Abstract
Background
There is considerable interest in a neoadjuvant approach for resectable pancreatic ductal adenocarcinoma (PDAC). This study evaluated perioperative gemcitabine + erlotinib (G+E) for resectable PDAC.
Methods
A multicenter, cooperative group, single-arm, phase II trial was conducted between April 2009 and November 2013 (ACOSOG Z5041). Patients with biopsy-confirmed PDAC in the pancreatic head without evidence of involvement of major mesenteric vessels (resectable) were eligible. Patients (n = 123) received an 8-week cycle of G+E before and after surgery. The primary endpoint was 2-year overall survival (OS), and secondary endpoints included toxicity, response, resection rate, and time to progression. Resectability was assessed retrospectively by central review. The study closed early due to slow accrual, and no formal hypothesis testing was performed.
Results
Overall, 114 patients were eligible, consented, and initiated protocol treatment. By central radiologic review, 97 (85%) of the 114 patients met the protocol-defined resectability criteria. Grade 3+ toxicity was reported in 60% and 79% of patients during the neoadjuvant phase and overall, respectively. Twenty-two of 114 (19%) patients did not proceed to surgery; 83 patients (73%) were successfully resected. R0 and R1 margins were obtained in 67 (81%) and 16 (19%) resected patients, respectively, and 54 patients completed postoperative G+E (65%). The 2-year OS rate for the entire cohort (n = 114) was 40% (95% confidence interval [CI] 31–50), with a median OS of 21.3 months (95% CI 17.2–25.9). The 2-year OS rate for resected patients (n = 83) was 52% (95% CI 41–63), with a median OS of 25.4 months (95% CI 21.8–29.6).
Conclusions
For resectable PDAC, perioperative G+E is feasible. Further evaluation of neoadjuvant strategies in resectable PDAC is warranted with more active systemic regimens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
For patients with resectable pancreatic ductal adenocarcinoma (PDAC), postoperative adjuvant therapy remains the standard approach.1,2,3 Neoadjuvant chemotherapy for PDAC offers potential advantages, including early treatment of micrometastatic disease, delivery of systemic therapy to a greater number of patients, and better patient selection for surgery. In addition, with this approach, patients with rapidly progressive disease could be spared unnecessary surgery. There is growing evidence to support a neoadjuvant approach for resectable PDAC.4
Resection for localized PDAC is indicated when a negative gross margin is anticipated. Over the past decade, the nature of pancreatectomy has changed due to the increased use of en bloc vascular resections. As a result, the ability to classify tumors as resectable versus unresectable is best considered on a spectrum. Various classification systems are in use, but, in general, resectable PDAC does not abut or encase the major mesenteric vasculature. Unresectable tumors have arterial encasement of the celiac and/or superior mesenteric arteries. Borderline resectable tumors may abut or encase portovenous structures and/or have abutment of the celiac and/or superior mesenteric arteries or their tributaries.5 Most studies to date have reported the use of neoadjuvant strategies in borderline and/or locally advanced PDAC.4,6,7,8,9,10,11
In 2008, the time of inception of this study, gemcitabine + erlotinib (G+E) was regarded as an active regimen for PDAC. The National Cancer Institute (NCI) of Canada trial PA.3 evaluated G+E versus single-agent gemcitabine in a phase III trial with 569 unresectable patients. A small but statistically significant increase in 1-year overall survival (OS) was observed with G+E when compared with gemcitabine (23 vs. 17%).12 Herein, we report the results of a single-arm, phase II study of perioperative G+E and pancreaticoduodenectomy (PD) in patients with resectable PDAC.
