Abstract
Background
Although self-expanding metallic stents (SEMS) are useful tools for relieving large bowel obstructions in patients with colorectal cancer (CRC), their efficacy in a palliative setting has not been validated. This meta-analysis aimed to evaluate the feasibility of SEMS as a palliation for unresectable CRC patients with bowel obstructions and to determine their contribution to the prognosis of CRC, compared with surgical intervention.
Methods
We conducted a literature search of the PubMed and Cochrane Library databases. We selected all controlled trials that compared SEMS with surgical interventions as palliative treatments in unresectable obstructive CRC patients. The primary outcome was early complications, and the secondary outcomes were mortality, other morbidities, and long-term survival rates.
Results
Ten studies met our inclusion criteria. SEMS significantly reduced the risk of early complications (odds ratio [OR] 0.34; 95 % confidence interval [CI] 0.20–0.58 %; P < 0.01), mortality (OR 0.31; 95 % CI 0.15 %–0.64 %; P < 0.01), and stoma creation (OR 0.19; 95 % CI 0.12–0.28 %; P < 0.01). Although SEMS placement was significantly associated with a higher risk of perforation of the large bowel (OR 5.25 95 % CI 2.00–13.78 %; P < 0.01) and late complications (OR 1.94; 95 % CI 0.90–4.19 %; P = 0.03), it also contributed significantly to better long-term survival (hazard ratio 0.46; 95 % CI 0.31–0.68 %; P < 0.01).
Conclusions
Compared with surgical intervention, SEMS could provide feasible palliation for patients with bowel obstructions and unresectable CRC, because of their acceptable morbidity rates and better patient prognoses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is one of the most common malignant diseases and accompanied by acute colonic obstruction in approximately 20 % of CRC patients [1, 2]. In these cases, surgical decompression is one of the conventional intervention strategies. However, the high morbidity and mortality rates remain critical issues, because many of the patients tend to be older and due to the emergent nature of the surgical intervention.
Since Dohmoto reported the use of metallic stents in 1991, self-expanding metallic stents (SEMS) have become a widely accepted means of alleviating malignant bowel obstructions [3–5]. Some meta-analyses have shown that SEMS are applicable as bridge to surgery [6], because they might increase the success rates for primary anastomoses, lower the demand for stoma creation, and lead to less morbidity. Some reports have demonstrated that SEMS could be promising in the palliative treatment of unresectable obstructive CRC patients and have low rates of morbidity and mortality [4, 5, 7, 8]; however, randomized control trials are hardly planned. The aim of this meta-analysis was comparing SEMS with surgical intervention in relation to patient mortality, morbidity, and prognoses, and demonstrating the value of SEMS as a palliation for patients’ unresectable obstructive CRC.
Materials and methods
Information sources and data extraction
The PubMed database was searched from 1946 to October 2014, and the Ovid MEDLINE® database was searched from 1950 to October 2014, to identify clinical studies that compared short-term or long-term outcomes, or both, in patients with unresectable obstructive CRC who had either undergone SEMS placement or surgical intervention as palliation. The following medical subject headings were used: ‘‘stents’’ or ‘‘colorectal cancer’’ or ‘‘intestinal obstruction’’ or ‘‘large bowel obstruction’’. Two of the authors (H.T. and M.Y.) performed the literature searches independently, and disagreements between them were resolved by a third author (K.O.). Data were extracted from each study independently by H.T. and M.Y. using a predesigned review form. Where data disagreed, another co-author (M.T.) decided which data should be adopted. Where possible, the following details were recorded from each study: first author, publication date, study design, patient characteristics, including the numbers of patients and the sex ratios, mortality, early complications, late complications, stoma creations, SEMS-specific complications, including stent migration, stent failure, and re-obstruction, bowel perforation rates, and long-term survival rates.
Study selection
The meta-analysis included all studies in every language that had been published until October 2014, and it compared the outcomes from patients who had undergone SEMS placement and those who had undergone surgical intervention as palliations for unresectable obstructive CRC.
