Abstract
Predation strategies are driven by habitat structure, prey’s nutritional value, and/or by predator characteristics such as developmental stage. Here, we evaluated the feeding habits of the social pseudoscorpion Paratemnoides nidificator in two areas of a Brazilian Cerrado savanna. These pseudoscorpions live under tree bark trunks of varying sizes; habitat structure could interfere with pseudoscorpion ambushing behavior and prey accessibility. We therefore assessed the hypotheses that (i) small and large colonies of P. nidificator will capture prey of distinct amounts and sizes; (ii) habitat structure will limit the captured prey size; (iii) there will be an age-dependent prey choice in P. nidificator. We evaluated the prey items, colony composition, and habitat structure of pseudoscorpions and determined whether P. nidificator presents age-dependent feeding preferences by offering prey items of different sizes. Colonies with more individuals captured more prey items and those prey presented a wider size variety. P. nidificator can capture a high variety of prey sizes by using openings in tree bark as a trap; however, only tree barks of intermediate size amplitude may be used for trapping most prey. Nymphs showed no preference for prey size, while adults mainly fed on larger ants. Tree bark may play a role as a phenotype extension by easing the process of large prey capture, which is considered a crucial factor for social species. Small prey might be a complementary food resource for nymphs, reducing intraspecific competition and their exposure to larger, dangerous prey.
Significance statement
Habitat structure and prey’s traits such as size affect the predation strategies of several animals. How these features interfere in the feeding habits and prey accessibility of social arachnids is a matter of question. We showed that habitat structure and colony size drive the prey size preference of a social pseudoscorpion. Paratemnoides nidificator lives under tree trunk barks that vary in size, depth, and shape. The tree bark openings may play a crucial role by easing the capture process of different prey sizes, including large prey, which is considered a crucial factor for social species. According to the prey size hypothesis, social species require, collectively, higher amounts of food energy. Thus, we propose that the bark openings are related to the evolution of P. nidificator’s social behavior as they potentially allow the capture of larger and more nutritious prey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat structure is considered to play an important role regarding the evolution of countless species. According to McCoy and Bell (1991), habitat structure is defined as the set of physical structures constituted of biotic and abiotic components that support both the animal and vegetal community. Many studies demonstrated the positive effects of highly structured habitats on predators, as they affect the food encounter frequency and food handling time (Vincent et al. 1996) and provide better conditions—specially to ambush species—to approach elusive prey (Folsom and Collins 1984; Manatunge et al. 2000; Shochat et al. 2004). There are several examples of predators that rely on habitat characteristics to ambush prey that otherwise would be difficult or even impossible to be captured (e.g., Higgins and Buskirk 1992; González-Bernal et al. 2011; Scharf et al. 2011).
Besides the countless strategies applied by predators, they must also decide which prey is worth or not to eat (Stephens and Krebs 1986; Houston and McNamara 1999; Dugatkin 2014). Prey size is an important factor as it defines how much energy a predator obtains from consumption (Harper and Blake 1988; Křivan 1996; Brechbühl et al. 2011). However, large prey can cause injury to the predator (Forbes 1989; Dietl 2003; Rutten et al. 2006) and the time spent weakening and handling it may also be excessive (Rovero et al. 2000). Conversely, small prey may be easier to kill and handle, but the amount of energy they provide to certain predators may be insufficient (Chen and Jiang 2006; Fossette et al. 2011; Tizo-Pedroso and Del-Claro 2018).
The combination of a suitable habitat structure and predation strategy may allow the capture of difficult large prey, which is especially important for social species as they experience a higher competition for food (Ward and Webster 2016). Yip et al. (2008) found that large colonies of the social spider Anelosimus eximius catch a smaller number of per capita preys than small colonies due to their lower surface area/volume ratio. However, this loss is contrasted by a large prey size that big colonies are able to capture, increasing the amount of energy obtained. These findings support the recent prey size hypothesis (Avilés et al. 2007; Powers and Avilés 2007; Purcell 2011), which proposes that a given abundance of large insects is required in order to energetically support large colonies of spiders.
The amount of energy provided by prey involves not only its size but also the assimilation ability of its predators, which can also vary intraspecifically (Krebs and Davies 1993; Chase et al. 2002; Gonzaga and Vasconcellos-Neto 2002). Factors such as age are related to shifts in species’ diets, which are determined by the relative costs and benefits that vary with differences in morphology, physiological needs, experience, and competitive ability (Hamilton and Barclay 1998). Furthermore, age-shifts in diet can also benefit populations that share the same space. For instance, many species that change the prey items over their lives may benefit from avoiding intraspecific competition (e.g., Winemiller 1989; Field et al. 2007).
Therefore, prey size and age-shifts in diet may play an important role on the maintenance of social species (O’Brien et al. 1990; Byk and Del-Claro 2011). Despite being well studied in diverse groups such as birds, mammals, fishes, and insects (e.g., Field et al. 2007; Boggs 2009; Belleggia et al. 2014; Alonso et al. 2015), age-shift diets and prey size effects are less explored in arachnids, especially in social species such as the Paratemnoides nidificator (Balzan, 1888) pseudoscorpion, in which these effects should be more evident (Tizo-Pedroso and Del-Claro 2011).
