Abstract
Background
Pancreatectomy with regional lymphadenectomy remains the only curative treatment option for pancreatic cancer. There is no clear consensus on what type of adjuvant therapy should be used for patients with pancreatic cancer.
Objective
Our objective was to retrospectively evaluate whether postoperative adjuvant chemotherapy using S-1 is clinically beneficial in managing resectable pancreatic cancer.
Methods
Patients were divided into three groups: those undergoing surgery alone, those receiving gemcitabine infusion, and those receiving S-1 orally.
Results
Of 189 studied patients, the median overall survival was 15.0 months after surgery alone, 33.0 months in the gemcitabine group, and 45.0 months in patients receiving S-1. A multivariate analysis identified regional lymph node metastasis, positive surgical margins, and absence of adjuvant chemotherapy as independent negative prognostic factors. S-1 was not inferior to gemcitabine in terms of survival outcomes and showed a favorable hazard ratio compared with gemcitabine in the subsets of patients with positive vascular invasion.
Conclusions
There was no difference between adjuvant chemotherapy with S-1 and gemcitabine in overall survival for patients with curative pancreatic cancer. Our results suggested that S-1 can be used as a second agent to gemcitabine after surgical resection for ordinary adenocarcinoma of the pancreas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic adenocarcinoma is a highly aggressive and often fatal human malignancy [1–3]. Pancreatectomy with regional lymphadenectomy remains the only curative treatment option for pancreatic cancer, although the extent of lymphadenectomy is of no clinical benefit according to randomized studies [4–6]. However, even when surgery is a treatment option for pancreatic cancer, the 5 year survival rate rises to only around 20.3 % [7–9]. This poor prognosis is attributed to a high incidence of local recurrence and the development of distant metastases. Over the last decade, adjuvant therapy for pancreatic carcinoma has become an accepted recommendation, with current standards reflecting the use of single-agent gemcitabine or modulated fluoropyrimidine therapy [10–12]. Current major questions include what kind of chemotherapy impacts on overall survival, accepting a proven impact on local disease control, and whether use of a second agent following gemcitabine in the adjuvant setting improves outcome.
Recent studies have demonstrated that fluorouracil/leucovorin plus irinotecan plus oxaliplatin (FOLFIRINOX), a gemcitabine-free combination regimen, provided a clear survival benefit compared with gemcitabine for patients with metastatic pancreatic cancer, with a performance status of 0 or 1 [13]. In Japan, clinical trials of S-1 (TS-1; Taiho Pharmaceutical, Tokyo, Japan) have been conducted since the early 2000 s for patients with pancreatic cancer. Phase II studies of S-1 as first-line therapy for unresectable pancreatic cancer resulted in a good response rate of 21.1–37.5 % [14, 15]. Consequently, S-1 was approved for the indication of pancreatic cancer in Japan in 2006. Furthermore, GEST (gemcitabine and S-1 Trial) verified the comparability of S-1 to gemcitabine, supporting S-1 as a first-line therapy option for patients with unresectable pancreatic cancer [16]. However, the impact of S-1 as a second agent to gemcitabine after surgical resection for ordinary adenocarcinoma of the pancreas is unclear. In the present study, we retrospectively evaluated whether postoperative adjuvant chemotherapy using S-1 is clinically beneficial in managing resectable pancreatic cancer.
Patients and methods
Patients
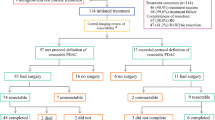
The initial diagnosis of pancreatic cancer was made following imaging and was confirmed by pathological analysis. We retrospectively reviewed the surgical pathology database of Kochi Health Sciences Center and Kochi Medical School to identify patients who underwent resection for pancreatic neoplasms from April 2006 to December 2011. Clinical characteristics evaluated included age, gender, part of the tumor, size of the tumor, operative procedures, pathological data, and postoperative chemotherapy. Location of the pancreatic cancer, size of the tumor, stage, degree of differentiation, vascular invasion, lymphatic permeation, perineural invasion, and lymph node metastasis were assessed according to the TNM committee of the American Joint Committee on Cancer-Union for International Cancer Control (UICC) staging system [17]. Our department followed the prognosis of each case and obtained accurate outcome details. This series included patients with ordinary invasive ductal carcinoma of the pancreas and excluded those with invasive pancreatic carcinoma derived from both intraductal papillary mucinous neoplasm and mucinous cystic neoplasm, acinar cell carcinoma, or adenosquamous cell carcinoma. The study was approved by the ethics committee of the Kochi Health Sciences Center and Kochi Medical School. All patients provided written informed consent.
