract
Background/purpose
Both curative resection and minimized in-hospital mortality offer the only chance of long-term survival in patients with hilar cholangiocarcinoma. The reported resectability rates for hilar cholangiocarcinoma have increased by virtue of combined major hepatectomy, but this procedure is technically demanding and still associated with a significant morbidity and mortality that must be carefully balanced against the chances of long-term survival.
Methods
Between January 2001 and December 2008, 350 patients with hilar cholangiocarcinoma underwent exploration for the purpose of potentially curative resection, of whom 302 (86.3%) were resected in the Department of Hepato-Biliary Surgery and Liver Transplantation, Asan Medical Center, University of Ulsan College of Medicine. Combined hepatectomy was carried out in 268 (88.7%) of 302 resected patients. Major hemihepatectomy and parenchyma-preserving hepatectomy were performed in 257 and 11 patients, respectively. Portal vein resection was associated in 40 (14.9%) of 268 hepatectomized patients. To control preoperative cholangitis and reduce risk of postoperative hepatic failure, biliary decompression through endoscopic and/or percutaneous transhepatic drainage and portal vein embolization were preoperatively applied in 329 (94.0%) of 350 explored patients and in 91 (54.2%) of 168 extended hepatectomized patients (154 right hemihepatectomy, 9 right trisectionectomy, 5 left trisectionectomy), respectively. Liver transplantation was not performed as primary treatment for hilar cholangiocarcinoma.
Results
There were 5 cases (1.7%) of in-hospital death after resection and 1 postoperative liver failure that was successfully treated with liver transplantation. Major complications were encountered in 23 patients (7.0%), and the overall morbidity rate was 43%. In 302 resections, 214 (70.9%) were curative resections (R0) and 88 (29.1%) were palliative resections (R1). The overall 1-, 3- and 5-year survival rates after resection, including in-hospital deaths, were 84.6, 50.7 and 47.3% in the R0 group and 69.9, 33.3 and 7.5% in the R1 group, respectively. The 5-year survival rate of extended hemihepatectomy of 36.4% was better than that of parenchyma-preserving hepatectomy at 10.5%. Two significant predictive factors adversely affecting survival after resection were lymph node metastasis and incurability of surgery (P < 0.001). Two patients with vascular involvement who underwent concomitant hepatic artery and portal vein reconstruction are alive after more than 3 years.
Conclusion
Preoperative biliary decompression and portal vein embolization enabled us to reduce in-hospital deaths associated with extended hepatectomy for hilar cholangiocarcinoma. Major hemihepatectomy offers an increased survival because of the higher possibility of curative resection than bile duct resection alone and parenchyma-preserving hepatectomy, but it still carries a certain mortality. Less extensive procedures can be conducted safely and are beneficial for aged patients in poor condition with a less advanced tumor stage if tumor-free resectional margins are obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In many leading centers treating hilar cholangiocarcinoma, major hemihepatectomy with combined en bloc resection of the caudate lobe is accepted as a standard surgical strategy to increase resectability and improve long-term results [1–3]. However, the reported 5-year survival rate of 20–30% after resection is far from encouraging. The low survival rates have been mainly attributed to the persisting postoperative mortality after extended hepatectomy in the liver with cholestatic injury and the high frequency of local recurrence of tumor originating from positive resection margins [4]. Curative hepatobiliary resection with minimizing postoperative mortality offers the only chance for long-term survival. To reduce the risk of lethal postoperative hepatic failure in patients with obstructive jaundice, adequate biliary drainage and portal vein embolization (PVE) have been practiced before major hepatectomy [1, 3, 5]. Because of the close proximity between tumor and vascular structures of the hepatic hilum, curative resection (R0) often requires combined portal vein (PV) and hepatic artery (HA) reconstruction. PV invasion was previously a main cause of unresectability of hilar cholangiocarcinoma. HA invasion has been considered a contraindication of surgical intervention. However, these concepts have now become outdated with the advancement of surgical techniques and introduction of microvascular surgery for HA anastomosis [6]. In 2000, we reported our results of 111 patients who underwent liver resection for hilar bile duct cancer with mortality of 6.3% [7]. The aim of this study was to review the short- and long-term results of our patients in whom hepatobiliary resection was conducted to treat hilar cholangiocarcinoma arising from the large hilar bile duct (EHC, the extrahepatic type of hilar cholangiocarcinoma) over the last 8 years.
