Abstract
Background
Advanced thymomas with disseminated nodules are difficult to manage, and the treatment strategy remains undefined.
Methods
A total of 28 thymoma patients with pleural and/or pericardial disseminated nodules were treated at Nagoya City University Hospital. Among them, 21 patients underwent resection of thymoma and pleural disseminated nodules. These patients were reviewed in the present study.
Results
Preoperative steroid pulse therapy was performed in 14 patients. Macroscopic total resection of all tumors was achieved in 15 patients. Postoperative adjuvant radiotherapy was performed for the mediastinum in 20 patients and hemithoracic irradiation (HTR) in 11 patients. The overall survival rate of operated 21 patients was 73.1% at 5 years. The patients who underwent resection showed a better prognosis than the patients without resection (p = 0.0006). Relapse was diagnosed in 14 of 21 patients who underwent resection. Disease-free survival was 67.5% at 1 year, 39.8% at 3 years, and 13.3% at 5 years. HTR alone did not improve the disease-free survival. Among the patients who underwent total resection, relapse-free survival was better than in the patients with subtotal resection (p = 0.009). Achievement of a trimodality therapy with preoperative steroid pulse, total resection, and postoperative HTR was associated with prolonged relapse-free survival in the operated patients (p = 0.027, hazard ratio 6.452).
Conclusions
Pursuing total resection for thymoma and disseminated nodules may be beneficial for stage IV thymoma. The combination of preoperative steroid pulse therapy, macroscopic total resection, and postoperative HTR may prolong the interval to relapse, but it did not lead to cure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thymomas are neoplasms arising from thymic epithelial cells with nonneoplastic T lymphocytes to varying degrees. Surgical resection has been advocated as the principal treatment, and completeness of resection has been considered the most important determinant of long-term survival for patients with thymoma [1–4]. Nevertheless, advanced-stage diseases such as tumors with pleural and/or pericardial disseminated nodules are difficult to manage, and the treatment strategy for those diseases remains undefined [5, 6]. Chemotherapy has shown significant antitumor activity against unresectable, recurrent, or metastatic thymomas [7, 8]. Preoperative chemotherapy for advanced thymomas has not been established, although neoadjuvant chemotherapy has been attempted and multimodality therapy using chemotherapy has been reported [9, 10]. There is also no consensus statement or randomized trial data to support adjuvant radiation therapy for advanced thymomas. Recent reports have shown that adjuvant radiation for the tumor bed did not prevent pleural recurrences even if it decreased local recurrences [11–13].
We have previously reported on the efficacy of steroid pulse therapy for advanced thymomas [14, 15]. We proposed it as induction therapy for advanced thymomas. In addition, we have used postoperative low-dose hemithoracic irradiation to prevent pleural recurrence. In the present study, we retrospectively analyzed 21 thymoma patients with pleural disseminated nodules who underwent resection to determine the prognostic factors and efficacy of resection and multimodality therapy that included preoperative steroid pulse therapy and postoperative hemithoracic irradiation.
Patients and methods
We performed a retrospective review of clinical and pathologic data for all thymoma patients undergoing treatment at Nagoya City University Hospital between January 1994 and March 2008. The study was approved by the institutional review board of Nagoya City University Hospital. Written informed consent was obtained from all of the patients.
During this time period, 149 patients underwent treatment for thymoma. There were 28 patients had World Health Organization (WHO) stage IV thymoma with disseminated nodules; and 21 of these patients underwent resection. The 2004 revision of the WHO histologic TNM classification and stage grouping of thymic epithelial tumors [16] was utilized in the present study. Pretreatment evaluation of the tumor was done by physical examination, chest radiography, and chest computed tomography (CT) scanning to evaluate the resectability of the lesions. Resection status was regarded as total resection when all visible nodules were resected macroscopically.
Response to preoperative therapy was graded according to the Response Evaluation Criteria In Solid Tumors (RECIST) criteria. Partial response (PR) was defined as a 30% decrease and progressive disease (PD) as a 20% increase. Preoperative and perioperative adverse events and adverse events arising from adjuvant radiation therapy were identified from the medical records and graded according to the Common Terminology Criteria for Adverse Events, version 3.
Survival analysis was performed using the Kaplan–Meier method and the univariate log-rank test; it was calculated from the beginning of primary treatment to death. Disease-free survival was determined in patients who had undergone total or subtotal resection and was calculated from the date of surgery to the date of the first radiographic study demonstrating recurrent disease or most recent study demonstrating absence of disease. Prognostic factors and relapse-related factors were analyzed with the Cox multivariate regression analysis using hazard ratio with 95% confidence intervals (CI). Statistical significance was defined as p < 0.05.