Treatment
After curative surgical resection, patients were divided into three groups: those treated by surgery alone, those who received gemcitabine infusion, and those who received oral S-1. Patients allocated to gemcitabine alone as an adjuvant chemotherapy after curative surgical management received 800 mg/m2 intravenously over 30 min on day 1, 8, and 15 of a 28 day cycle. Patients allocated to S-1 alone as an adjuvant chemotherapy after curative surgical management received S-1 orally twice daily at a dose according to the body surface area (BSA) (<1.25 m2, 60 mg/day; >1.25 to <1.5 m2, 80 mg/day; >1.5 m2, 100 mg/day) on days 1 through 14 of a 21 day cycle. All patients received adjuvant chemotherapy using either gemcitabine or S-1 within 2 months after curative surgical resection for pancreatic carcinoma.
Assessments
This is a study of prospectively collected, retrospectively analyzed data analyzed by a biostatistician (TI). Overall survival, defined as time from date of pancreatic resection to date of death from any cause, was investigated. The prognostic factors after intent-to-cure surgical resection for pancreatic adenocarcinoma were evaluated by assessing age, gender, location of the tumor, tumor size, type of operation, pathological findings, adjuvant chemotherapy, and UICC staging system. Furthermore, we evaluated whether postoperative adjuvant chemotherapy using S-1 is clinically beneficial for the management of resectable pancreatic cancer.
Statistics
Survival curves were generated using the Kaplan–Meier method and compared using the log-rank test [18]. Patients alive as of 31 December 2012 were censored at the time of follow-up. A multivariate Cox regression analysis identified factors that were independently associated with mortality [19]. Differences in proportions were evaluated by Pearson’s Chi-square test. A p value <0.05 was considered statistically significant. All analyses were performed using SPSS® (SPSS; Chicago, IL, USA).
Results
Patients
A total of 189 patients who underwent surgery as an initial treatment for pancreatic carcinoma between April 2006 and December 2011 at Kochi Health Sciences Center and Kochi Medical School were studied. Of these patients, 102 were men and 87 were women, ranging in age from 34 to 88 years (mean 68.4) (Table 1). Curative resection was the operative aim for all patients. No significant differences were observed in age, gender, tumor location, or pathological background among the three groups. In the postoperative pathological stage, according to the UICC classification, patients who received surgery alone were surgically treated at an earlier stage than those in the gemcitabine and S-1 groups (Table 1). The type of operation was not significantly different between the three groups; however, there was a significant difference in portal vein transection, with combined resection performed in 7.7 % of patients not subjected to adjuvant chemotherapy, 32.3 % of patients administered gemcitabine, and 37.1 % of patients administered S-1 (Table 1).
Study treatment
Patients in adjuvant groups (gemcitabine and S-1) received adjuvant chemotherapy immediately after the curative surgical resection for pancreatic adenocarcinoma and chemotherapy using gemcitabine drip infusion or S-1 oral administration, and this was continued for as long as possible. The median duration of treatment was 24.0 months in the gemcitabine group and 20.0 months in the S-1 group. The main reasons for treatment discontinuation were recurrent disease (36 patients [58.1 %] in the gemcitabine group and 26 patients [41.9 %] in the S-1 group) or adverse events (eight patients [12.9 %] in the gemcitabine group and one patient [1.6 %] in the S-1 group). In this study, the rate of treatment withdrawal due to adverse events in the gemcitabine group was greater than that in the S-1 group (p = 0.038).
Survival
There was no mortality in this series. Patient follow-up as of December 2011 ranged from 0.5–130.0 months, with a median of 18.0 months (mean 24.8). The analysis of overall survival was based on 114 deaths (60.3 %) among the 189 patients. Overall 1-, 3-, and 5 year survival rates after surgery were 78.0, 42.9, and 31.6 %, respectively. Median overall survival of patients who underwent curative surgical resection for pancreatic adenocarcinoma was 27.5 months (Fig. 1). Comparing the survival rate among the subgroups identified by each predictive factor identified the following factors as significantly associated with a poor outcome after surgery: positive lymph node metastases; positive surgical margin; positive lymphatic permeation; advanced tumor status (stage IIB and III) according to UICC classification; and no postoperative adjuvant chemotherapy (Fig. 1). Multivariate analysis revealed the following factors to be independently associated with poor survival: positive surgical margin; presence of metastatic lymph node; and no adjuvant chemotherapy (Table 2). Although the postoperative pathological values of tumor stage according to the UICC guidelines and presence of vascular invasion were significant prognostic factors by univariate analysis, these factors were not significant in the multivariate context. The size of the tumor, location of the pancreatic carcinoma, type of operation, pathological differentiation, and the invasion to portal vein by the tumor were also not significant as prognostic factors.
