Abstract
Background
We reported a novel technique of sentinel lymph node (SLN) identification using fluorescence imaging of indocyanine green injection. Furthermore, to obtain safe and accurate identification of SLN during surgery, we introduce the image overlay navigation surgery and evaluate its efficacy.
Methods
This study enrolled 50 patients with a tumors <2 cm in diameter. Initially, we obtained three-dimensional (3-D) imaging from multidetector-row computed tomography (MD-CT) by volume rendering. It was projected on the patient’s operative field with the clear visualization of lymph node (LN) through projector. Then, the dye of indocyanine green (ICG) was injected subdermally in the areola. Subcutaneous lymphatic channels draining from the areola to the axilla were visible by fluorescence imaging immediately. Lymphatic flow was reached after LN revealed on 3-D imaging. After incising the axillary skin on the point of LN mapping, SLN was then dissected under the guidance of fluorescence imaging with adequate adjustment of sensitivity and 3-D imaging.
Results
Lymphatic channels and SLN were successfully identified by Photodynamic eye (PDE) in all patients. And the sites of skin incision also were identical with the LN being demonstrated by 3-D imaging in all patients. The mean number of SLN was 3.7. The image overlay navigation surgery was visually easy to identify the location of SLN from the axillary skin. There were no intra- or postoperative complications associated with SLN identification.
Conclusions
This combined navigations of fluorescence and 3-D imaging revealed more easy and effective to detect SLN intraoperatively than fluorescence imaging alone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sentinel lymph node biopsy (SLNB) of the patients with breast cancer was introduced by Krag et al. using a radioactive colloid in 1993, consecutively followed by Giuliano et al. using a blue dye in 1994 [1, 2]. Recently, many studies have reported the usefulness of SLNB, including high detection rate, low false-negative rate, and the avoidance of axillary lymph node dissection [3–8]. The combination method of radioactive colloid and dye showed high detection and low false-negative rates in SLNB [9–11]. However, it needs the learning curve of procedural skill by dye alone and the equipment of nuclear medicine facilities by radioactive colloid. Furthermore, the lymphatic flow to SLN is uncertain during SLNB in the method of dye or radioactive colloid. From those findings, Kitai et al. reported the SLNB of visible lymphatic pathway using an ICG [12]. We also confirmed that their method was feasible and safe for an intraoperative detection of SLN allowing real-time observation without any need for training, and obtained high detection and low false-negative rates [13, 14]. Therefore, to obtain easy, immediate, and accurate identification of SLN during surgery, we introduced a novel approach for SLN identification by fluorescence imaging using ICG and image overlay navigation surgery in patients with breast cancer.
Patients and methods
All had breast cancer that had been histologically confirmed by core needle biopsy of lesions < 2 cm without axillary lymph nodes involvement and were scheduled to receive standard care in accordance with the designated clinical pathway. This study was approved by the Institutional Review Board of Dokkyo Medical University. Informed consent was obtained from all patients. Patients with a history of allergy to iodine or shellfish were excluded from the study.
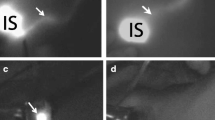
Initially we obtained 3-D imaging from MD-CT by volume rendering of Osirix imaging software (DICOM viewer, Macintosh, Apple Inc., U.S.A.). Its software can be purchased for free by the download from the website of Apple Mackintosh. The 3-D image was projected on the operative field of the patient’s chest with the clear visualization of LN through the microprojector (M-pro 110; 115 × 50 × 22 mm, 152 g, Sumitomo 3 M, Tokyo, Japan) connected with a personal computer (Fig. 1). The image on the skin surface was adjusted to the size of patient according to the site of nipple and the line of lateral edge of major pectoral muscle (Fig. 2). Then the dye mixture of 0.5 ml (1.25 mg) of ICG (Daiichi-Sankyo Pharmaceutical, Tokyo, Japan) and 0.5 ml of indigo carmine (Daiichi-seiyaku, Osaka, Japan) was injected subdermally into the areola. Subcutaneous lymphatic channels draining from the areola to the axilla were visible as a fluorescence imaging produced by PDE (Photodynamic eye, Hamamatsu Photonics Co., Hamamatsu, Japan) immediately [4]. This apparatus is equipped with a light-emitting diode that produces light with a wavelength of 760 nm and also a charged-coupled device (CCD) camera with filter. Lymphatic flow was reached after LN revealed on 3-D imaging of the patient, and we drew it onto the skin to confirm the accurate location of the SLN (Fig. 3). In a case of invisible lymphatic flow to SLN, the surface of the breast or axillary skin was compressed by a transparent hemisphere device to observe the lymphatic flow (Fig. 4). After incising the axillary skin on the point of LN mapping of the patient, SLN was dissected under the guidance of fluorescence imaging with adequate adjustment of sensitivity and 3-D imaging (Fig. 5). The PDE was used to confirm whether the resected SLNs were fluorescent. All LNs showing fluorescence were investigated for the presence of metastasis in the pathology department intraoperatively.