Methods
The American College of Surgeons Oncology Group (ACOSOG) Z5041 study was an NCI-sponsored, cooperative group, prospective, multicenter, non-randomized, single-arm, phase II trial of perioperative G+E for resectable PDAC requiring PD (ACOSOG is now part of Alliance for Clinical Trials in Oncology). The inclusion criteria included biopsy-confirmed PDAC arising in the pancreatic head; potentially resectable disease based on radiologic criteria [i.e. no evidence of tumor extension to the celiac, hepatic, or superior mesenteric arteries; no evidence of tumor encasement (> 180° contact between the vein and the tumor) or occlusion of the superior mesenteric vein (SMV) or the SMV/portal vein confluence; and no evidence of visceral or peritoneal metastases]; Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1; adequate hematologic, renal, and hepatic function; < 15% premorbid weight loss; no prior therapy for pancreatic cancer or prior epidermal growth factor receptor therapy; no prior malignancy in the past 5 years; and absence of active infection. Each participant signed an Institutional Review Board-approved, protocol-specific informed consent document in accordance with federal and institutional guidelines. The trial was registered with ClinicalTrials.gov (NCT00733746).
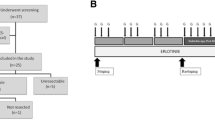
Patients received an 8-week cycle of G+E, both pre- and postoperatively. Gemcitabine 1000 mg/m2 was administered on days 1, 8, 15, 29, 36, and 43, and erlotinib 100 mg was administered orally on days 1–43 of each cycle (electronic supplementary Fig. 1). Surgery was performed 3–6 weeks after the neoadjuvant phase following restaging. At the time of surgery, the abdominal cavity was explored and PD was performed after resectability was confirmed. Postoperative therapy was initiated within 5–10 weeks after PD. Patients were followed with imaging and CA19-9 every 3 months for 2 years, and then every 6 months for 4 years or until disease relapse. The primary endpoint was 2-year OS, defined as the proportion of patients alive 2 years from the date of registration. Secondary endpoints included response rate, resection rate, toxicity, and relapse/progression-free survival (PFS). Radiologic response rate was defined as the proportion of patients with complete response (CR) or partial response (PR) per RECIST 1.1 criteria.13 Resection rate was defined as the proportion of patients who had PD, and PFS was defined as the time from the date of registration to the date of disease recurrence/progression or death due to any cause, whichever occurred first. Protocol-defined resectability status and radiologic response to treatment were confirmed retrospectively by central radiologic review for quality assurance purposes.
CONSORT diagram.
 Central review completed retrospectively for quality assurance. *Reasons for not completing the protocol treatment included adverse events, disease progression, refused further treatment, performance status, physician decision, and alternate treatment sought. CONSORT Consolidated Standards of Reporting Trials, PDAC pancreatic ductal adenocarcinoma
Central review completed retrospectively for quality assurance. *Reasons for not completing the protocol treatment included adverse events, disease progression, refused further treatment, performance status, physician decision, and alternate treatment sought. CONSORT Consolidated Standards of Reporting Trials, PDAC pancreatic ductal adenocarcinoma
A minimum of 78 patients, defined as those eligible for resection per retrospective central review, consenting and undergoing PD with R0/R1 intent (the original analysis population for the primary endpoint) were required to provide 90% power (one-sided alpha 0.1) to detect an improvement in 2-year OS of 15% (from 45 to 60%). The study was closed early due to slow accrual. No formal hypothesis testing was performed due to insufficient power. Sensitivity analysis was performed using the cohort defined as resectable using retrospective central review radiologic definitions. A full description of the statistical methods can be found in the Electronic Supplementary Appendix.
Results
Study Cohort
From April 2009 to November 2013, 123 patients were registered from 31 centers. Of these, 114 (93%) consented, were deemed eligible, and were treated (Fig. 1). Demographic characteristics are shown in Table 1. A preplanned interim analysis was performed in May 2013. Based on the interim efficacy decision rules, the regimen did not cross the preset futility thresholds, and study accrual continued. The study was closed early due to slow accrual, thus no formal hypothesis testing was performed. The median follow-up was 4 years.