Results
Clinical and pathologic data
All 21 patients with WHO stage IV thymoma with pleural disseminated nodules underwent resection. There were 8 men and 13 women with a mean age of 53 years (median 49 years; range 33–78 years). Of the 21 patients, 11 had no symptoms before treatment. Symptoms of myasthenia gravis (MG) were recognized in two patients at the time of treatment. One of these patients showed only MG symptoms, and the other patient had MG symptoms and cough. The other chief clinical symptoms were chest pain (n = 4), cough (n = 3), and dyspnea (n = 2). Anti-acetylcholine receptor (AChR) antibodies were positive in eight patients. Pretreatment biopsy of the mediastinal tumor or disseminated lesions was performed in all patients. A pathological diagnosis was obtained before initial treatment in all patients.
Preoperative chemotherapy with a platinum-based regimen was performed in three patients: adriamycin/cisplatin/vincristine/cyclophosphamide (ADOC) in two patients and cisplatin/vincristine/cyclophosphamide in one patient. Preoperative irradiation was performed in one patient at another institution. Preoperative administration of steroid was performed in 17 patients. Steroid pulse therapy with venous infusion of 1 g of methylprednisolone over 3 days was performed in 14 patients [15]. In the other 3 patients, oral low-dose prednisolone was used.
Pleural disseminated nodules were recognized in all 21 patients. The most common surgical approach was a median sternotomy for extended thymothymomectomy and posterolateral thoracotomy to resect pleural disseminated nodules. Visceral and parietal pleural disseminated lesions were resected with small margins. Intercostal or pulmonary parenchymal involvement was managed with intercostal muscle resection or wedge resections of the involved lung (n = 9). Lobectomy was performed in four patients and bilobectomy (right upper and middle lobes) in three patients. Other organs that were resected include the pericardium in 13, diaphragm in 10, phrenic nerve in 7, and left brachiocephalic vein in 6. In two patients the superior vena cava was resected and reconstructed with a prosthetic material. Because of chest wall invasion, partial resection of the rib was required in one patient. Macroscopic total resection of all tumors was achieved in 15 (71%) patients. In six patients the main tumors and most of the disseminated nodules were resected, but visible, small disseminated nodules remained. These were regarded as subtotal resections. In one patient, a pericardial disseminated nodule was recognized and regarded as unresectable.
Postoperative adjuvant radiotherapy was performed targeting the mediastinum in 20 patients (30–52 Gy). Postoperative radiotherapy was not performed in any patient who underwent preoperative radiotherapy. In addition, low-dose hemithoracic radiotherapy (HTR) (10–16 Gy) was performed in 11 patients [17]. Trimodality therapy—preoperative steroid pulse, total (n = 5) or subtotal (n = 5) resection, postoperative HTR—was accomplished in 10 patients.
Using the WHO histopathologic classification of tumors [16], thymomas were diagnosed as type AB (n = 1), B1 (n = 3), B2 (n = 7), B2–B3 (n = 3), and B3 (n = 7). Four patients were diagnosed pathologically as having Masaoka IVb disease. Of these four patients, pulmonary metastasis was seen in one and anterior mediastinal lymph node metastasis in the other three.
Efficacy of preoperative treatment
Three patients received preoperative chemotherapy. Two of them were treated at another institution, however, so we had no detailed data for these patients. One patient underwent preoperative chemotherapy (cisplatin/vincristine/cyclophosphamide) in our institution, and the effect was stable disease (SD). Of the 14 patients who received preoperative steroid pulse therapy, 6 achieved a partial response (PR). The response rate was 42.9%. The rate of reduction in size with RECIST (RRIS) for steroid pulse therapy was 25.4%. RRISs in the WHO histopathological classification were 19.5% for type AB (n = 1), 38.5% for type B1 (n = 2), 33.0% for type B2 (n = 4), 20.1% for type B2-3 (n = 3), and 16.8% for type B3 (n = 4). Another eight tumors were diagnosed as SD, although six tumors showed a size reduction of more than 10% (23.8%, 22.0%, 19.9%, 19.5%, 16.5%, 12.0%, 3.0%, and –8%). No information was available regarding the preoperative irradiation performed on one patient at another institution.