Results
This study enrolled 50 patients with a tumor <2 cm in diameter and no axillary lymph nodes involvement preoperatively. The setup time, consisting of the connection of personal computer, setting of the microprojector, and adjustment of 3D-image on the patient’s operation field, took 5–10 min. Lymphatic channels and SLN were successfully identified by PDE in all patients (detection rate: 100%). The sites of skin incision also were identical with the LN being demonstrated by 3-D imaging in all patients. The mean number of SLN was 3.7 (range, 1–7). Eight patients (16%) were found to have lymph node metastases pathologically. The image overlay navigation surgery was visually easy to identify the location of SLN on the axillary skin even in obese patients. The adjustment of sensitivity of PDE and the image overlay navigation provided the reduction of operation time. The mean procedural time of SLNB was 10.3 (range, 6–18) min. The adjustment of sensitivity of PDE ranged from 20–25% to identify the SLN. Furthermore, the compression of the axillary skin by a transparent hemisphere device was effective to identify the location of SLN in nonobese patients. However, 18 patients (36%) were still invisible on the axillary skin after the compression by PDE alone. The combination of image overlay navigation using 3D-image was very helpful for those patients to identify the location of SLN. There were no intra- or postoperative complications associated with SLN identification and ICG itself. ICG tattooing around the areola disappeared spontaneously within 2 weeks.
Discussion
Image overlay means a display technique that superimposes medical images over the direct view of viewer in the real world. The images are transformed in real-time so they appear to the user to be an integral part of the surrounding environment. By using an image overlay with 3-D medical images, such as computed tomography (CT) reconstructions, surgeons can visualize the data in vivo, exactly positioned within the patient’s anatomy, and potentially enhance the surgeon’s ability to perform a complex procedure [15]. Furthermore, by localizing the targeted lesion and the critical lesion that should be avoided, the surgical navigation helps to achieve effective and safe surgery while minimizing the invasiveness of the surgery [16]. Therefore, we applied the image overlay navigation system to obtain safe and early identification of SLN for the patients with breast cancer. This is the first report to introduce image overlay navigation surgery used to identify the SLN in patients with breast cancer.
Recently 3D-images have been relatively easily reconstructed from CT, magnetic resonance imaging, or ultrasonography. The guidance of 3-D ultrasound image is very helpful for surgeons in determining the resection line of breast conservation surgery according to the superimposed image [17, 18]. However, there are some unresolved problems consisting of the low quality of image due to some particular noise caused by two-dimensional ultrasound image, the requirement of prolonged creation time of 3-D image, and the elasticity of the breast. To solve those problems, we reconstructed 3D-CT image by the volume rendering of Osirix. It provides a high-quality image and immediate reconstruction of 3-D imaging. Liao et al. [16] reported an autostereoscopic image overlay technique that is integrated into a surgical navigation system to superimpose a real 3-D image onto the patients via a half-silvered mirror; its use would increase surgical accuracy and reduce invasiveness. Furthermore, we combined the fluorescence imaging of real-time lymphatic flow with the image overlay technique and confirmed to reach the lymphatic flow to LN superimposed on the patient’s axilla. We think that this finding reveals the best navigation system for detecting SLN at this time.
