Abstract
Background
Thymoma patients with pleural dissemination are difficult to manage, and their treatment strategy remains undefined. This study aimed to investigate the clinicopathologic features of these patients, focusing on the association between the depth of pleural invasion and prognosis.
Methods
Between 2003 and 2019, the study identified 120 disseminated lesions in 20 thymoma patients. Seven patients had de novo stage IVa thymoma and 13 were recurrent cases. Extrapleural pneumonectomy was performed for 8 patients and debulking surgery for 12 patients. Invasion depth of pleural tumors was classified into two groups: when the disseminated tumors invaded the pleura beneath the elastic layer, the tumor was diagnosed as Da, and when the disseminated tumors invaded the pleura beyond the elastic layer, the tumor was diagnosed as Db.
Results
Of 120 nodules, 31 (26%), found in eight patients with recurrent malignancies, were classified as Db. The pathologic status of the surgical margin (PSM) was positive in eight patients, seven of whom had Db nodules. The 5-year overall survival (OS) rate was 100% in the Da group and 75% in the Db group (P = 0.02). The 5-year progression-free survival (PFS) rate was 66.7% in the Da group and 25% in the Db group (P = 0.02). Cox univariate analysis showed that PFS was significantly influenced by the depth of invasion (P = 0.04) and PSM (P = 0.03).
Conclusion
Depth of pleural invasion may influence survival outcomes for thymoma patients with pleural dissemination. The patients in this study with Da-disseminated nodules had an increased probability of a longer OS and PFS and tended to achieve negative PSM compared with the patients with Db.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Thymomas are thymic neoplasms arising from thymic epithelial cells. Although the progression of thymomas is relatively slow, they sometimes show aggressive malignant behavior.1 In addition to direct invasion into adjacent structures, thymomas can metastasize to distant sites including pleural or pericardial tissues and the lung, or infrequently, to the mediastinal lymph nodes and extra-thoracic organs.2,3,4 For patients with early-stage thymoma, primary resection is recommended, and completeness of the resection is considered the most important factor for achieving long-term, recurrence-free patient survival.5
Advanced-stage thymomas are difficult to manage, and the treatment strategy remains undefined. For advanced disease with pleural dissemination, classified as stage IVa thymoma according to the current tumor-node-metastasis (TNM) classification of the Union for International Cancer Control,6 macroscopic complete resection (MCR) may provide a favorable prognosis.7,8,9 Although MCR may be associated with a positive outcome, complete resection of disseminated nodules generally is difficult to attain. Several studies with limited patient numbers have investigated the efficacy of surgery for treating pleural dissemination of thymoma.
Three types of operations have been reported: debulking surgery (DS),10,11,12 pleurectomy/decortication (P/D),13,14 and extrapleural pneumonectomy (EPP),15,16,17,18,19 each with its own advantages and disadvantages. Debulking surgery is a surgical removal of as many visible nodules as possible, whereas P/D is defined as removal of the parietal and visceral pleurae. The third thymoma-linked surgical type, EPP is en bloc resection of the parietal and visceral pleurae with ipsilateral lung and/or pericardium and hemidiaphragm.
In addition to the different surgical approaches, the thymoma treatment outcomes have been associated with several pathologic characteristics. However, the prognostic significance of pathologic markers such as depth of invasion, site of dissemination, tumor growth pattern, and surgical margin status of pleural disseminated nodules remains to be investigated. Moreover, evidence-based treatment strategies have not been established in association with the pathologic etiology for thymoma patients with pleural dissemination.
In this study, detailed pathologic observation of the disseminated nodules showed that nodules sometimes invade the resected adjacent tissues deeper than expected. We hypothesized that the pleural invasion depth was associated with recurrent events and patient prognosis. Using retrospective analysis, we investigated the clinicopathologic characteristics of thymomas from patients with pleural dissemination. We assessed the association between depth of invasion and prognosis of the patients to identify predictive factors and establish treatment strategies.