Adverse events associated with preoperative treatment and operation
One patient who underwent preoperative chemotherapy showed grade 3 leukocytopenia. Grade 1 insomnia appeared in all of the 14 patients receiving steroid pulse therapy. There were no operative deaths, and the 30-day mortality was 0%. The mean operating time was 424 ± 173 min (150–800 min). The mean blood loss was 2040 ± 1834 ml (205–7600 ml). Blood transfusions of >1000 ml were required in five patients. Perioperative adverse events occurred in 4 (19%) of the 21 patients. Intraoperative cardiac arrest of unknown etiology occurred in one patient who was successfully resuscitated and subsequently discharged with no sequelae. Grade 2 chylothorax appeared in two patients, and pleural drainage was required. Grade 3 postoperative pneumonitis appeared in one patient who underwent postoperative mediastinal irradiation without HTR.
Adverse events associated with postoperative radiotherapy
Three months after mediastinal irradiation and HTR, one patient developed pneumonitis and pyothorax, for which open drainage thoracotomy was required. Two years later the patient died of bleeding from the thoracic cavity. In the other 18 patients, adverse events were limited to grade 1 or 2 skin reactions, nausea, esophagitis, and pneumonitis. One long-term adverse event, pulmonary honeycomb change and Aspergillus infection, appeared in one patient. Antimycotic therapy was given over a period of 11 years after operation. This patient did not undergo HTR.
Overall survival and disease-free survival
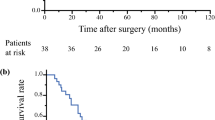
The observation period was 2 to 142 months postoperatively. Nine patients died of tumor (n = 7) or related morbidity (n = 2). The latter group included meningitis during chemotherapy (n = 1) and bleeding from the pyothorax cavity (n = 1). The overall survival for the 21 patients was 87.5% at 3 years, 73.1% at 5 years, and 37.6% at 10 years (Fig. 1).
Clinicopathologic factors that could influence prognosis were analyzed (Table 1). No prognostic factors emerged during the univariate analysis. The 21 patients who underwent resection showed a better prognosis than the patients with dissemination who did not undergo resection (n = 7) (p = 0.0006) (Fig. 2).
Relapse of the thymoma was diagnosed in 14 of 21 patients who underwent resection, with 13 of them occurring within 5 years after operation. For the 21 patients, disease-free survival was 67.5% at 1 year, 39.8% at 3 years, and 13.3% at 5 years, with a median disease-free survival of 37.1 months (Fig. 3). For treatment of relapse, re-resections of metastases were performed in four patients, chemotherapy in seven patients, and radiotherapy in one patient. Best supportive care was selected for two patients.
Clinicopathologic factors were assessed to determine the cause of the relapse (Table 2). Postoperative HTR did not prolong disease-free survival ((p = 0.720). Relapses were less frequent in patients who underwent total resection than in the patients with subtotal resection (p = 0.009) (Fig. 4). Relapse-free survival in patients who underwent trimodality therapy (preoperative steroid pulse, total resection, postoperative HTR) was better than in the patients without such therapy (p = 0.0035) (Fig. 5). Trimodality therapy with preoperative steroid pulse, total resection, and postoperative HTR was the only significant factor for prolonged disease-free survival in the operated patients in the multivariate Cox regression analysis (p = 0.027), and the hazard ratio was 6.452 (Table 3).
Disease-free survival and trimodality therapy, which included preoperative steroid pulse therapy, total resection, and postoperative hemithoracic irradiation. Relapses in patients receiving trimodality therapy (n = 5) were clearly less frequent than in those without the therapy (n = 16) (p = 0.0035)
Discussion
A retrospective analysis was conducted for a series of thymoma patients with pleural disseminated nodules. This disease was classified as T4 and stage IV in the TNM classification and stage grouping, respectively, of the WHO [16]. The prognostic factors of T4 thymoma and optimal treatment strategy have not yet been determined. In the present study we demonstrated the efficacy of surgical treatment. The 21 patients who underwent resection showed a better prognosis than the 7 patients with dissemination who did not undergo resection. As the surgical procedures, we selected total resection of the thymoma and disseminated lesions. Unfortunately, in 6 of 21 patients, small but visible tumors were left unresected (subtotal resection). The disease-free interval in these six patients was shorter than in patients who underwent macroscopic total resection, although relapse has been observed even in patients with total resection. Some reports have proposed extrapleural pneumonectomy (EPP) for complete resection of pleural disseminated nodules [10, 18]. EPP may be an option, but the indication for EPP is limited because of high operative mortality and low postoperative quality of life.
To increase the resectability of locally advanced thymoma, we explored induction steroid pulse therapy. A significant reduction in size (PR) was achieved in 6 of 14 patients who received a preoperative steroid pulse. More than 10% size reduction, which did not reach PR, was observed in six patients. The rates of reduction in size seemed to depend on the WHO classification of the tumor. It should be noted that the effect of steroid pulse therapy is transient, and the operation should be planned within 14 days of the last steroid pulse. Steroid pulse therapy can be performed without serious complications.