Studies were included in the meta-analysis if (1) they described the reasons underlying the bowel obstructions, (2) they compared SEMS and surgical intervention in palliative settings, (3) the SEMS were applied using either endoscopic guidance or fluoroscopic radiologic guidance, or both, (4) the surgical procedures included primary tumor resection, bypass creation, or stoma creation, or some of these, and (5) at least one of the outcomes of interest were reported. Studies were excluded if (1) it was impossible to calculate the outcomes from the published results and (2) the study reported on a patient group undergoing SEMS acts as a bridge to surgery.
Outcomes of interest
The primary outcome was early complications, which were defined as adverse events occurring within 30 days after the intervention that includes peri-interventional complications, specific complications associated with the stent including migration, re-obstruction, perforation and stent failure, or specific complications associated with surgical intervention including ileus, bleeding, wound infections, anastomotic leaks, pulmonary thromboembolisms, myocardial infarctions, urinary tract infections, strokes, hepatic failures, and pneumonia. The secondary outcomes included the clinical success rates, mortality, late complications, stoma creation, and long-term survival. Late complications were defined as those events that occurred 30 days after the procedure, and they included all of those complications that occurred where the interval between the intervention and the occurrence of the complication was not noted. In the SEMS group they included perforation, pan-peritonitis, stent migration, re-obstruction, infections, and cancer bleeding, whereas, cancer bleeding, enterocutaneous fistulas, small-bowel obstructions, incisional hernias, and colostomy prolapses consisted in the surgical intervention group.
Quality of the studies
The quality of each of the studies was assessed using the Newcastle–Ottawa Scale [9, 10], which was developed to assess the quality of non-randomized studies in relation to their design, content, and their ease of use when directed to the task of incorporating the quality assessments into the interpretation of meta-analytic results. Studies that achieve five or more stars are considered to be of high quality.
Statistical analysis
Statistical analyses were conducted in line with the recommendations from the Cochrane Handbook for Systematic Reviews of Interventions [11]. Odds ratios (OR) with their 95 % confidence intervals (CI) were determined as summary statistics for dichotomous outcomes [12]. Given the inherent heterogeneity associated with the studies, we assumed the presence of statistical heterogeneity and decided to only use a random-effects model before pooling the data, because a random-effects model can adjust according to the variability of the results among trials, and it provides a more conservative estimate of an effect using a wider CI. Heterogeneity among the studies was assessed using the I 2 statistic to measure the proportion of the total variation in the estimates that is caused by heterogeneity, where I 2 values of 50 and 75 % correspond to cut-off points for low, moderate, and high degrees of heterogeneity, respectively [13]. We tested for funnel plot asymmetry using Egger’s linear regression method to assess publication bias across all of the studies included in the analysis [14, 15]. For long-term survival analysis, hazard ratios (HR) were extracted or computed from each study as an effect size by applying the statistical model described by Tierney et al. [16]. The statistical analyses were performed using R version 2.15.2 and the Metafor Meta-Analysis Package for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Study selection
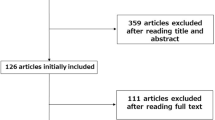
The literature searches identified 2076 studies which matched the initial search criteria. A review of the titles and abstracts excluded 2043 articles of them, because the studies did not assess SEMS as a palliative therapy for unresectable obstructive CRC patients. The full text of the remaining articles was reviewed, and a further 33 studies were retrieved from the references and the “related articles” sections on PubMed. A total of 10 studies [17–26] were assessed for eligibility, and these met the inclusion criteria and were used in the meta-analysis (Fig. 1).
The characteristics of all of the registered studies and their outcomes are summarized in Tables 1 and 2. Nine studies were retrospective cohort studies and one was a prospective study. All of the studies compared the outcomes from SEMS placement with those from surgical intervention for unresectable obstructive CRC patients in palliative settings. The SEMS were placed under fluoroscopic guidance in nine studies, but the remaining study did not mention about the way the SEMS were placed. The surgical interventions included resection of the primary tumor with anastomosis in one study, colostomy in one study, and either of them in the remaining studies. Two of the studies were limited to primary tumors on the left side of the colon and rectum.