Materials and Methods
Patients
For this study, 316 successive cases of surgically excised thymomas performed between 2003 and 2019 were retrieved from the thoracic surgical pathology files of the Nagoya University Hospital. Microscopic sections from these patients were re-reviewed by one pathologist (H.T.) to confirm the diagnosis of thymoma without prior knowledge of the clinical data. The study excluded nine patients whose resected specimens were not applicable to this study (6 specimens were not definitively proven to be thymomas, and 3 specimens could not be evaluated due to marked necrosis). In the remaining 307 thymoma patients, we identified 20 cases that had 120 lesions with pleural disseminations. All the selected cases/specimens had adequate clinical information and histologic material for examination. In addition to hematoxylin and eosin (H&E)-stained specimens from each case, the relevant sections were submitted for Elastica van Gieson staining to detect the presence of elastic layers.
All available clinical and follow-up information was obtained from the patients’ charts. This retrospective study protocol was approved by the institutional review boards of the Nagoya University Hospital (2017-0125). Pathologic staging was based on TNM classification,6 and histologic tumor type was determined according to the World Health Organization classification.20
Definition of the Invasion Depth of Pleural Tumors
The current TNM classification for thymic tumors6 has no definition or classification regarding invasion depth of the disseminated pleural nodules. The TNM classification of lung cancer defines the depth of visceral pleural invasion in detail, and patients are classified in each T descriptor as described previously.5 Furthermore, the Japan Lung Cancer Society for domestic cancer registration subclassifies T3 into three subcategories according to invasion depth on the parietal pleura.20,21 Both definitions are classified in terms of whether the tumor invades the pleura beyond the elastic layer or not.
In this study, following the definition style for lung cancer invasions, we identified the invasion depth of pleural tumors according to the following specifications. In cases with pleural nodules on visceral pleura, when the tumor had invaded the pleura beneath the inner elastic layer, the tumor was diagnosed as Da; when the tumor had invaded the lung parenchyma beyond the inner elastic layer, the tumor was diagnosed as Db (Fig. 1A and B). In the case with pleural nodules on the parietal pleura or diaphragm, when the tumor had invaded the parietal pleura beneath the elastic layer, the tumor was diagnosed as Da; when the tumor had invaded the muscle layer (chest wall or diaphragm) beyond the elastic layer, the tumor was diagnosed as Db (Fig. 1C and D).
(a) Scheme showing the visceral pleural layers corresponding to the pathologic image in (b). (b) Elastica van Gieson staining showing the elastic layers of the visceral pleura. a Submesothelial layer. b Outer elastic layer. c Interstitial layer. d Inner elastic layer. e Alveoli (lung parenchyma). (c) Scheme showing the parietal or diaphragm pleural layers corresponding to the pathologic image in (d). (d) Elastica van Gieson staining showing the elastic layers of the diaphragm. f Fascia. g Muscle layer.
When the tumor cells appeared to be entrapped within the thick elastic layer, the tumor was diagnosed as Da. When different criteria of invasion depth were seen in different nodules in a patient, the deepest one was applied regardless of the site of dissemination.
Statistical Analysis
To identify prognostic factors, we selected 12 categorical variables: age (<55 vs ≥55 years), sex (male vs female), histologic subtype (B1 vs B2–B3), surgical procedure (DS vs EPP), MCR (achieved vs none), pathologic status of the surgical margin (PSM) (positive vs negative), depth of pleural invasion (Da vs Db), invasive growth pattern (expansile vs infiltrative), tumor size (<4.0 vs ≥4.0 cm), induction chemotherapy (not performed vs performed), dissemination status (primary vs recurrent), and number of disseminated nodules (1–10 vs ≥11).
Analyses were performed using commercially available statistical software: SPSS version 26 (IBM Corp., Amonk, NY, USA). Statistical differences in clinicopathologic correlations were examined using the chi-square test. Differences between mean values of continuous variables were examined using the t test. Overall survival (OS) rates were calculated from the date of resection to the date of the last follow-up visit or death.