Materials and methods
Patients
Between January 2001 and December 2008, surgical interventions with curative intent were performed on 350 patients who had hilar cholangiocarcinoma arising from the large hilar bile duct (EHC, the extrahepatic type of hilar cholangiocarcinoma). Eventually, 302 patients underwent hepatobiliary resection (resectability rate 86.3%), and 48 (13.7%) did not undergo any resectional surgery because of unexpected peritoneal dissemination, liver metastasis, lymph node metastasis far beyond regional nodes and extensive local invasion of major vessels. Besides 34 (11.3%) bile duct resections alone (BDR), combined hepatectomy was carried out in 268 (88.7%) of 302 resections. Major hepatectomy, including right or left hemihepatectomy and trisectionectomy, was performed in 257 of the 268 liver resections (Table 1). Parenchyma-preserving hepatectomy, including Couinaud’s segment 1 only, or 4 + 1 or 5 + 8 + 1 or 4 + 5+8 + 1, was selected for the remaining 11 patients because of poor liver function and comorbidity of patients, or limited tumor extent. The 64 cases of intrahepatic cholangiocarcinoma involving the hepatic hilus (IHC, the intrahepatic type of hilar cholangiocarcinoma) that underwent hepatic resection (Table 1) were excluded in this review because the prognosis of IHC is still believed to be different from that of EHC [8]. This study comprises 302 resected cases of EHC of hilar cholangiocarcinoma at our department. There were 83 women and 219 men, with a median age of 61 years (range 24–83 years).
Preoperative evaluation and management
Initially, the location and extent of the disease were roughly evaluated by ultrasonography and computed tomography. Positron emission tomography (PET) scan is currently added to assess for the presence of extrahepatic metastasis. Patients with distant metastasis, ascites and overt para-aortic lymph node metastasis (size >2 cm, oval shaped) were not included as candidates for resectional surgery. If a patient showed evidence of jaundice or there were dilated bile ducts in the future remnant liver (FRL), a selective biliary decompression only to the FRL was established principally using percutaneous transhepatic biliary drainage (PTBD). When segmental cholangitis could not be controlled after hemihepatic biliary drainage, another PTBD was added to resolve infection in the future resecting area. The proximal end of the obstruction was investigated by cholangiography via PTBD. The distal end was evaluated in detail with endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP). While waiting for jaundice to disappear, further investigation was processed using selective angiography or multiphasic helical computed tomography with a bolus injection of a contrast medium to obtain precise information about vascular involvements and to define vascular anatomic details. All patients also underwent other generalized preoperative tests to assess their operative fitness. The 329 (94.0%) of 350 surgically explored patients for resectional intent underwent biliary decompression by preoperative PTBD (182, 52.0%) or ENBD alone (64, 18.3%) or both PTBD and ENBD (83, 23.7%). Percutaneous transhepatic cholangioscopy (PTCS) was selectively carried out in papillary tumors of its cholangiographic feature to discriminate multiple foci. The volume of the whole liver and the part of the hepatic segment to be resected were calculated using CT volumetry. PVE via the percutaneous transhepatic route for the liver segment to be resected was indicated 2–4 weeks before operation if the estimated resecting volume exceeded 60% of the whole liver volume. However, while taking into consideration the liver functional reserve (ICGR15 >20%, delayed clearance of jaundice after biliary drainage, moderate steatosis), the associated comorbidities, such as DM and cardio-pulmonary dysfunction, advanced age (>70 years) or invasiveness of the potentially concomitant vascular resection/pancreatoduodenectomy, PVE was carried out even if the estimated liver volume to be resected exceeded 50%. PVE was performed in 91 (54.2%) of 168 patients who underwent 154 right hemihepatectomy, 9 right and 5 left trisectionectomy. After PVE, 8 patients underwent additional right hepatic vein embolization (HVE), aiming at further atrophy of the liver to be resected, and compensatory hypertrophy of the FRL [9].
Surgical procedure
Resection procedures were selected according to tumor extent of the bile duct and involvement of PV and HA as determined by preoperative and intraoperative evaluation. A microscopically tumor-free surgical margin of the bile duct is absolutely necessary for prolonged survival in hilar cholangiocarcinoma. To accomplish this, at least a 5-mm margin has been the goal for curative resection in our practice. In principle, more extensive resection than right hemihepatectomy was performed when the serum total bilirubin level had decreased to ≤2 mg/dl, but left hemihepatectomy can be advocated in minimally jaundiced liver (TB < 4 mg/dl). Initially, systematic lymphadenectomy for nodes at the hepatoduodenal ligament, behind the pancreas head, along with the common hepatic artery, and at the right side of celiac axis was performed, followed by skeletonization resection of the hepatic hilus. Routine para-aortic lymph node dissection discontinued since 2000 because the increasing number of living donor liver transplantations, more than 100 cases per year, had made heavy demands on our surgeons. The results of this study were accomplished by 5 attending surgeons. En-bloc caudate lobectomy was performed routinely in all liver resections. Frozen section pathologic examination of cut ends of ducts was routinely used to guide resection; additional ductal resection inside the pancreas was performed if the distal bile duct margin was initially tumor-positive. Meanwhile, hepatopancreatoduodenectomy (HPD) was performed in 7 (2.6% of combined hepatectomy) selected patients with diffuse bile duct cancer to secure a negative distal bile duct margin. The surgical procedures are summarized in Table 2. The longitudinal extents of resected tumors along bile duct were classified according to the Bismuth–Corlette classification