Study characteristics
A total of 793 patients were included in this meta-analysis. Of these, 375 (47.3 %) patients underwent SEMS placements and 418 (52.7 %) patients underwent surgical interventions. Almost all of the patients were diagnosed with stage IV CRC, except for one patient who had local cancer recurrence. Five studies described the chemotherapy that followed the restoration of bowel obstruction [20, 21, 23, 25, 26]. One study described the first-line regimen as irinotecan-based chemotherapy or oxaliplatin-based chemotherapy [26]. In one study, 5-fluorouracil (5-FU)/leucovorin was administered with oxaliplatin or irinotecan [25], and another study used only oxaliplatin-based chemotherapy [21].
Early complications
Nine studies assessed early complications. The weighted average rates of early complications were 12.3 % (range 8.8–15.7 %) in the SEMS group and 29.7 % (range 25.2–34.3 %) in the surgical intervention group. SEMS placement significantly reduced the risk of early complications (OR 0.34; 95 % CI 0.20–0.58 %; P < 0.01, Fig. 2). The heterogeneity among the studies included in this analysis was high (I 2 = 88.7 %), although the cause of this was unclear. Tests to measure funnel plot asymmetry using Egger’s linear regression test did not identify any significant publication bias (bias = 0.43; P = 0.67).
Mortality
All of the studies assessed mortality. The weighted average rates of mortality were 2.1 % (range 1.4–2.8 %) in the SEMS group and 8.6 % (range 7.2–10.0 %) in the surgical intervention group. SEMS was associated with a lower mortality rate compared with surgical intervention. Although no studies declared significant difference between them, this meta-analysis showed that SEMS significantly reduced the risk of mortality compared with surgical intervention, (OR 0.31; 95 % CI 0.15–0.64 %; P < 0.01, Fig. 3). The heterogeneity was low (I 2 = 39.8 %). Egger’s linear regression test did not identify any significant publication bias (bias = −0.0133; P = 0.99).
Late complications
Eight studies assessed late complications. The weighted average rates of late complications were 24.3 % (range 19.2–29.3 %) in the SEMS group and 13.6 % (9.7–17.4 %) in the surgical intervention group. SEMS placement was significantly associated with a higher risk of late complications (OR 1.94; 95 % CI 0.90–4.19 %; P = 0.03, Fig. 3). The heterogeneity was moderate (I 2 = 59.0 %). Egger’s linear regression test did not identify any significant publication bias (bias = 1.221, P = 0.22).
Long-term survival
Eight studies assessed long-term survival. SEMS was associated with better patient prognoses than surgical intervention (HR 0.46; 95 % CI 0.31–0.68 %; P < 0.01, Fig. 4). The heterogeneity was high (I 2 = 88.0 %), however, no reason was detected for the high level of heterogeneity. Egger’s linear regression test did not identify any significant publication bias (bias = −0.0133, P = 0.99).
Permanent stoma
SEMS placement significantly reduced the risk for stoma creation (OR 0.19; 95 % CI 0.12–0.28 %; P < 0.01, the weighted average rates were 10.9 % (range 7.6–14.1 %) versus 40.9 % (range 36.0–45.8 %)) (Fig. 3). The heterogeneity was high (I 2 = 82.4 %); however, no reason was detected for the high level of heterogeneity. A significant publication bias was identified (bias = −2.394, P = 0.02).
Perforation rates
SEMS placement was significantly associated with a higher risk of perforation (OR 5.25; 2.00–13.78 %; P < 0.01, the weighted average rates were 7.4 % (range 4.6–10.3 %) versus 0.5 % (range 0.2–1.3 %)) (Fig. 3). The heterogeneity was low (I 2 = 18.2 %). No significant publication bias was identified (bias = −0.672, P = 0.50).