Progression-free survival rates (PFS) were calculated from the date of resection to the date of the last follow-up visit or the date of recurrence detection using the Kaplan-Meyer method. The PFSs were compared using the log-rank test. For thymoma patients with pleural dissemination, PFS is considered to be a reliable measure of outcomes when the disease is thought to be still present after treatment.22 To eliminate the arbitrary nature of setting a threshold, the effect of variables on PFS were evaluated using the Cox proportional hazards model. The differences were considered to be significant when the P value was lower than 0.05.
Results
Clinical Features
Patients’ clinicopathologic characteristics and their associations with the depth of pleural invasion are presented in Table 1. The median patient age of the 20 patients (9 men and 11 women) was 55 years (range, 31–77 years). Of the 20 patients, 7 had de novo stage IVa thymoma, and 13 had recurrent thymoma with pleural disseminations. The median interval from the initial resection to recurrence was 51 months (range, 19–144 months).
Clinically, almost all the patients were asymptomatic, and abnormal shadows were pointed out from routine follow-up computed tomography images. Two patients were symptomatic, one with exacerbation of myasthenia gravis (MG) and one with chest pain. Three patients had histories of MG, and one patient had pure red aplasia. Preoperatively, 11 patients received neoadjuvant chemotherapy, and 1 patient received adjuvant chemotherapy postoperatively. The median follow-up period was 82 months (range, 17–194 months).
Surgical Procedures
The EPP operation was performed for selected young patients who had cardiopulmonary function sufficient to undergo pneumonectomy and stable MG under good control, and DS was performed for patients with a general and/or disease condition not indicated for EPP. In this cohort, EPP with or without thymectomy was performed for eight patients. For 12 patients, DS with or without thymectomy was performed. For 17 patients (7 patients with EPP and 10 patients with DS), MCR was achieved. Two of the remaining three patients with DS had too many disseminated nodules to achieve MCR, and one patient with EPP had a nodule deeply infiltrating the intervertebral foramen. No perioperative deaths occurred. Postoperative complications were observed in two patients (17%) with DS (MG crisis and massive pleural effusions) and in two patients (25%) with EPP (bleeding and heart failure).
Histologic Features
Pathologic examination led to a diagnosis of type B1 thymoma for 2 patients, type B2 thymoma for 11 patients, and type B3 thymoma for 7 patients. The histology of the disseminated nodules was identical to that of the primary tumor in all the patients. Almost all the disseminated nodules, except for four lesions, showed an expansile growth pattern, with the nodules relatively well-circumscribed and generally arranged in the form of distinct lobules. The excepted four lesions, all of which involved the diaphragm, showed an infiltrative growth pattern with poorly circumscribed margins and irregular nests infiltrating the normal tissue.
Of 17 patients who achieved MCR, 8 had microscopic tumor cells at the surgical margin of at least one disseminated nodule removed (positive PSM) (1 patient with EPP and 7 patients with DS). According to surgical records, the number of disseminated nodules ranged from 1 to innumerable, with 11 cases having 10 nodules or fewer and 9 cases having more than 10 nodules.
Depth of Invasion
The penetration sites of the 120 lesions in the thoracic cavity included the chest wall (n = 42), the lung (n = 54), the diaphragm (n = 23), and the pericardium (n = 1). In terms of location, 32 Da and 10 Db nodules were on the chest wall, 41 Da (Fig. 2A and B) and 13 Db (Fig. 2C and D) nodules were on the visceral pleura, and 16 Da (Fig. 3A and B) and 7 Db (Fig. 3C and D) nodules were on the diaphragm. One Db nodule was invading the pericardium.
(a, b) Da and (c, d) Db type disseminated nodules on the visceral pleura. (a, b) Type B3 thymoma tumor cells invading the outer elastic layer of the visceral pleura (black arrow), but not the inner elastic layer (white arrow). (c, d) Type B2 thymoma tumor cells invading the lung parenchyma beyond the inner elastic layer (white arrow). (a, c) Hematoxylin and eosin staining and (b, d) Elastica van Gieson staining.