Low-dose HTR was used to prolong the relapse-free interval in some patients. Nevertheless, no benefit was found in overall survival or relapse-free survival in the patients given low-dose HTR. The intent of applying low-dose HTR was to suppress microscopic residual tumors. In the present study, the combination of macroscopic total resection and postoperative low-dose HTR prolonged relapse-free intervals. Furthermore, combined treatments of preoperative steroid pulse therapy, macroscopic total resection, and low-dose postoperative HTR prolonged relapse-free intervals. A prospective trial is needed to confirm these observations.
Conclusions
Pursuing total resection for thymoma and disseminated nodules may be beneficial for patients with stage IV thymoma. In addition, macroscopically total resection of pleural disseminated nodules plus the combination of preoperative steroid pulse therapy, macroscopic total resection, and postoperative low-dose HTR may prolong the relapse-free interval but did not lead to cure.
References
Masaoka A, Monden Y, Nakahara K et al (1981) Follow-up study of thymomas with special reference to their clinical stages. Cancer 48:2485–2492
Blumberg D, Port JL, Weksler B et al (1995) Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg 60:908–913
Kondo K, Monden Y (2003) Therapy for thymic epithelial tumors: a clinical study of 1, 320 patients from Japan. Ann Thorac Surg 76:878–884
Nakagawa K, Asamura H, Matsuno Y et al (2003) Thymoma: a clinicopathologic study based on the new World Health Organization classification. J Thorac Cardiovasc Surg 126:1134–1140
Lucchi M, Ambrogi MC, Duranti L et al (2005) Advanced stage thymomas and thymic carcinomas: results of multimodality treatments. Ann Thorac Surg 79:1840–1844
Lucchi M, Melfi F, Dini P et al (2006) Neoadjuvant chemotherapy for stage III and IVA thymomas: a single-institution experience with a long follow-up. J Thorac Oncol 1:308–313
Evans TL, Lynch TJ (2005) Role of chemotherapy in the management of advanced thymic tumors. Semin Thorac Cardiovasc Surg 17:41–50
Fornasiero A, Daniele O, Ghiotto C et al (1991) Chemotherapy for invasive thymoma: a 13-year experience. Cancer 68:30–33
Yokoi K, Matsuguma H, Nakahara R et al (2007) Multidisciplinary treatment for advanced invasive thymoma with cisplatin, doxorubicin, and methylprednisolone. J Thorac Oncol 2:73–78
Huang J, Rizk NP, Travis WD et al (2007) Feasibility of multimodality therapy including extended resections in stage IVA thymoma. J Thorac Cardiovasc Surg 134:1477–1483
Mangi AA, Wain JC, Donahue DM et al (2005) Adjuvant radiation of stage III thymoma: is it necessary? Ann Thorac Surg 79:1834–1839
Kundel Y, Yellin A, Popovtzer A et al (2007) Adjuvant radiotherapy for thymic epithelial tumor: treatment results and prognostic factors. Am J Clin Oncol 30:389–394
Eng TY, Thomas CR Jr (2005) Radiation therapy in the management of thymic tumors. Semin Thorac Cardiovasc Surg 17:32–40
Tateyama H, Takahashi E, Saito Y et al (2001) Histopathologic changes of thymoma preoperatively treated with corticosteroids. Virchows Arch 438:238–247
Kobayashi Y, Fujii Y, Yano M et al (2006) Preoperative steroid pulse therapy for invasive thymoma: clinical experience and mechanism of action. Cancer 106:1901–1907
Travis WD, Brambilla E, Muller-Hermelink HK et al (2004) World Health Organization classification of tumours: pathology and genetics of tumours of the lung, pleura, thymus and heart. International Agency for Research on Cancer, Lyon, Lyon, pp 146–151
Sugie C, Shibamoto Y, Ikeya-Hashizume C et al (2008) Invasive thymoma: postoperative mediastinal irradiation, and low-dose entire hemithorax irradiation in patients with pleural dissemination. J Thorac Oncol 3:75–81
Wright CD. Pleuropneumonectomy for the treatment of Masaoka stage IVA thymoma. Ann Thorac Surg 82:1234–1239
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yano, M., Sasaki, H., Yukiue, H. et al. Thymoma with Dissemination: Efficacy of Macroscopic Total Resection of Disseminated Nodules. World J Surg 33, 1425–1431 (2009). https://doi.org/10.1007/s00268-009-0069-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-009-0069-4