Stent-specific complications
The weighted average rates of stent failure, migration, and re-obstruction were 4.5 % (range 2.3–6.8 %), 8.4 % (range 5.5–11.3 %), and 13.1 % (range 9.6–16.6 %), respectively (Table 3). The heterogeneity was low (I 2 = 28.4 %), and no significant publication bias was identified (bias = 0.013, P = 0.99).
Discussion
The results from this meta-analysis suggest that SEMS could offer a promising approach to the management of unresectable obstructive CRC patient, because of the low morbidity rate and the better prognoses compared with surgical intervention, and these findings are compatible with those from other previous studies [24, 27, 28]. Since SEMS placement does not involve general anesthesia or surgery, SEMS placement should be less invasive than surgery. Moreover, compared with surgical intervention, SEMS placement could reduce the time required before induction of chemotherapy. Thus, SEMS should achieve better prognoses than with surgical intervention. Whereas, Maruthachalam et al. suspected that SEMS were oncologically unsafe, because SEMS appeared to increase the levels of circulating neoplastic cells [29], however, a slight increase in the levels of circulating tumor cells may not have a large impact on advanced unresectable CRC patients.
Although SEMS placement appears to be less invasive, there are several potential inherent defects associated with SEMS. Perforation occurred in around 4 % of patients, and stent migration and re-obstruction rates were 10 %, which are comparable with other studies [5, 12, 17, 18, 27, 30–34]. Acute perforation usually occurs as a mishandling of guide wire and catheter manipulation during endoscopy [23]. Therefore, strict and careful attention should be given to patients during this procedure. While, erosion of the colonic wall by the edges of the stents mainly results in late colonic perforation; however, some of the newer stents with looped edges, are expected to overcome such shortfalls [23, 35, 36]. Furthermore, novel drugs targeting specific molecules, including bevacizumab, may also increase the risk of late perforation and these drugs should be withheld from the patients following SEMS placement. Stent migration occurs within a few days or a few months after SEMS placement [21, 23, 25]. Early stent migrations are caused by unsuitable placement or by placement in an unsuitable area for SEMS. Late migration is a consequence of tumor shrinkage caused by chemotherapy [4, 23, 25]. Though surgical intervention is considered the appropriate strategy against these SEMS-specific complications, some have been reported that an additional SEMS was effective or no treatment was required. Exacerbation of the primary tumor can cause re-obstruction, which has been observed from 4 to 15 months after SEMS insertion [17–26]. Lee et al. reported that second stents are comparable to surgical intervention in terms of their patency [25].
In terms of patient satisfaction, Nagula et al. suggested that SEMS could offer a better quality of life (QOL) than surgical intervention [37]. The patients’ QOL immediately recovered and they had longer durations of improved QOL following SEMS placement, while the QOL recovery was slower and the durations of improved QOL were shorter in patients who had undergone surgical intervention [37]. These findings are consistent with those from other studies that found that SEMS could provide immediate relief from the suffering associated with obstructed bowels and that they could prevent the need to create stomas, both of which can destroy the quality of the rest of a patient’s life [7, 28, 38–40].
The success rate associated with SEMS placement was over 90 % in this meta-analysis, which is considered acceptable. However, sometimes it is technically challenging to deploy SEMS because of the location of the tumor or as a result of complications, and it is time consuming to position SEMS in these patients. This meta-analysis included two studies in which the patients were limited with left-sided CRC [17, 18], and subgroup analysis of these patients was performed (data not shown). Although there were no obvious differences between the results from our left-sided Lt-CRC subgroup analysis and those from the All-CRC analysis, further investigations are required to clearly define the types of CRC that are suitable for SEMS placement (Table 4).
There were some limitations to this meta-analysis. First, the definition of bowel obstruction was obscure. Only six studies specified that the obstructions were diagnosed according to clinical or radiological signs [20–23, 25, 26]. Second, this meta-analysis was limited to primary CRC. SEMS might be a promising palliative strategy for bowel obstructions caused by other malignancies [41–43]. Third, the heterogeneity among the studies included in this analysis was high in relation to the analysis of early complications, mortality, and stoma creation. Although this should be taken into consideration, the heterogeneities were not large enough to negate the results from this meta-analysis.