Fluorescence imaging in breast cancer surgery is advantageous in allowing real-time observation of the lymphatic streams on the skin, and superior recognition of lymphatic vessels and LNs in comparison with dye staining alone; furthermore, there is no specific need for specialized training for detection of SLNs and no requirement for a radioisotope facility [12, 13]. However, this method has several problems, including the need to turn the shadowless light on and off during the procedure, limitation for detection of SLNs due to easy spreading of ICG through the subcutaneous tissue, its inapplicability to patients with iodine hypersensitivity, and residual dye tattooing that persists for 10 to 14 days [12, 13]. Nevertheless, we think that the advantages of this method outweigh its disadvantages and that ICG fluorescence imaging provides better outcomes of SLNB.
In SLN identification using ICG, Motomura et al. [19] reported that the SLN detection rate using ICG alone was 73.8% and that the mean number of SLNs was 1.7. Kitai et al. [12] reported that the SLN detection rate using both ICG and fluorescence imaging was 94% and that the mean number of SLNs was 2.8. The present study showed that the detection rate was 100% and the mean number of SLNs was 3.7. However, with ICG alone, the detection rate was 75.2% and the mean number of SLNs was 2.1. These results were similar to those of Motomura et al. with the use of dye alone without fluorescence imaging. In general, the detection rate using radioactive colloid has ranged from 87 to 98% (mean, 92.3%), and the mean number of SLN ranged from 1.4 to 2.6 (mean, 1.95) [20–25]. The introduction of fluorescence imaging has yielded a detection rate equal to that of radioactive colloid. Florescence imaging using a PDE has high sensitivity for ICG. The combination of fluorescence imaging with a dye is more advantageous for SNNS at institutions that lack suitable facilities for handling radioisotopes. However, the number of SLNs identified using florescence imaging in our study was still higher than that achievable using radioactive colloid. We think that unnecessary removal of some LNs is currently a problem with this method. In fact, the number of LNs demonstrated on 3D-imaging ranged from 1 to 3, and these LNs were identical with SLNs visualized by fluorescence. We think that removal of these LNs alone is reasonable in actual clinical practice.
The number of SLNs detected using ICG fluorescence imaging depends on the time taken to identify them. In our series, the number of SLNs in the initial 20 patients was more than twice that in the last 20. At the time of introduction of SLNB using ICG fluorescence imaging, identification of SLNs took 20 min or more. However, as the identification time increased, more LNs were found by fluorescence imaging. Because PDE has high sensitivity for ICG, the injected ICG easily spreads through the subcutaneous tissue, including the lymph vessels and LNs during SLNB, even in cases of SLN metastasis. Therefore, we think that the optimum time required for SLN identification is approximately 10 min, based on the results of recent practice. Furthermore, the outcome also depends on adjustment of the PDE sensitivity. At full power (100%), the PDE showed strong fluorescence from all parts of the surgical field, which hindered identification of SLNs. Therefore, adjustment of the PDE sensitivity for identifying SLNs ranged from 20–25%.
One notable feature was that identification of SLNs in obese patients became relatively easy after the introduction of image overlay navigation. Image overlay surgery with fluorescence imaging is very helpful for residents or medical students to comprehend the lymphatic flow and the correlation between SLNs and axillary vessels. Furthermore, the recognition of the optimal site for a skin incision is identical with that using radioactive colloid and methods such as pinpoint marking. These results represent a considerable advantage of image overlay navigation surgery during SLNB. Recently we have come to consider that image overlay navigation surgery can eliminate the use of indigo carmine with this procedure, as all patients with pathologically positive lymph nodes were recognized by fluorescence imaging, but some were not identified using a vital dye. Although compression of the axillary skin by a transparent hemispheric device was effective for identifying the location of SLNs in nonobese patients, there was an absolute need for the image overlay navigation system in obese patients due to the high rate of invisible nodes (36%) in our present series. Because fluorescence can be observed from ICG solution located at a depth of 1 cm in material with optical properties compatible with human tissue [12], fluorescence through bulky connective tissue in the axilla is invisible at the axillary skin. A further disadvantage of this system is the time required to setup the projector and personal computer preoperatively. To solve this problem, a dedicated operating room equipped with a we will need an exclusive operating room with a remote-controlled bar and projector will be required.