as type I (n = 16, 5.3%), type II (n = 41, 13.6%), type IIIA (n = 131, 43.4%), type IIIB (n = 62, 20.5%) and type IV (n = 52, 17.2%). The hepatic parenchyma was divided by CUSA and/or Kelly clamp crushing method close to the hepatic vein using Pringle’s maneuver. PV resection and reconstruction were performed in 40 patients, including 36 segmental resections with 29 end-to-end anastomoses and 5 interposition vein grafts (1 autogenous external iliac vein, 2 autogenous left renal veins, 2 homologous cadaveric iliac veins) and 4 wedge resections with 3 primary closures and 1 autogenous saphenous vein patching (Table 3). The right hepatic artery was resected in 5 patients who underwent 3 left-sided hepatectomies and two BDRs. Arterial reconstruction was performed under the microscope using the right gastroepiploic artery in 1 patient, rotating left hepatic artery in 1 patient and direct end-to-end anastomosis in 1 patient. The right hepatic artery was resected, but not reconstructed in 2 patients with BDR. They did not develop any complications. Combined IVC resection with direct suture closure was performed in 1 patient (Table 3). All PV reconstructions with interposition vein grafts were encountered in left-sided hepatectomy. The biliary tract was reconstructed by bilio-enteric anastomosis using a Roux-en-Y loop, and short internal stents were placed across the anastomosis. But, in patients with previous PTBDs, a long external stent was placed via the PTBD tract, which was removed 4–8 weeks after the operation. The term “extended right lobectomy” defines resection of the right liver lobe, the inferior part of Couinaud’s segment IV and the total caudate lobe. “Extended left lobectomy” included the left liver lobe, the hilar part of segment V and most of the caudate lobe. Surgical curability was defined by residual tumor status: R0 (n = 214, 70.9%) indicated no residual tumor (negative histologic margins); R1 (n = 88, 29.1%) indicated microscopic residual tumor. Pathologic tumor staging was carried out according to the TNM classification of the International Union Against Cancer (UICC) [10].
In principle, adjuvant chemotherapy and external or internal radiation treatment were not given to patients who underwent R0 and R1 resection in the current study.
Statistical analysis
Figures are expressed as median values with the range in brackets, unless otherwise indicated. Patient survival was analyzed using the Kaplan–Meier method, and univariate analysis was performed using the log-rank test. Multivariate analysis was performed using the Cox proportional hazard model. A P value of ≤0.05 was considered statistically significant. Patient survival was measured in days or months from resectional surgery until death.
Results
Operative procedures performed
Depending on the longitudinal cancer spread, 4 types of operative procedures were employed: right hemihepatectomy (n = 164) mainly for Bismuth–Corlette type IIIA or IV and II tumors, left hemihepatectomy (n = 93) mainly for type IIIB or IV and II tumors, parenchyma-preserving hepatectomy from isolated caudate lobectomy to central bisectionectomy (n = 11) mainly for type IV or I and II tumors, and BDR (n = 34) mainly for type I and II or IIIA and IV tumor for palliation (Table 2). All hepatectomies included caudate lobectomy and resection of extrahepatic bile ducts. Nine right trisectionectomies (resection of Couinaud’s segments 4, 5, 6, 7, 8 and 1) were performed with 6 combined portal vein resections and 1 pancreatoduodenectomy. Five left trisectionectomies (resection of Couinaud’s segments 1, 2, 3, 4, 5 and 8) were performed with 3 combined PV resections. Trisectionectomy was indicated only for patients for whom preoperative imaging evaluation demonstrated longitudinal tumor spread extending beyond the potential separation lines of the proximal bile duct from the underlying vasculature by the conventional right or left hemihepatectomy. Concomitant vascular resection was carried out in 43 patients, with PV resection alone in 38 patients, HA alone in 3 and both procedures in 2 patients (Table 3). There was zero mortality and no thrombotic complication related to 45 vascular reconstruction procedures, except 1 portal vein thrombosis (PVT) developing after 5-cm segmental resection and end-to-end anastomosis with left trisectionectomy. This patient underwent re-exploration on the 5th postoperative day with thrombectomy and intraoperative metallic PV stenting via the inferior mesenteric vein to relieve the tension of previous PV anastomosis that might cause the relative anastomotic stricture and the subsequent PVT. She recovered slowly, but is alive with normal liver function. HPD was performed in 7 selected patients with extensive bile duct cancer to obtain a negative distal bile duct margin. All hepatectomies of HPD were major hepatectomies (6 right and 1 left hemihepatectomy). Intraoperative P-RBC transfusion was used in 32.1% of hepatic resection cases.
Pathologic findings of tumor
Pathologic staging of tumors was determined according to the International Union Against Cancer (UICC) staging system [10]. Infiltrating tumor was the most common type (n = 197, 65.2%), followed by polypoid tumor (n = 62, 20.6%) than nodular tumor (n = 43, 14.2%), classified by macroscopic appearance. The histologic type of lesion reflects the predominant type. The histologic grade was well-differentiated carcinoma in 82 patients (27.2%), moderately differentiated carcinoma in 182 patients (60.2%) and poorly differentiated carcinoma in 38 patients (12.6%). Twenty-five (8.3%), 161 (53.5%), 103 (34.2%) and 12 (4%) patients had T1, T2, T3 and T4 tumors, respectively. One hundred fifty-four (51.2%), 130 (43.2%), 12 (4.0%) and 5 (1.7%) patients had stage IA and B, IIA and B, III and IV tumors, respectively. Perineural invasion, lymphovascular invasion and lymph node metastasis were found in 218 (72.2%), 85 (28.2%) and 73 (24.2%) patients, respectively. Histopathologic examination of the respected portal vein revealed actual tumor invasion in 23 (57.2%) of 40 specimens. R0 curative resection was achieved in 214 (70.9%) patients. The remaining 88 (29.1%) patients underwent R1 palliative resection.