Given the aforementioned benefits of SEMS, we believe that SEMS should be the first-choice palliative strategy for unresectable CRC patients with bowel obstructions.
References
Ohman U (1982) Colorectal carcinoma in patients with ulcerative colitis. Am J Surg 144:344–349
Deans GT, Krukowski ZH, Irwin ST (1994) Malignant obstruction of the left colon. Br J Surg 81:1270–1276
Tack J, Gevers AM, Rutgeerts P (1998) Self-expandable metallic stents in the palliation of rectosigmoidal carcinoma: a follow-up study. Gastrointest Endosc 48:267–271
Camunez F, Echenagusia A, Simo G et al (2000) Malignant colorectal obstruction treated by means of self-expanding metallic stents: effectiveness before surgery and in palliation. Radiology 216:492–497
de Gregorio MA, Mainar A, Tejero E et al (1998) Acute colorectal obstruction: stent placement for palliative treatment–results of a multicenter study. Radiology 209:117–120
Zhang Y, Shi J, Shi B et al (2012) Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: a meta-analysis. Surg Endosc 26:110–119
Cole SJ, Boorman P, Osman H et al (2000) Endoluminal stenting for relief of colonic obstruction is safe and effective. Colorectal Dis 2:282–287
Fernandez LR, Pinto I, Paul L et al (1999) Self-expanding prostheses as a palliative method in treating advanced colorectal cancer. Int Surg 84:159–162
Castillo JJ, Dalia S, Pascual SK (2010) Association between red blood cell transfusions and development of non-Hodgkin lymphoma: a meta-analysis of observational studies. Blood 116:2897–2907
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions, version 5.1.0. The Cochrane Collaboration. doi:10.1002/9780470712184.fmatter
Fiori E, Lamazza A, Schillaci A et al (2012) Palliative management for patients with subacute obstruction and stage IV unresectable rectosigmoid cancer: colostomy versus endoscopic stenting: final results of a prospective randomized trial. Am J Surg 204:321–326
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses BMJ 327:557–560
Sterne JA, Egger M (2001) Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 54:1046–1055
Sterne JA, Gavaghan D, Egger M (2000) Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 53:1119–1129
Tierney JF, Stewart LA, Ghersi D et al (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16
Law WL, Choi HK, Lee YM et al (2004) Palliation for advanced malignant colorectal obstruction by self-expanding metallic stents: prospective evaluation of outcomes. Dis Colon Rectum 47:39–43
Carne PW, Frye JN, Robertson GM et al (2004) Stents or open operation for palliation of colorectal cancer: a retrospective, cohort study of perioperative outcome and long-term survival. Dis Colon Rectum 47:1455–1461
Ptok H, Marusch F, Steinert R et al (2006) Incurable stenosing colorectal carcinoma: endoscopic stent implantation or palliative surgery? World J Surg 30:1481–1487. doi:10.1007/s00268-005-0513-z
Karoui M, Charachon A, Delbaldo C et al (2007) Stents for palliation of obstructive metastatic colon cancer: impact on management and chemotherapy administration. Arch Surg 142:619–623 discussion 623
Faragher IG, Chaitowitz IM, Stupart DA (2008) Long-term results of palliative stenting or surgery for incurable obstructing colon cancer. Colorectal Dis 10:668–672
Suarez J, Jimenez J, Vera R et al (2010) Stent or surgery for incurable obstructive colorectal cancer: an individualized decision. Int J Colorectal Dis 25:91–96
Vemulapalli R, Lara LF, Sreenarasimhaiah J et al (2010) A comparison of palliative stenting or emergent surgery for obstructing incurable colon cancer Dig Dis Sci 55:1732–1737
White SI, Abdool SI, Frenkiel B et al (2011) Management of malignant left-sided large bowel obstruction: a comparison between colonic stents and surgery. ANZ J Surg 81:257–260