Kaplan–Meier estimates of a overall survival, as well as survival rates compared with b pathological findings, c lymph node metastases, d surgical margin, e lymphatic permeation, f vascular invasion, g UICC classification staging system, and h adjuvant chemotherapy. LN lymph node, Mod moderately differentiated adenocarcinoma, Poor poorly differentiated adenocarcinoma, UICC Union for International Cancer Control, Well well differentiated adenocarcinoma
Subgroup analysis
The subgroup analyses of survival according to postoperative pathological characteristics showed significant differences between surgery alone and adjuvant chemotherapy in those patients with positive surgical margins, positive lymph node metastases, and final UICC classification system stage IIA and IIB (Fig. 2). Although S-1-treated patients showed a favorable outcome compared with the gemcitabine group in the subsets of patients, there was a significant difference among those with positive surgical margins (Fig. 2). In addition, the Forest plots of S-1 treatment effects on overall survival in the subgroup analyses showed a favorable hazard ratio (HR), with both S-1 and gemcitabine in the subsets of patients with positive vascular invasion; however, S-1 failed to improve overall survival at a statistically significant level compared with gemcitabine (HR 0.56; 65 % confidence interval [CI] 0.27–1.13; p = 0.106) (Fig. 3).
Discussion
In this study of pancreatic cancer patients, the overall survival curves were virtually identical between those administered S-1 and those administered gemcitabine for adjuvant chemotherapy. Toxicity profiles of these two drugs differed slightly in that gemcitabine tended to show hepatic toxicity, although both S-1 and gemcitabine were generally well tolerated. Furthermore, the subgroup analyses demonstrated that S-1 and gemcitabine were equivalent. Overall, our results suggested that S-1 could be used in first-line adjuvant chemotherapy as a convenient oral alternative for pancreatic adenocarcinoma after curative surgical resection.
In a relatively large multicenter phase III study from Japan in patients with stages I–III pancreatic cancer, Uesaka et al. [20] demonstrated both equivalence and superiority of S-1 compared with gemcitabine in the adjuvant setting. This phase III study thus sought to clarify the comparison of S-1 with gemcitabine as adjuvant chemotherapy for resected pancreatic cancer with respect to overall survival. The toxicities were comparable in both arms, with less myelosuppression in patients receiving S-1. A longer follow-up (such as 5 years) is warranted to ascertain whether the superiority of S-1 over gemcitabine lasts beyond 2 years and translates into long-term survival [20]. The pancreatic cancer community throughout the world is awaiting the final publication of this study, which will ultimately inform study designs, settings, participants, methodologies, outcome measures, results, and the study relevance to patients with pancreatic cancer [21].
At the time of evaluation in this study, the participants included only patients with resectable pancreatic adenocarcinoma. Interestingly, although the surgery alone group consisted of patients with early-stage pancreatic cancer, overall survival was worse in that group than in either the S-1 or gemcitabine groups. Our study thus suggested that adjuvant chemotherapy should be adopted as a standard treatment after surgical resection of pancreatic carcinoma, even if the pancreatic cancer is diagnosed as UICC classification stage I or II. In addition, the lack of a significant difference in overall survival between gemcitabine and S-1 indicates that gemcitabine and S-1 could be used sequentially rather than concurrently. Moreover, the S-1 group showed a favorable HR compared with gemcitabine for overall survival in patients with positive vascular invasion after curative surgical management. We therefore speculate that S-1 adjuvant chemotherapy could be a viable option in such patients, depending on the profile of the patients and further investigations.
A major limitation of our study is uncertainty over whether our results could be extrapolated to Western patients, because the pharmacokinetics and pharmacodynamics of S-1 may differ between Westerners and East Asians [22, 23]. Although S-1 is available for pancreatic carcinoma only in Japan, we would suggest that S-1 could be tested in Western patients with careful monitoring and appropriate adjustment of the dose. Another potential limitation is that the dosage of both gemcitabine and S-1 in this study was relatively small, while the periods of adjuvant chemotherapy administration were lengthy. To date, the optimal timing of adjuvant chemotherapy and administration duration following the surgical resection of pancreatic carcinoma with respect to prognosis remains unclear [24], although adjuvant chemotherapy is standard care after curative surgical resection for pancreatic carcinoma [25, 26]. Our results herein suggest that the prospective large randomized controlled trials should be reprogrammed to evaluate both dose and periods of adjuvant chemotherapy after curative surgical resection for pancreatic carcinoma, considering the balance between cost effectiveness and patient prognosis.