(a, b) Da and (c, d) Db type disseminated nodules on the diaphragm. (a, b) Type B3 thymoma tumor cells invading the fibroadipose tissue of the diaphragm, but not the elastic layer (white arrow). (c, d) Type B2 tumor cells invading the muscular layer beyond the elastic layer (white arrow). (a, c) Hematoxylin and eosin (H&E) staining and (b, d) Elastica van Gieson staining.
In the whole group, 31 (26%) of 120 disseminated nodules invaded beyond the elastic layer into the pulmonary parenchyma or the muscular layer of the chest wall or diaphragm. The depth of invasion did not differ significantly among the penetration sites including the chest wall, the visceral pleura, and the diaphragm. In eight patients, Db nodules were found, all of which were recurrent cases. Seven patients with Db nodules showed positive PSM.
Follow-up and Prognostic Factor Analysis
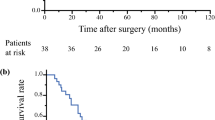
Disease progression was noted in 12 patients (60%). After 5 years, the OS was 95% and the PFS was 50% (Fig. 4A and B). The depth of invasion was associated negatively with the 5-year OS and PFS outcomes. The OS at 5 years was 100% in the Da group and 75% in the Db group (P = 0.02). The PFS was 66.7% in the Da group and 25% in the Db group (P = 0.02) (Fig. 4C and D).
(a, b) The 5-year overall survival (OS) and progression-free survival (PFS) curves for all the enrolled patients. (c, d) The 5-year OS and PFS analyses divided into two groups according to the depth of pleural invasion (Da vs Db). The depth of invasion was associated with 5-year OS and PFS using the Kaplan-Meyer method.
The two groups differed significantly in both OS and PFS. The PSM was associated with the 5-year PFS (P = 0.01), but not with the OS (P = 0.20). The depth of invasion was significantly correlated with surgical procedure (P = 0.04), PSM (P = 0.01) and progression of disease (P = 0.02). Cox univariate analysis showed that the depth of pleural invasion (P = 0.03) and PSM (P = 0.02) significantly influenced the PFS (Table 2). The variables age, sex, histologic type, surgical procedure, MCR, growth pattern, number of disseminated nodules, preoperative induction chemotherapy, dissemination status, and tumor size were not associated with PFS. Multivariate analysis was not performed due to the small number of cases.
Discussion
Thymomas show relatively slow progression and rarely invade neighboring structures or metastasize to other organs, in contrast to thymic carcinomas. Thymomas can sometimes show pleural disseminations at the initial diagnosis or during follow-up evaluation after treatment.23,24
The optimal management strategy for thymomas with dissemination remains a matter of debate. The current study investigated 120 lesions from 20 thymoma patients with pleural dissemination to determine the relationship between clinicopathologic factors and prognosis. The findings showed that the invasion depth of disseminated nodules was associated with the OS and PFS. Additionally, about one fourth of disseminated nodules invaded beyond the elastic layer, deeper than surgeons generally expect. Patients with disseminated nodules classified as Da (less invasive spreading) had a higher probability of prolonged OS and PFS and tended to achieve negative PSM compared with patients who had Db (more invasive spreading beyond the inner elastic layer).
In non-small cell lung cancer, visceral pleural invasion increases T category and is one of the most important prognostic factors.25,26,27,28 Extent of visceral invasion affects patient prognosis. Despite some small degree of variation, the visceral pleura consists of five layers: mesothelial, sub-mesothelial, outer elastic, interstitial, and inner elastic layers.28,29,30,31 Invasion through the elastic layer correlates with aggressive behavior and prognosis for lung cancers.