Lee HJ, Hong SP, Cheon JH et al (2011) Long-term outcome of palliative therapy for malignant colorectal obstruction in patients with unresectable metastatic colorectal cancers: endoscopic stenting versus surgery. Gastrointest Endosc 73:535–542
Lee WS, Baek JH, Kang JM et al (2012) The outcome after stent placement or surgery as the initial treatment for obstructive primary tumor in patients with stage IV colon cancer. Am J Surg 203:715–719
Khot UP, Lang AW, Murali K et al (2002) Systematic review of the efficacy and safety of colorectal stents. Br J Surg 89:1096–1102
Xinopoulos D, Dimitroulopoulos D, Theodosopoulos T et al (2004) Stenting or stoma creation for patients with inoperable malignant colonic obstructions? Results of a study and cost-effectiveness analysis Surg Endosc 18:421–426
Maruthachalam K, Lash GE, Shenton BK et al (2007) Tumour cell dissemination following endoscopic stent insertion. Br J Surg 94:1151–1154
Johnson R, Marsh R, Corson J et al (2004) A comparison of two methods of palliation of large bowel obstruction due to irremovable colon cancer. Ann R Coll Surg Engl 86:99–103
Bhardwaj R, Parker MC (2003) Palliative therapy of colorectal carcinoma: stent or surgery? Colorectal Dis 5:518–521
Suzuki N, Saunders BP, Thomas-Gibson S et al (2004) Colorectal stenting for malignant and benign disease: outcomes in colorectal stenting. Dis Colon Rectum 47:1201–1207
Aviv RI, Shyamalan G, Watkinson A et al (2002) Radiological palliation of malignant colonic obstruction. Clin Radiol 57:347–351
Meisner S, Hensler M, Knop FK et al (2004) Self-expanding metal stents for colonic obstruction: experiences from 104 procedures in a single center. Dis Colon Rectum 47:444–450
Small AJ, Baron TH (2008) Comparison of Wallstent and Ultraflex stents for palliation of malignant left-sided colon obstruction: a retrospective, case-matched analysis. Gastrointest Endosc 67:478–488
van Hooft JE, Fockens P, Marinelli AW et al (2008) Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy 40:184–191
Nagula S, Ishill N, Nash C et al (2010) Quality of life and symptom control after stent placement or surgical palliation of malignant colorectal obstruction. J Am Coll Surg 210:45–53
Nugent KP, Daniels P, Stewart B et al (1999) Quality of life in stoma patients. Dis Colon Rectum 42:1569–1574
Sideris L, Zenasni F, Vernerey D et al (2005) Quality of life of patients operated on for low rectal cancer: impact of the type of surgery and patients’ characteristics. Dis Colon Rectum 48:2180–2191
Krouse R, Grant M, Ferrell B et al (2007) Quality of life outcomes in 599 cancer and non-cancer patients with colostomies. J Surg Res 138:79–87
Pothuri B, Guirguis A, Gerdes H et al (2004) The use of colorectal stents for palliation of large-bowel obstruction due to recurrent gynecologic cancer. Gynecol Oncol 95:513–517
Shin SJ, Kim TI, Kim BC et al (2008) Clinical application of self-expandable metallic stent for treatment of colorectal obstruction caused by extrinsic invasive tumors. Dis Colon Rectum 51:578–583
Caceres A, Zhou Q, Iasonos A et al (2008) Colorectal stents for palliation of large-bowel obstructions in recurrent gynecologic cancer: an updated series. Gynecol Oncol 108:482–485
Conflict of interest
All authors have received none were declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, H., Okabayashi, K., Tsuruta, M. et al. Self-Expanding Metallic Stents Versus Surgical Intervention as Palliative Therapy for Obstructive Colorectal Cancer: A Meta-analysis. World J Surg 39, 2037–2044 (2015). https://doi.org/10.1007/s00268-015-3068-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3068-7