Morbidity and in-hospital death rates
Following the criteria of complications proposed by Sano et al. [3], complications were defined as major when they resulted in organ failure or required another surgery or interventional radiology. On the other hand, complications such as pleural effusion necessitating thoracocentesis, wound infection, intra-abdominal infection with positive culture of the drainage fluid, delayed gastric emptying, anastomotic leakage, clinically silent pancreatic fistula with amylase-rich serous or contaminated fluid with positive culture, and bile leakage from the raw surface of the liver healing spontaneously or responding to conservative treatment were classified as minor. Operative death was defined as death at any time during the first hospitalization, regardless of the length of the hospitalization. One hundred thirty patients developed postoperative complications, resulting in a morbidity rate of 43%. Major complications were encountered in 23 patients (7.0%) and were the cause of 5 in-hospital deaths. Major complications comprised 4 cases of hepatic failure, 5 bilomas and intra-abdominal abscesses because of hepaticojejunostomy leakage and missing bile ducts, 3 intra-abdominal bleedings, 3 abdominal wound dehiscences, 2 pseudoaneurysm ruptures of the common hepatic artery, 1 jejunojejunostomy bleeding and subsequent necrosis by arterial embolization, 1 colon perforation by careless abdominal wall closure, 1 PVT, 1 massive gastric ulcer bleeding, 1 persisting septic cholangitis and shock caused by inadequate preoperative biliary drainage, and 1 aspiration pneumonia. All 5 in-hospital deaths occurred in aged patients (>65 years) who underwent major hemihepatectomies (Table 4). The in-hospital death rate was 1.7% of 302 patients who underwent resection and 1.94% of 257 patients who underwent major right or left hemihepatectomy. After the more extensive resections than right hemihepatectomy, including 154 right hemihepatectomies and 9 right and 5 left trisectionectomies, in-hospital death developed in 4 (2.4%) of these 168 patients.
Overall survival
Curability according to surgical procedure is shown in Table 5. R0 resection was achieved far less frequently in patients who underwent BDR alone than BDR with combined hepatectomy (26.4 vs. 76.5%, P < 0.001), even though we took into consideration that 13 (38.2%) of 34 BDRs alone were performed on Bismuth type IIIA, IIIB and IV tumors as palliative resections because of poor liver function or the poor general condition of patients. Overall 1-, 3- and 5-year survival rates after 302 resections, including in-hospital death, were 80.2, 40.6 and 32.5%, respectively. Mean survival time with 1-, 3- and 5-year survival rates in patients treated by BDR alone and BDR with combined hepatectomy were 28.5 ± 3.6 and 47.7 ± 3.1 months, with 82.4, 26.3 and 17.5 and 81.5, 43.4 and 35.5%, respectively. Patient survival with respect to the curability of resection is shown in Fig. 1. Overall 1-, 3- and 5-year survival rates after resection, including 5 in-hospital deaths, were 84.6, 50.7 and 47.3% in the R0 group and 69.9, 33.3 and 7.5% in the R1 group, respectively.
Univariate and multivariate analyses
The results of a univariate analysis for the relations among the variables of clinical, surgical and histologic factors and patient survival after resectional surgery are shown in Table 6. Three significant parameters associated with patients’ long-term survival were revealed: curability (P < 0.001), lymph node metastasis (P < 0.001) and lymphovascular tumor emboli (P = 0.001). Curability was the only significant prognostic factor in a multivariate analysis using parameters that had low P values in the univariate analysis: lymph node metastasis, curability and histologic differentiation (Table 7).