Conclusion
This study verified the equivalent value of S-1 and gemcitabine, and supports the use of S-1 as a second agent to gemcitabine after surgical resection for ordinary adenocarcinoma of the pancreas. S-1-based regimens for treating pancreatic cancer should be developed in the future to improve the management of this formidable disease.
References
Oettle H, Post S, Neuhaus P et al (2007) Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297:267–277
Neoptolemos JP, Stocken DD, Friess H et al (2004) European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350:1200–1210
Choti MA (2004) Adjuvant therapy for pancreatic cancer–the debate continues. N Engl J Med 350:1249–1251
Michalski CW, Kleeff J, Wente MN et al (2007) Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg 94:265–273
Konstantinidis IT, Warshaw AL, Allen JN et al (2013) Pancreatic ductal adenocarcinoma: is there a survival difference for r1 resections versus locally advanced unresectable tumors? what is a “true” r0 resection? Ann Surg 257:731–736
Mollberg N, Rahbari NN, Koch M et al (2011) Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg 254:882–893
Jemal A, Siegel R, Ward E et al (2006) Cancer statistics. CA Cancer J Clin 56:106–130
Birkmeyer JD, Stukel TA, Siewers AE et al (2003) Surgeon volume and operative mortality in the United States. N Engl J Med 349:2117–2127
Yeo CJ, Cameron JL, Lillemoe KD et al (2002) Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 236:355–366
Neoptolemos JP, Stocken DD, Bassi C et al (2010) European study group for pancreatic cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 304:1073–1081
Regine WF, Winter KA, Abrams RA et al (2008) Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 299:1019–1026
Berger AC, Garcia M Jr, Hoffman JP et al (2008) Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol 26:5918–5922
Conroy T, Desseigne F, Ychou M et al (2011) Groupe tumeurs digestives of unicancer; prodige intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Ueno H, Okusaka T, Ikeda M et al (2005) A phase I study of combination chemotherapy with gemcitabine and oral S-1 for advanced pancreatic cancer. Oncology 69:421–427
Okusaka T, Funakoshi A, Furuse J et al (2008) A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol 61:615–621
Ueno H, Ioka T, Ikeda M et al (2013) Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 31:1640–1648
Cascinu S, Jelic S (2009) ESMO guidelines. Working group pancreatic cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 20(Suppl 4):37–40
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457
Cox DR (1972) Regression models and life tables. J R Stat Soc 187:34B
Uesaka K, Fukutomi A, Boku N et al (2012) Randomized phase III trial of adjuvant chemotherapy with gemcitabine versus S-1 for patients with resected pancreatic cancer (JASPAC-01 study). J Clin Oncol 30 (Suppl 34; abstract 145)
Chaulagain CP, Ng J, Goodman MD et al (2013) Adjuvant therapy of pancreatic cancer. JOP 14:119–122
Haller DG, Cassidy J, Clarke SJ et al (2008) Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol 26:2118–2123
Chuah B, Goh BC, Lee SC et al (2011) Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci 102:478–483
Murakami Y, Uemura K, Sudo T et al (2013) Early initiation of adjuvant chemotherapy improves survival of patients with pancreatic carcinoma after surgical resection. Cancer Chemother Pharmacol 71(2):419–429
Murakami Y, Uemura K, Sudo T et al (2012) Long-term results of adjuvant gemcitabine plus S-1 chemotherapy after surgical resection for pancreatic carcinoma. J Surg Oncol 106:174–180
Neoptolemos JP, Cunningham D, Friess H et al (2003) Adjuvant therapy in pancreatic cancer: historical and current perspectives. Ann Oncol 14:675–692
Acknowledgment
This work was supported by the Kochi Organization for Medical Reformation and Renewal Grants.
Conflict of interest
The authors state that there are no conflicts of interest or financial disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okabayashi, T., Shima, Y., Iwata, J. et al. S-1 vs. Gemcitabine as an Adjuvant Therapy after Surgical Resection for Ductal Adenocarcinoma of the Pancreas. World J Surg 38, 2986–2993 (2014). https://doi.org/10.1007/s00268-014-2703-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-014-2703-z