Neoadjuvant Phase
Neoadjuvant chemotherapy was administered in 114 patients. The median dose percentage of preoperative chemotherapy received was 98.2% for G and 100% for E. Grade 3+ adverse events (AEs) occurred in 68/114 patients (60%) (Table 2). The most common AEs were elevated alanine aminotransferase (17%) and aspartate aminotransferase (16%). There were no Grade 5 AEs in the neoadjuvant phase.
Following the neoadjuvant phase, 22/114 patients (19%) did not go on to surgery due to disease progression (n = 12), AEs/medical problems (n = 4), declined surgery (n = 3), or physician decision (n = 3) (Table 2). Response rate after neoadjuvant therapy was available for 107 patients; an objective radiologic response (CR+PR) was seen in six patients (6%).
Surgery
Surgery was undertaken in 92/114 patients (80.7%), and 83 (90%) underwent PD (Table 3); nine patients were found to have advanced disease at the time of operation. The overall resectability rate in the eligible and treated population was 73% (83/114). Concomitant venous resection was performed in 30/83 patients (36%). Median tumor size was 2.7 cm (Q1, Q3 = 2.1 cm, 3.6 cm), and 71% of patients had positive lymph nodes. An R0 resection was completed in 67/83 patients (81%), and 16 patients (19%) had an R1 resection. Pathologic treatment effect was graded using the methods of Staley et al.14 and was available for 69 patients; six patients (9%) had major/complete response (grade 3 or 4), 17 (25%) had partial response (grade 2a or 2b), and 46 (67%) had minor or no response (grade 1).
Perioperative outcomes were available for 86/92 patients. Grade 3+ AEs were reported in 50/86 patients (58%) (Table 2). There were two deaths within 90 days of surgery—one as a result of aspiration and one due to myocardial ischemia. Pancreatic fistulae occurred in four patients (5%).
Adjuvant Phase
Following PD, 68/83 patients (82%) commenced postoperative therapy. The most common reason for G+E discontinuation was AE/complication (15%). The median dose percentage of adjuvant chemotherapy received was 99.5% for G and 100% E. In the adjuvant phase, 31/68 patients (46%) encountered grade 3+ AEs (Table 2). The total number of patients experiencing grade 3+ AEs during the entire study was 90/114 (79%). Of the entire study cohort (n = 114), 54 patients (47%) completed all phases of the protocol treatment.
Survival Data
OS 2 years following registration was 40% [46/114, 95% confidence interval (CI) 31–50] by intention-to-treat, 48% (44/92, 95% CI 37–39) for those who underwent surgery, 52% (43/83, 95% CI 41–63) for those who were resected, and 59% (32/54, 95% CI 45–72) for patients who completed all protocol therapy. The median survival for these categories was 21.3 (95% CI 17–26), 23.2 (95% CI 21–29), 25.4 (95% CI 22–30), and 27.3 (95% CI 23–40) months, respectively (Fig. 2).
The median PFS was 10.8 months (95% CI 9–15) by intention-to-treat, 12.0 months (95% CI 11–18) for those who underwent surgery, 14.5 months (95% CI 11–19) for resected patients, and 14.8 months (95% CI 11–24) for those who completed all protocol therapy. Following resection, 72 of the 83 resected patients (87%) had developed recurrent disease at the time of last follow-up (Table 3).
Sensitivity Analysis
By retrospective central radiologic review, 97/114 patients (85%) met the protocol resectability criteria, and 17 had localized disease beyond protocol definitions. The most common reason for exceeding protocol definition was vascular abutment and/or encasement (8/17, 47%).
Sensitivity analysis was performed for the 97 patients who met the retrospective central review definition of resectable disease. Using this definition, 81 patients (84%) proceeded to surgery, and seven were found to have advanced disease at the time of surgery. PD was performed in 74/97 patients (76%). Surgery was not performed in 16 patients following the neoadjuvant phase, most commonly due to disease progression. The 2-year OS was 43%, with a median OS of 21.8 months (95% CI 19–27) and PFS of 11.6 months (95% CI 10–26). The resection rate was 76% (75/97), with R0 and R1 rates of 81% (60/74) and 19% (14/74), respectively.