Discussion
Curative surgery for patients with hilar cholangiocarcinoma often necessitates extensive hepatic resection to achieve a tumor-negative resection margin because characteristics of its growth pattern include longitudinal intraductal extension and direct hepatic invasion [1–3]. Meanwhile, most surgical treatment of hilar cholangiocarcinoma with extrahepatic BDR has been shown to result in recurrence early after surgery due to positive surgical margins at the hepatic edge of the bile duct (17.5% of BDR vs. 35.5% of hepatectomy in 5-year survival rate) (Table 6) [7]. Extended hemihepatectomy is currently recognized as a curative treatment of hilar cholangiocarcinoma, but it is not always safe because postoperative hepatic failure is a common cause of in-hospital death after major hepatectomy in patients with compromised liver function caused by obstructive jaundice [2, 4, 11]. Therefore, surgical treatment of hilar cholangiocarcinoma still remains a challenge for the surgeon, and the encouraging feature of this current study comparing our previous study (Table 8) was that 257 (95.9%) of the 268 liver resections in the current series consisted of extended hemihepatectomy with 1.94% (5 of 257) rate of in-hospital death and 4 occurrences of postoperative hepatic failure. In 2000, we reported our in-hospital death rate of 6.2% (6 of 97) after extended hemihepatectomy for hilar cholangiocarcinoma [7]. A common cause of death was hepatic insufficiency that developed after complications that were considered to be recoverable, such as hepatic failure after extended surgery in case 1, persistent bile leak from hepaticojejunostomy and sepsis in case 2, and jejuno-jejunostomy pseudoaneurysm rupture and subsequent perforation of bowel by arterial embolization in case 5 (Table 4). All 3 patients underwent preoperative PTBDs and right percutaneous PVE, but their successive volumetric CT did not demonstrate the sufficient compensatory hypertrophy (>15% increase) of FRL volume after a 4–6-week waiting period with PVE. Case 5 showed only atrophy of the future resecting liver and no hypertrophy of the FRL, even though the volume ratio of the FRL:the whole liver increased from 31 to >40% after PVE. Limited or no regeneration of FRL after PVE in advanced aged (>70 years) patients despite the sufficient atrophy in the future resecting liver may carry a high frequency of postoperative mortality. Several authors reported that the extent of hepatic resection was closely related to the occurrence of postoperative complications, such as liver failure, sepsis and persistent anastomotic leakage [12, 13]. Parenchymal-preserving hepatectomy with a smaller hepatic resected mass for hilar cholangiocarcinoma could tolerate major postoperative complications and was accompanied by zero mortality in our study, although the 5-year survival rate is lower than that of extended hemihepatectomy (10.5 vs. 36.4%) (Table 6) [13]. Therefore, for operative procedures, limited as much as possible to what is necessary for curative resection chosen according to the tumor extent as well as the liver functional reserve (high ICGR15 value, delayed clearance of jaundice after biliary drainage, steatotic liver), and advanced age (>70 years), the general condition and the associated comorbidities of the patient, such as DM and cardio-pulmonary dysfunction, should be individually selected to minimize in-hospital death. We think the low morbidity and in-hospital death rates experienced in this study basically originated from the routine application of preoperative biliary drainage and PVE after a precise evaluation of the hepatic lobar volume before major hepatectomy, as this preoperative management has already been done in many leading Japanese centers [1, 3, 5]. To avoid cather-related infectious complications and enhance further atrophy of the future resecting liver lobe, we have made it a principle to drain only the hemihepatic lobe destined to remain after hepatectomy, as suggested by Makuuchi’s group [3]. Because of potential difficulties in effective multiple endoscopic drainages, the risk of post-endoscopic nasobiliary drainage (ENBD) ascending cholangitis and to optimally define the intrahepatic biliary anatomy, biliary decompression has been principally performed by PTBD in our department, although ENBD has some advantages over PTBD as a less invasive procedure with a low possibility of cancer cell spread along the draining tract. Preoperative biliary decompression by single ENBD directed to the FRL was applied only in 64 (18.3%) of our 350 patients who underwent exploratory laparotomy mainly for Bismuth–Corlette type I and II tumors, and was rarely complicated with ascending segmental cholangitis. The application of ENBD as the first step of biliary decompression for Bismuth type I and II tumors is an acceptable option. PVE, which induces atrophy of the embolized lobe and compensatory hypertrophy of the FRL, has contributed to the improvement of postoperative outcomes in patients undergoing extended hemihepatectomy. Although PVE has beneficial effects on postoperative outcomes, it does not always induce sufficient regeneration of the nonembolized lobe in all patients [5]. Subsequent embolization of the ipsilateral hepatic artery in a small number of patients did not yield satisfactory results and increased the risk of liver abscess [14]. Sequential preoperative ipsilateral hepatic vein (right hepatic vein) embolization 10 weeks after right PVE to induce further liver regeneration was first applied on August 2006 in our department, and 7 weeks after HVE, the patient underwent successful extended right lobectomy with caudate lobectomy and segmental PV resection. In our small series of HVE for hilar cholangiocarcinoma, most patients demonstrating limited liver regeneration after PVE resulted in a significant final increase in FRL volume from sequential HVE after PVE, but its routine application and timing of HVE after PVE need further evaluation [9]. Curability of the resectional surgery was the most significant prognostic factor in achieving patients’ long-term survival in our univariate and multivariate analysis (Tables 6, 7). A microscopically tumor-free surgical margin of the proximal and distal bile ducts is absolutely necessary to accomplish curability. Despite the almost routine application of extended hemihepatectomy (Table 1), the