Although the recognition of pleural invasion is very important for the strategy used to treat lung cancer patients, the elastic layer of the visceral pleura is not defined by H&E staining alone. Elastic stain is known to be very helpful in identifying the elastic layer and is recommended for the pathologic examination of lung cancer.32 Although the visceral pleura usually shows two elastic layers (inner thin and outer thick elastic layers), the layers are not always distinct. Therefore, the dominant (most visible) elastic layer is the focus for assessment of visceral pleural invasion in such circumstances.
The structure of parietal pleura is similar to that of the visceral pleura.28 An outer elastic layer consists of loose discontinuous elastic fibers and is sometimes difficult to identify, even if elastic stain is used.
In the current study, the inner elastic fibers that present above the fascia of skeletal muscle of the chest wall or diaphragm were used to evaluate the depth of invasion. We found that the OS and PFS differed significantly between the Da and Db groups, with the former showing a better prognosis than the latter. Our findings indicated that the microscopically determined depth of pleural invasion (disseminated nodules) is important for evaluating patient prognosis. Accordingly, the elastic layers of both the visceral and parietal pleurae can be used as key landmarks of prognosis.
In the current cohort, two types of surgical procedures were performed: DS and EPP. The DS procedure needs a relatively short operation time and resection of a small volume of the neighboring structures. Therefore, this procedure is less invasive, and the patient’s quality of life (QoL) is preserved compared with other procedures. In contrast, although MCR can be achieved with DS procedures, complete pathologic resection without microresidual tumor is extremely difficult to obtain because invisible microscopic tumor cells cannot be completely resected. Actually, several patients have received repeated resections for repeated recurrences of pleural disseminations in this cohort. Patients who underwent two or more repeated resection procedures showed a better prognosis than those without repeated resection.12,33 Yano et al.7 reported that a longer DFS was associated with multidisciplinary treatment using a steroid pulse, MCR with DS, and postoperative radiotherapy. However, they also mentioned that their treatment strategy was not curative.
On the other hand, the EPP procedure makes it possible to achieve not only MCR but also complete pathologic resection, especially for patients with the Da type of disseminated nodules, which also may lead to a longer PFS. Conversely, QoL is occasionally lower than that of other procedures because pneumonectomy is a highly invasive procedure. Ishikawa et al.17 reported that patients who received EPP instead of DS had a more favorable prognosis. Fabre et al.18 also reported the long-term outcome for 17 patients with stage IVa thymoma who received EPP. It was concluded that EPP, as part of multidisciplinary treatment, may provide good long-term survival for highly selected patients despite the association of significant EPP morbidity and mortality rates.18
In the current study, 5 of 12 patients with DS and 7 of 8 patients with EPP achieved complete resection with a negative PSM. Although the type of surgical procedure was not found to be significantly associated with PFS, EPP tended to achieve complete resection of disseminated nodules with negative PSM. Postoperative complications were observed in two patients with EPP.
Previous studies with a limited number of patients who had stage IVa or recurrent thymoma with pleural dissemination reported a wide range of 5-year OS, from 60% to 93%.7,12,17,19,33 In accordance with previous reports,18,33 dissemination status (primary or recurrent tumor) in our study was not significantly associated with the prognosis of the patients, although the disseminated nodules in recurrent cases tended to invade beyond the elastic layer.
According to a recent study based on a large-scale database of thymoma patients with pleural dissemination from the Japanese Association for Research on the Thymus, the 5-year OS and recurrence-free survival rates were respectively 89% and 30%.7 The 5-year OS and PFS rates in the current study were respectively 95% and 50%, which are comparable with previous findings.
In the current study, the growth pattern of thymoma-derived pleural-disseminated nodules tended to be well-circumscribed, expansile, and lobular rather than infiltrative. This indicates that the individual disseminated nodules can be resected with adequate surgical margins. However, tumor cells were observed sometimes infiltrating beyond the elastic layer into the underlying parenchyma, especially in recurrent cases.