In conclusion, the combined navigation of fluorescence and 3-D imaging was an easier and more effective method to detect SLN intraoperatively than fluorescence imaging alone. The operator’s demand was satisfied with the introduction of image overlay navigation surgery, and it may be practical in various surgical fields.
References
Krag DN, Weaver DL, Alex JC et al (1993) Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol 2:335–339
Giuliano AE, Kirgan DM, Guenther JM et al (1994) Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 220:391–398
Giuliano AE, Jones RC, Brennan M et al (1997) Sentinel lymphadenectomy in breast cancer. J Clin Oncol 15:2345–2350
Veronesi U, Paganelli G, Gallmberti V et al (1997) Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 349:1864–1867
Krag D, Weaver D, Ashikaga T et al (1998) The sentinel node in breast cancer: a multicenter validation study. N Engl J Med 339:941–946
Borgstein PJ, Pijpers R, Comans EF et al (1998) Sentinel lymph node biopsy in breast cancer: guidelines and pitfalls of lymphoscintigraphy and gamma probe detection. J Am Coll Surg 186:275–283
Nwariaku FE, Euhus DM, Beitsch PD et al (1998) Sentinel lymph node biopsy, an alternative to elective axillary dissection for breast cancer. Am J Surg 176:529–531
Bass SS, Cox CE, Ku NN et al (1999) The role of sentinel lymph node biopsy in breast cancer. J Am Coll Surg 189:184–194
Cox CE, Pendas S, Cox JM et al (1998) Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer. Ann Surg 227:645–653
McMasters KM, Tuttle TM, Carlson DJ et al (2000) Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol 18:2560–2566
Tafra L, Lannin DR, Swanson MS et al (2001) Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg 233:51–59
Kitai T, Inomoto T, Miwa M et al (2005) Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 12:211–215
Tagaya N, Yamazaki R, Nakagawa A et al (2008) Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am J Surg 195:850–853
Ogasawara Y, Ikeda H, Takahashi M et al (2008) Evaluation of breast lymphatic pathways with indocyanine green florescence imaging in patients with breast cancer. World J Surg 32:1924–1929
Blackwell M, Nikou C, DiGioia AM, Kanade T (2000) An image overlay system for medical data visualization. Med Image Analysis 4:67–72
Liao H, Hata N, Nakajima S et al (2004) Surgical navigation by autostereoscopic image overlay of integral videography. IEEE Trans Inf Technol Biomed 8:114–121
Sato Y, Nakamoto M, Tamaki Y et al (1998) Image guidance of breast cancer surgery using 3-D ultrasound images and augmented reality visualization. IEEE Trans Med Imaging 17:681–693
Tamaki Y, Sato Y, Nakamoto M et al (1999) Intraoperative navigation for breast cancer surgery using 3D ultrasound images. Comp Aid Surg 4:37–44
Motomura K, Inaji H, Komoike Y et al (1999) Sentinel node biopsy in breast cancer patients with clinically negative lymph-nodes. Breast Cancer 6:259–262
Krag D, Weaver D, Ashikaga T et al (1998) The sentinel node in breast cancer: a multicenter validation study. N Engl J Med 339:941–946
Veronesi U, Paganelli G, Gallmberti V et al (1997) Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 349:1864–1867
Cox CE, Pendas S, Cox JM et al (1998) Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer. Ann Surg 227:645–653
McMasters KM, Tuttle TM, Carlson DJ et al (2000) Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol 18:2560–2566
Tafra L, Lannin DR, Swanson MS et al (2001) Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg 233:51–59
Motomura K, Inaji H, Komoike Y et al (2001) Combination technique is superior to dye alone in identification of the sentinel node in breast cancer. J Surg Oncol 76:95–99
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tagaya, N., Aoyagi, H., Nakagawa, A. et al. A Novel Approach for Sentinel Lymph Node Identification Using Fluorescence Imaging and Image Overlay Navigation Surgery in Patients with Breast Cancer. World J Surg 35, 154–158 (2011). https://doi.org/10.1007/s00268-010-0811-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-010-0811-y