apparently high frequency of a positive ductal margin, which is 23.5% of the R1 resection rate even in patients undergoing combined hepatectomy (Table 5), can be explained by many resectional approaches to patients with locally advanced Bismuth type IV tumors (17.2% of our patients). To secure negative proximal ductal margins, additional resection of the liver volume beyond the hemihepatectomy by application of a trisectionectomy that allows a division site of the intrahepatic bile duct more peripherally may seem to be ideal, but would be a burden, especially for patients with compromised liver function. In this study, the number of trisectionectomies confined to Bismuth type IV tumors was small (5.2%, 14 of the 268 hepatic resections), but morbidity and mortality rates were lower than in extended hemihepatectomies because all trisectionectomies were performed after sufficient regeneration of the FRL with PVE, and the operation itself with a smaller parenchymal cut surface and low number of bile duct anastomoses was technically easier than extended right hemihepatectomy. To achieve more R0 resection rates for Bismuth type IV tumors, “anatomic” right hepatic trisectionectomy, recommended by Nagino et al. [15], in which the bile duct of the left lateral section is divided at the left side of the umbilical plate, will be the most appropriate procedure if there is no preoperative CT evidence of liver parenchymal atrophy in the future remaining lateral section. Controversy appears to exist regarding the selection of operative procedures for patients with Bismuth–Corlette type I and II tumors. Several authors have reported that patients with type I and II tumors can undergo BDR [4, 12]. Other authors have recommended left hemihepatectomy, as this resection is the most versatile procedure for hilar cholangiocarcinoma, affording high resectability and safety [3]. Others recommend routine right hemihepatectomy because the right hepatic artery passes behind the proximal part of the common hepatic duct, and therefore, is often invaded by cancer [1]. Nagoya's group recommended that the surgical approach to Bismuth type I and II hilar cholangiocarcinoma should be determined according to preoperative cholangiographic features [16]. For nodular and infiltrating tumors, right hemihepatectomy is essential; for papillary tumors, bile duct resection with or without limited hepatectomy is adequate. Table 2 shows that bile duct resections with or without parenchymal preserving hepatectomy were performed in 13 of the 16 Bismuth type I tumors, and 15 right and 14 left hemihepatectomies were performed in 29 of the 41 Bismuth type II tumors. The prevalence of polypoid (papillary) tumors by macroscopic type was 14.6% (44 of our 302 resected patients) (Table 6). Our operative approach to Bismuth type I and II tumors has resembled that of Nagoya's group. Portal vein invasion is still a major obstacle to resection for advanced hilar cholangiocarcinoma, but PV resection and reconstruction are now recognized as a means to increase the respectability, and in turn, may offer a better chance of long-term survival [17–19]. The mortality of hepatectomy with PV resection and reconstruction for hilar cholangiocarcinoma in our past study between June 1989 and June 2005 was 9.8% (5 of the 51 patients), but only 1 of 5 mortalities was directly related to PV resection [20]. All of these in-hospital mortalities occurred in the 1990s. There was no mortality and 1 PVT related to 40 PV resection and reconstruction in our current study between January 2001 and December 2008. The general improvement of our technique and knowledge concerning vascular surgery by large experience in adult living-donor liver transplantation has contributed to the excellent outcomes of vascular procedures for patients with hilar cholangiocarcinoma in the current study. Neuhaus et al. [2] proposed routine PV resection with right trisectionectomy as part of “no-touch” resection of the tumor and adjacent tissue to increase R0 resection rates with overall 60-day mortality of 17%. However, in actual clinical practice, the “no-touch technique” for extensive surgical resection of pancreatobiliary malignancies has failed to show a short-term survival benefit because of the high postoperative mortality, and we do not use the routine “no-touch” resection of hilar cholangiocarcinoma [21]. Despite the suggestion of macroscopic PV involvement during intraoperative inspection, histological examination of the resected portal vein revealed no actual tumor invasion in 30.8% in the Nagoya group [17] and in 45.1% in our study. Although the portal vein resection group demonstrated a low survival rate compared to the non-resection group, portal vein resection could offer long-term survival in more than 1 of 10 locally advanced hilar cholangiocarcinoma patients in our study [20]. Table 9 shows the clinicopathologic data of 10 long-term survivors after portal resection with major hepatectomy for hilar cholangiocarcinoma in our department. According to Miyazaki’s reports of 9 combined hepatic artery and portal vein resections, there was no benefit in terms of survival (1- and 3-year survival rate; 17 and 0%, respectively) and it led to an increase in operative mortality (33%) [22]. In contrast, portal vein resection alone can be used aggressively in advanced cases of hilar cholangiocarcinoma in patients without lymph node metastases. In our series, combined HA and PV resection was carried out only in 2 patients (Table 3), but they are alive now with tumor recurrence 38 months and 47 months after operation (Fig. 2). With the introduction of microvascular surgery for HA reconstruction, combined HA and PV resection seems to be acceptable from a operative risk perspective and to improve the outcome in the selected patients with locally advanced hilar cholangiocarcinoma, but our experience is still small to justify the combined HA and PV resection. HPD was performed in 7 selected patients (2.3% of the 302 resected cases) with extensive bile duct cancer to secure a negative distal ductal margins. All combined liver resections were extended hemihepatectomies, resulting in 1 mortality (Table 4). As substantiated by several reports, the morbidity and mortality associated with HPD for hilar cholangiocarcinoma still remains considerably high [3, 7]. In the current study, the number of HPD cases decreased compared