In the 74 patients who met protocol-defined resectability on retrospective central review and underwent PD with R0/R1 intent (i.e. the defined evaluable population for analysis of the primary endpoint), the 2-year OS for resected patients was 54%, with a median OS of 25.9 months (95% CI 22–31). Median PFS for this group was 14.5 months (95% CI 11–19). The targeted 78 evaluable patients needed to power the study was not met and thus no formal hypothesis testing was performed.
Discussion
Proponents of neoadjuvant treatment for PDAC point to several possible benefits. For one, preoperative systemic treatment may identify patients with early metastatic disease resistant to chemotherapy who are unlikely to benefit from surgery. In addition, upfront systemic treatment can ensure that a greater proportion of patients receive multimodal therapy compared with a surgery-first approach. Currently, adjuvant chemotherapy following pancreatectomy is received by fewer than 70% of patients.15,16 The American Society of Clinical Oncology clinical practice guideline for PDAC recommends neoadjuvant therapy as an acceptable option.17
Few neoadjuvant studies have been undertaken in accurately staged patients with PDAC. Most studies have included a heterogeneous population of resectable, borderline, and/or locally advanced patients.4,6,7,8,9,11 As such, resectability status is not clear and outcomes are difficult to translate to patients who could be managed with upfront surgery. Interpretation is further complicated by the changing definitions of surgical resectability. Use of vascular reconstruction has increased, and thus patients previously considered unresectable now undergo routine resection. Despite the limitations of the data, most studies with neoadjuvant therapy with or without the addition of radiation report that treatment is tolerable. Analyses of fluoropyrimidine and/or gemcitabine-based regimens report a median OS of 21–27 months, with resectability rates of up to 80%.7,10,18,19,20,21
Our study incorporated a clear definition of radiologic resectability and a standardized approach to surgery. This included a protocol description of the extent of retroperitoneal dissection required along the edge of the uncinate process and lateral border of the superior mesenteric artery with frozen section evaluation of surgical margins. This is one of the few studies that prospectively addressed key surgical quality issues within the study design. In addition, this trial included central radiologic review, although logistic constraints meant that imaging was retrospectively interpreted. Our results highlight the difficulty in defining resectability and emphasize the importance of central review for resectability early in the study process. Lessons learned from this study have since been incorporated in successive cooperative studies. Another key strength of our study was that it is one of the earliest multicenter trials of neoadjuvant therapy for resectable PDAC to have been undertaken by a cooperative group.
We report that G+E was a tolerable regimen, with toxicity similar to other gemcitabine-based regimens administered postoperatively.2,22 There did not appear to be intra- or postoperative complications related to preoperative G+E. Postoperative adjuvant chemotherapy was initiated in 82% of the 83 patients who completed surgery. It is difficult to compare toxicity profiles between pre- and postoperative therapy; previous studies with postoperative therapy are prone to selection bias as they exclude patients at higher risk of toxicity, such as those with early metastatic disease, poor performance status, and surgical complications that preclude chemotherapy. These are precisely the circumstances where neoadjuvant therapy may prove optimal. Despite the theoretical advantages of neoadjuvant therapy with surgery, only 54/114 patients (47%) received all intended therapy. The ability to deliver multiple treatments in series, particularly when one of the intended treatments is a major operation, remains a concern. Our results support the feasibility of a perioperative strategy with a neoadjuvant phase; however, the role of neoadjuvant therapy in the resectable group of PDAC patients will require further trials to clarify.