Based on the current findings, to promote prolonged OS and PFS, surgeons should aim to achieve complete resection of the nodules with a negative PSM. However, surgeons find it difficult to discern during surgery whether the disseminated lesion is confined beneath the elastic layer or invading beyond the elastic layer (Da or Db).
Notably, the size of disseminated nodules was not associated with the depth of pleural invasion. Therefore, to determine the depth of invasion during surgery, it is important to assess the mobility of the nodule with palpation. When the nodule has limited mobility from the surrounding tissue, it has likely invaded beyond the elastic layer. In such cases, if the tumor is deemed resectable, surgeons should attempt to remove it together with underlying tissue to enhance the possibility of negative surgical margins, which may improve patient prognosis.
The current study had several limitations. It was a retrospective investigation, and the rarity of thymoma with pleural dissemination made it difficult to collect a sufficient number of patients from a single institution for evaluation of prognostic factors. Because of the small patient cohort, multivariate analysis was not performed. The results were not compared with those of patients who received nonsurgical treatments. A better prognosis for patients who underwent resection than for those who did not has been previously reported.6 Finally, the follow-up period for our cohort was short, and 10-year OS and PFS rates were not available for analyses. We hope the results of the current study will stimulate further investigation regarding invasiveness of nodules from thymic malignancies, especially the depth of pleural invasion.
In conclusion, this study demonstrated that the depth of pleural invasion of disseminated nodules was significantly associated with prognoses. The patients who had disseminated nodules entrapped within the thick elastic layer had a higher probability of prolonged OS and PFS and tended to achieve negative pathologic status of the surgical margin compared with the patients who had disseminated nodules invading beyond the elastic layer. The disseminated nodules sometimes invaded the muscle layer or pulmonary parenchyma beyond the elastic layer, generally showing expansile growth pattern.
Surgeons should aim at complete pathologic resection with negative surgical margins for the disseminated nodules, which may contribute to improved patient prognosis. Further studies with larger numbers of thymoma patients are needed to confirm the results of the current study.
References
Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J. 2013;126:2186–91.
Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–92.
Weissferdt A, Kalhor N, Bishop JA, et al. Thymoma: a clinicopathological correlation of 1470 cases. Hum Pathol. 2018;73:7–15.
Vladislav T, Jain RK, Alvarez R, et al. Extrathoracic metastasis of thymic origin: a review of 35 cases. Mod Pathol. 2012;25:370–7.
Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1320 patients from Japan. Ann Thorac Surg. 2003;76:878–85.
Brierley JD, Gospodarowicz MK, Wittekiind C. UICC, TNM Classification of Malignant Tumours. 8th edn. New York, NY: Wiley; 2017.
Yano M, Sasaki H, Yukiue H, et al. Thymoma with dissemination: efficacy of macroscopic total resection of disseminated nodules. World J Surg. 2009;33:1425–31.
Okuda K, Yano M, Yoshino I, et al. Thymoma patients with pleural dissemination: nationwide retrospective study of 136 cases in Japan. Ann Thorac Surg. 2014;97:1743–9.
Moser B, Fadel E, Fabre D, et al. Surgical therapy of thymic tumours with pleural involvement: an ESTS thymic working group project. Eur J Cardiothorac Surg. 2017;52:346–55.
Liu HC, Chen YJ, Tzen CY, et al. Debulking surgery for advanced thymoma. Eur J Surg Oncol. 2006;32:1000–5.
Lucci M, Davini F, Ricciardi R, et al. Management of pleural recurrence after curative resection of thymoma. J Thorac Cardiovasc Surg. 2009;137:1185–9.
Murakawa T, Karasaki T, Kitano K, et al. Invasive thymoma disseminated into the pleural cavity: mid-term results of surgical resection. Eur J Cardiothorac Surg. 2015;47:567–72.
Imanishi N, Nabe Y, Takenaka M, et al. Extended pleurectomy decortication for thymoma with pleural dissemination. Gen Thorac Cardiovasc Surg. 2019;67:814–7.