to that of the previous study (Table 8). In the treatment of hilar cholangiocarcinoma, total hepatectomy and orthotopic liver transplantation (OLT) offers the advantage of resecting all structures that may be involved by the tumor, including the bilateral hepatic ducts, portal vein and hepatic artery. Therefore, OLT seems to permit R0 resection with “no-touch” isolation of cancer even in very locally advanced tumors that are beyond the indication criteria of resection. Unfortunately, the early experiences with OLT for hilar cholangiocarcinoma were discouraging: 20–30% 3-year survival [23]. Recently, the Mayo group introduced preoperative neoadjuvant chemoradiation followed by OLT to selected patients with hilar cholangiocarcinoma arising in the setting of primary sclerosing cholangitis or unresectable hilar cholangiocarcinoma not showing regional lymph node metastasis; the reported 5-year patient survival was 82% [24]. In our department, 3 patients with Bismuth type IV tumors were scheduled for living-donor liver transplantation with application of preoperative adjuvant chemoradiation according to the Mayo protocol, but they could not tolerate the intense and prolonged course of neoadjuvant therapy that seemed to be a heavy regimen for Asian patients. At present, liver transplantation is not considered a standard form of treatment for hilar cholangiocarcinoma in patients with resectable disease. We performed 1 urgent salvage liver transplantation on a 73-year-old male patient with postoperative hepatic failure who underwent extended right lobectomy with segmental PV resection after PVE for Bismuth type 1 tumor 1 month ago, and intraoperative frozen biopsy of his liver revealed 40% steatosis. The pathologic tumor staging of his hilar cholangiocarcinoma was T2N1M0, stage IIB cancer that belonged to the Mayo’s exclusion criteria of OLT, and finally he died of cancer recurrence 2 years after transplantation. Lymph node (LN) metastasis is an important predictor of long-term survival after resection in hilar cholangiocarcinoma. Survival was closely related to the extent of nodal involvement; the 3-year and 5-year survival rate with regional LN metastasis was reported as 31.8 and 14.7%, whereas the Nagoya group reported 12.3 and 12.3% with paraaortic LN metastasis [25]. However, many studies have shown poor survival for those with nodal metastasis beyond the hepatoduodenal ligament with 5-year survival of 0–6% [3, 4, 11]. Therefore, routine LN dissection beyond the hepatoduodenal ligament is not recommended, and patients with grossly involved LNs beyond the hepatoduodenal ligament are considered to have unresectable disease. However, the grossly enlarged LN does not always mean metastatic involvement, and patients with gross involvement of the paraaortic LN with size <2 cm should not be excluded as candidates for resectional surgery (Fig. 3). Only a limited number of 5-year survivors with LN metastasis have been reported to date, and Table 10 shows our 6 long-term survivors with LN metastasis after resection. Of note, one patients whose 3 of 8 paraaortic LNs showed metastasis (8 of a total of 24 excised LNs revealed metastasis) is alive more than 14 years with no sign of recurrence after modified hepato-ligamento-pancreatoduodenectomy (Table 10) [26]. The fact that long-term survival is possible even in pN1 or pM1 disease encourages surgeons to perform an aggressive surgical resection with extended LN dissection in selected patients with hilar cholangiocarcinoma. Thirteen patients survived longer than 10 years after surgical treatment for hilar cholangiocarcinoma in our series, and Table 11 details the clinicopathologic factors in chronological sequence and lists the outcome of each individual patient. In general, the prognosis after aggressive resection of hilar cholangiocarcinoma is better than often suspected. It is hoped that further improvement of the results of resectional surgery may develop using multimodal treatment strategies that combine new chemotherapy and local/external radiation therapy.
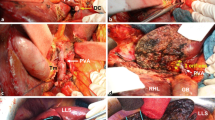
a, b Tumor involvement of the long segment of the right hepatic artery and right portal vein (indicated by white arrows). Proximal parts of left and middle hepatic arteries were also invaded by tumor. left portal vein was obliterated by tumor encasement, and left lobe of the liver was already atrophied. cleft hemi-hepatectomy with resection of caudate lobe and extrahepatic bile duct was performed with concomitant PV and HA resection. HA was reconstructed under the microscope using the mobilized right gastroepiploic artery (indicated by black arrows). d PV was segmentally resected and reconstructed with interposition vein graft that was obtained from autogenous left renal vein (indicated by black arrows). This patient is alive with recurrence 47 months after operation
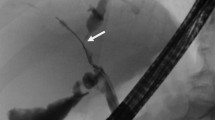
a, b A 1.8-cm, oval lymph node in the hepatic hilum (indicated by white arrow). Single left PTBD tube is shown in the future remnant left liver lobe (indicated by black triangle). c, d A 1-cm, oval lymph node in the aorto-caval area (indicated by white arrow). Despite the presumptive preoperative diagnosis of lymph node metastasis to the regional node (the hepato-duodenal ligament group) and the distant node (the aorto-caval group), this patient underwent right trisectionectomy, caudate lobectomy, bile duct resection and segmental portal vein resection with end-to-end anastomosis. Intraoperative frozen section and permanent pathology revealed that lymph node metastasis was not found in all 10 resected nodes, including the aorto-caval group
Conclusion
Curative resection with a low postoperative mortality offers the only chance for long-term survival in patients with hilar cholangiocarcinoma. Radical resection including extended hemihepatectomy offers the highest curability to patients with locally advanced disease, but this expected benefit must be balanced against an increased operative risk. Safe and curative surgery should be an aim of the hepatobiliary surgeons dealing with hilar cholangiocarcinoma. Surgeons must consider the following elements to be vital for such a strategy: preoperative treatment with adequate biliary decompression and selective PVE, a proper choice of operative procedure, and a careful but aggressive attitude with skillful surgical technique.