There are several study limitations. Slow accrual led to early study closure, and, as a result, formal hypothesis testing for the primary endpoint was not possible. At the time of study inception, the use of neoadjuvant chemotherapy was novel in this group of patients, and the logistics of accruing and completing therapy in this group of patients was a challenge. In addition, as the study evolved, the choice of G+E likely limited enthusiasm for participation. G+E has only modest efficacy in the metastatic setting, and during the study period G+E was replaced by more efficacious metastatic regimens, including 5-fluorouracil + irinotecan + oxaliplatin + leucovorin (FOLFIRINOX) and gemcitabine + nab-paclitaxel.23,24 The randomized phase III PRODIGE-24 trial found FOLFIRINOX to be significantly more effective in the adjuvant setting than any prior regimen reported.25 Results are pending for the randomized, multicenter, phase III APACT trial of gemcitabine/nab-paclitaxel versus gemcitabine.26
Another limitation included the lack of real-time central review to confirm resectability status prior to study enrollment. When central review of resectability was performed retrospectively, 97/114 patients (85%) met the protocol definitions. The most common reason for exceeding resectability was tumor relationship to major vascular structures and/or inclusion of pancreatic neck tumors. However, sensitivity analysis of the 97 patients who met the resectability criteria by central review demonstrated similar PD rates as the entire cohort of 114 patients (73% vs. 76%). The 2-year OS for those who underwent PD in the entire cohort was 52%, versus 54% in central review-defined patients. It should be noted that our study did not incorporate radiotherapy, yet outcomes are reasonable. The role of radiotherapy for these patients remains to be defined.
Interest in neoadjuvant strategies for PDAC is increasing. Current NCI National Clinical Trials Network trials in this area have accrued briskly. Alliance is currently undertaking a study with neoadjuvant mFOLFIRINOX ± hypofractionated radiation (A021501) in the borderline setting,27 and has a phase III protocol of neoadjuvant versus adjuvant mFOLFIRINOX for patients with resectable PDAC in development.
Conclusions
Our results demonstrate the safety and feasibility of preoperative therapy with G+E in patients with upfront resectable PDAC. It is possible that this approach avoided a non-therapeutic PD in 27% of patients; however, it is unknown whether earlier surgical intervention could have been beneficial in this subset. Now that more active systemic regimens are available, this question can be addressed in future randomized trials of neoadjuvant versus adjuvant therapy to confirm the value of a neoadjuvant approach.
References
Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473–81.
Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. The Lancet. 2017;389(10073):1011–24.
Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. The Journal of the American Medical Association. 2010;304(10):1073–81
Van Tienhoven G, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): a randomized, controlled, multicenter phase III trial. Paper presented at the ASCO Annual Meeting, 1–5 Jun 2018; Chicago (IL).
Chatzizacharias NA, Tsai S, Griffin M, et al. Locally advanced pancreas cancer: staging and goals of therapy. Surgery. 2018;163(5):1053–62.
Kim EJ, Ben-Josef E, Herman JM, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer. 2013;119(15):2692–700.
Assifi MM, Lu X, Eibl G, Reber HA, Li G, Hines OJ. Neoadjuvant therapy in pancreatic adenocarcinoma: a meta-analysis of phase II trials. Surgery. 2011;150(3):466–73.
Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. 2015;22(4):1153–9.
Jensen EH, Armstrong L, Lee C, et al. Neoadjuvant interferon-based chemoradiation for borderline resectable and locally advanced pancreas cancer: a phase II pilot study. HPB (Oxford). 2014;16(2):131–9.
O’Reilly EM, Perelshteyn A, Jarnagin WR, et al. A single-arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg. 2014;260(1):142–8.
Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with folfirinox followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncology. 2018;4(7):963–9.
Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Staley CA, Cleary KR, Abbruzzese JL, et al. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas. 1996;12(4):373–80.
Labori KJ, Katz MH, Tzeng CW, et al. Impact of early disease progression and surgical complications on adjuvant chemotherapy completion rates and survival in patients undergoing the surgery first approach for resectable pancreatic ductal adenocarcinoma—A population-based cohort study. Acta oncologica. 2016;55(3):265–77.