Miyahara R, Hasegawa S, Kono T, et al. Extended pleurectomy decortication for Masaoka stage IVa thymoma with massive pleural and pericardial dissemination. Gen Thorac Cardiovasc Surg. 2020;68:1569–72.
Wright CD. Pleuropneumonectomy for the treatment of Masaoka stage IVa thymoma. Ann Thorac Surg. 2006;82:1234–9.
Huang J, Rizk NP, Travis WD, et al. Feasibility of multimodality therapy including extended resections in stage IVa thymoma. J Thorac Cardiovasc Surg. 2007;134:1477–83.
Ishikawa Y, Matsuguma H, Nakahara R, et al. Multimodality therapy for patients with invasive thymoma disseminated into the pleural cavity: the potential role of extrapleural pneumonectomy. Ann Thorac Surg. 2009;88:952–7.
Fabre D, Fadel E, Mussot S, et al. Long-term outcome of pleuropneumonectomy for Masaoka stage IVa thymoma. Eur J Cardiothorac Surg. 2011;39:133–8.
Nakamura S, Kawaguchi K, Fukui T, et al. Multimodality therapy for thymoma patients with pleural dissemination. Gen Thorac Cardiovasc Surg. 2019;67:524–9.
Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. IARC, Lyon, 2015.
Yokoi K. Revision of the general rule for surgical record of lung cancer (in Japanese). Jpn J Lung Cancer. 2012;52:68–71.
The Japan Lung Cancer Society. General Rule for Clinical and Pathological Record of Lung Cancer (in Japanese), 8th ed. Kanehara, Tokyo, 2017.
Huang J, Detterbeck FC, Wang Z, et al. Standard outcome measures for thymic malignancies. J Thorac Oncol. 2010;5:2017–23.
Tateo V, Manuzzi L, Parisi C, et al. An overview on molecular characterization of thymic tumors: old and new targets for clinical advances. Pharmaceuticals. 2021;14:316.
Shimizu K, Yoshida J, Nagai K, et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovascular Surg. 2005;130:160–5.
Butnor KJ, Beasley MB, Cagle PT, et al. Protocol for the examination of specimens from patients with primary non-small cell carcinoma, small cell carcinoma, or carcinoid tumor of the lung. Arch Pathol Lab Med. 2009;133:1552–9.
Yoshida J, Nagai K, Asamura H, et al. Visceral pleura invasion impact on non-small cell lung cancer patient survival: its implications for the forthcoming TNM staging based on a large-scale nation-wide database. J Thorac Oncol. 2009;4:959–63.
Travis WD, Brambilla E, Rami-Porta R, et al. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol. 2008;3:1384–90.
Nagaishi C. Pulmonary Pleura: Functional Anatomy and Histology of the Lung. Chapter 6. Igaku Shoin, Tokyo, 1972, pp 254–61.
Dail DH, Hammer SP. Pulmonary Pathology. New York: Springer-Verlag Inc; 1988. p. 789–826.
Corrin B, Nicholson AG. Pathology of the Lungs E-Book: Expert Consult. Online and in print, Elsevier Health Sciences, Oxford, 2011.
Taube JM, Askin FB, Brock MV, et al. Impact of elastic staining on the staging of peripheral lung cancers. Am J Surg Pathol. 2007;31:953–6.
Kimura K, Kanzaki R, Kimura T, et al. Long-term outcomes after surgical resection for pleural dissemination of thymoma. Ann Surg Oncol. 2019;26:2073–80.
Acknowledgments
We thank Dr. Kota Ono (https://ono-biostat-consulting.com/), also an employee of AbbVie GK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakamura, S., Tateyama, H., Nakanishi, K. et al. Pleural Invasion Depth of Disseminated Nodules in Patients with Stage IVa or Recurrent Thymoma: Assessment, Curative Impact, and Surgical Outcomes. Ann Surg Oncol 29, 1829–1837 (2022). https://doi.org/10.1245/s10434-021-10888-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10888-0