References
Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238(1):84–92.
Neuhaus P, Jonas S, Settmacher U, Thelen A, Benckert C, Lopez-Hänninen E, et al. Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch Surg. 2003;388:194–200.
Sano T, Shimada K, Sakamoto Y, Yamamoto J, Yamasaki S, Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240–7.
Klempnauer J, Ridder GJ, Werner M, Weimann A, Pichlmayr R. What constitutes long-term survival after surgery for hilar cholangiocarcinoma? Cancer. 1997;79:26–34.
Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364–72.
Shimada H, Endo I, Sugita M, Masunari H, Fujii Y, T anaka K, et al. Hepatic resection combined with portal vein or hepatic artery reconstruction for advanced carcinoma of the hilar bile duct and gallbladder. World J Surg. 2003;27(10):1137–42.
Lee SG, Lee YJ, Park KM, Hwang S, Min PC. One hundred and eleven liver resections for hilar bile duct cancer. J Hepatobiliary Pancreat Surg. 2000;7:135–41.
Ebata T, Kamiya J, Nishio H, Nagasaka T, Nimura Y, Nagino M. The concept of perihilar cholangiocarcinoma is valid. Br J Surg. 2009;96:926–34.
Hwang S, Lee SG, Ko GY, Kim BS, Sung KB, Kim MH, et al. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg. 2009;249:608–16.
International Union Against Cancer (UICC). TNM classification of malignant tumors. 6th ed. New York: Wiley; 2002.
Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693–702.
Miyazaki M, Kimura F, Shimizu H, Yoshidome H, Otsuka M, Kato A, et al. Extensive hilar bile duct resection using a transhepatic approach for patients with hepatic hilar bile duct diseases. Am J Surg. 2008;196:125–9.
Shimada H, Endo I, Sugita M, Masunari H, Fujii Y, Tanaka K, et al. Is parenchyma-preserving hepatectomy a noble option in the surgical treatment for high-risk patients with hilar bile duct cancer? Langenbecks Arch Surg. 2003;388:33–41.
Nagino M, Kanai M, Morioka A, Yamamoto H, Kawabata Y, Hayakawa N, et al. Portal and arterial embolization before extensive liver resection in patients with markedly poor functional reserve. J Vasc Interv Radiol. 2000;11:1063–8.
Nagino M, Kamiya J, Arai T, Nishio H, Ebata T, Nimura Y. “Anatomic” right hepatic trisectionectomy (extended right hepatectomy) with caudate lobectomy for hilar cholangiocarcinoma. Ann Surg. 2006;243:28–32.
Ikeyama T, Nagino M, Oda K, Ebata T, Nishio H, Nimura Y. Surgical approach to Bismuth type I and II hilar cholangiocarcinomas—audit of 54 consecutive cases. Ann Surg. 2007;246:1052–7.
Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg. 2003;238:720–7.
Kondo S, Katoh H, Hirano S, Ambo Y, Tanaka E, Okushiba S. Portal vein resection and reconstruction prior to hepatic dissection during right hepatectomy and caudate lobectomy for hepatobiliary cancer. Br J Surg. 2003;90:694–7.
Hwang S, Ha TY, Jung DH, Park JI, Lee SG. Portal vein interposition using homologous iliac vein graft during extensive resection for hilar bile duct cancer. J Gastrointest Surg. 2007;11:888–92.
Song GW, Lee SG, Hwang S, Kim KH, Cho YP, Ahn CS, et al. Does Portal vein resection with hepatectomy improve survival in locally advanced hilar cholangiocarcinoma? Hepatogastroenterology. 2009;93:935–42.
Hirano S, Kondo S, Tanaka E, Shichinohe T, Tsuchikawa T, Kato K. No-touch resection of hilar malignancies with right hepatectomy and routine portal reconstruction. J Hepatobiliary Pancreat Surg. 2009;16:502–7.
Miyazaki M, Kato A, Ito H, Kimura F, Shimizu H, Ohtsuka M, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery. 2007;141:581–8.
Robles R, Figueras J, Turrión VS, Margarit C, Moya A, Varo E, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265–71.
Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts S, Kremers WK, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–61.
Kitagawa Y, Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233:385–92.
Maeba T, Maeta H, Wakabayashi H, Okada S, Mori S, Karasawa Y. Modified hepatoduodenal ligamentectomy for advanced carcinoma of the biliary tract: the importance of preservation of the replaced left hepatic artery. J Hepatobiliary Pancreat Surg. 1998;5(3):297–302.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lee, S.G., Song, G.W., Hwang, S. et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci 17, 476–489 (2010). https://doi.org/10.1007/s00534-009-0204-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-009-0204-5