Bergquist JR, Ivanics T, Shubert CR, et al. Type of resection (whipple vs. distal) does not affect the national failure to provide post-resection adjuvant chemotherapy in localized pancreatic cancer. Ann Surg Oncol. 2017;24(6):1731–8.
Khorana AA, Mangu PB, Berlin J, et al. Potentially curable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(21):2541–56.
Katz MH, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg. 2016;151(8):e161137.
Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20(8):2787–95.
Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14(7):2088–96.
de W March R, Talamonti MS, Baker MS, et al. Primary systemic therapy in resectable pancreatic ductal adenocarcinoma using mFOLFIRINOX: a pilot study. J Surg Oncol. 2018;117(3):354–62.
Neuhaus P, Riess H, Post S, et al. CONKO-001: final results of the randomized, prospective, multicenter phase III trial of adjuvant chemotherapy with gemcitabine versus observation in patients with resected pancreatic cancer (PC). J Clin Oncol. 2008;26(15 Suppl):LBA4504.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–406.
Margaret AT, Dana Backlund C, David G, et al. APACT: Phase III randomized trial of adjuvant treatment with nab-paclitaxel (nab-P) plus gemcitabine (Gem) versus Gem alone in patients (pts) with resected pancreatic cancer (PC). J Clin Oncol. 2016;34(4 Suppl):TPS473.
Katz MHG, Ou FS, Herman JM, et al. Alliance for clinical trials in oncology (ALLIANCE) trial A021501: preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer. 2017;17(1):505.
Acknowledgments
The authors would like to thank T.J. FitzGerald, MD, and staff at IROC Rhode Island [formerly the Quality Assurance Review Center (QARC)], especially Sandra Kessel and Deirdre Logan, for their support in acquisition of the diagnostic imaging and facilitation of the radiologic review. The following institutions enrolled patients to this study: University Health Network–Princess Margaret Cancer Centre, Laura and Isaac Perlmutter Cancer Center at NYU Langone, Vanderbilt University/Ingram Cancer Center, University of Chicago Comprehensive Cancer Center, UC Irvine Health/Chao Family Comprehensive Cancer Center, Medical College of Wisconsin, Memorial Sloan Kettering Medical Center, MD Anderson Cancer Center, West Virginia University Healthcare, University of Tennessee–Knoxville, Providence Portland Medical Center, Carolinas Medical Center/Levine Cancer Institute, UC San Diego Moores Cancer Center, Lakeland Regional Health Hollis Cancer Center, Ochsner Medical Center Jefferson, Saint Peter’s Health Partners, Piedmont Hospital, Henry Ford Hospital, University of Mississippi Medical Center, Kaiser Permanente Los Angeles Medical Center, Nebraska Methodist Hospital, Natalie Warren Bryant Cancer Center at Saint Francis, Loma Linda University Medical Center, Providence Sacred Heart Medical Center and Children’s Hospital, UNC Lineberger Comprehensive Cancer Center, Saint Luke’s University Hospital–Bethlehem Campus, University of Wisconsin Hospital and Clinics, Good Samaritan Hospital–Dayton, Miami Valley Hospital, Spartanburg Medical Center, and St. Vincent’s Medical Center.
Funding
Research reported in this publication was supported by the National Institutes of Health under award numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA180791, U10CA180836, U10CA180858, and U10CA180867, U24CA180803 (IROC), and U10CA29511 (QARC). This work was also supported in part by funds from OSI Pharmaceuticals, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wei, A.C., Ou, FS., Shi, Q. et al. Perioperative Gemcitabine + Erlotinib Plus Pancreaticoduodenectomy for Resectable Pancreatic Adenocarcinoma: ACOSOG Z5041 (Alliance) Phase II Trial. Ann Surg Oncol 26, 4489–4497 (2019). https://doi.org/10.1245/s10434-019-07685-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07685-1