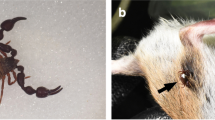
Paratemnoides nidificator (synonym of P. elongatus according to Judson 2016) is a social pseudoscorpion (4–8 mm) that lives under rough tree barks in the Brazilian savanna (Cerrado). These barks vary in depth and shape and there is no evidence of a bark structure preference by these pseudoscorpions. Its colonies can contain up to 300 individuals, and they exhibit high intraspecific tolerance and social features that are uncommon among other pseudoscorpion species, such as extended parental care, matriphagy (nymphs that feed on parts of adult females in starvation conditions), and cooperative hunting (Tizo-Pedroso and Del-Claro 2005, 2007, 2018). The social organization of P. nidificator enables it to capture a wide range of prey items, including ants, beetles, and spiders of different sizes (Tizo-Pedroso and Del-Claro 2007). They are sit-and-wait predators, often capturing prey by catching their legs or antennae and pulling them under the tree bark in which they live (Del-Claro and Tizo-Pedroso 2009; Tizo-Pedroso and Del-Claro 2018). The structure of bark openings seems to play an important role in P. nidificator predation process, as large prey frequently get trapped under the tree bark, allowing P. nidificator to feed on it. Despite the fact that adults perform most of the protective behavior and prey capture, nymphs also participate in hunts, sometimes in large numbers (Tizo-Pedroso and Del-Claro 2005, 2011). Nevertheless, we currently have only a poor understanding of how nymphs forage and feed on different prey items, and we do not know if their feeding behavior changes with age.
Given this, our main objective was to evaluate whether habitat structure, prey size, and age-based feeding preferences shape the predation strategy of P. nidificator. Given the higher energetic needs required by groups of social species (Avilés et al. 2007; Powers and Avilés 2007; Purcell 2011) and their known preference for larger prey (Tizo-Pedroso and Del-Claro 2007, 2011), we hypothesized that (i) small and large colonies of P. nidificator will capture prey of distinct amounts and sizes; we expect that large colonies will capture large and more numerous prey than small colonies. Considering the sit-and-wait behavior and foraging strategy of P. nidificator, we hypothesized that (ii) the width of openings in the tree bark influences the size of prey they can capture; larger openings might allow the capture of larger and more variable prey items (Fig. 1), which is expected from social sit-and-wait predators (Griffiths 1980; O’Brien et al. 1990; Barnard 2004; Yip et al. 2008; Purcell 2011). Finally, based on optimal foraging theory (Stephens and Krebs 1986; Kamil et al. 1987; Stephens 2008), we hypothesized that (iii) adults and nymphs of P. nidificator will show distinct interest on prey of different sizes. We predict that adults will show less interest in small prey as they should offer less energy to them. However, nymphs will show no preference over small and large prey, as both prey should offer enough energy to them.
Hypothetical use of bark openings in three capture processes of large prey by Paratemnoides nidificator. Illustrated ants have the same size. a P. nidificator can use a bark opening to trap prey. b In narrow openings, P. nidificator can still capture the prey, but is unable to trap it under the bark. c Excessively wide openings cannot be used as a trap by P. nidificator and in addition, some arthropods can fight back and cause them harm (Illustration by Jefferson Nascimento)
Methods
Field study
We undertook fieldwork in two study sites, the Sabiá Municipal Park (18° 55′ S, 48° 17′ W) and the Caça e Pesca Itororó de Uberlândia Reserve (18° 59′ S, 48° 18′ W), both at Uberlândia city, Minas Gerais state, Brazil. Sabiá Municipal Park covers an area of 18,500,000 m2 including 350,000 m2 of woods surrounded by urban development. Caça e Pesca Reserve is a private establishment within a conservation unity, consisting of 7,066,400 m2 of Cerrado vegetation, sustained by the Uberabinha River (for area characterization, see Vilela et al. 2014, Velasque and Del-Claro 2016). Both areas contain trees (Caesalpinia peltophoroides Benth. [Fabaceae]) in which colonies of P. nidificator are commonly found (Tizo-Pedroso and Del-Claro 2007).
Predation strategy and colony characterization
In July 2015, we located and marked 30 colonies at each area, one colony per tree. After capturing and killing prey, pseudoscorpions feed on the internal tissues by injecting a digestive secretion into the pleura and sucking their dissolved tissues. Most prey’s exoskeletons are discarded from the colonies almost intact, which allow for their collection and identification. Prey collectors (see Tizo-Pedroso and Del-Claro 2007) were attached on the tree trunks (two collectors per tree) with 30 previously identified colonies at each per area. The collectors were made of plastic bottles and then attached to the trees using pins and silicon glue to avoid prey of slipping between the collectors’ edges. One of the collectors was placed under a colony, so we could survey the discarded prey exoskeletons. The additional collector was positioned on the opposite side of the same colony at a similar height, but in a spot that did not contain any pseudoscorpion, so we could compare the samples between the two collectors. We assessed prey consumed by P. nidifcator by observing their legs’ disposition; when dead arthropods present very stretched legs, it is a sign that they were manipulated by P. nidificator.
At the beginning of the study, we measured two openings of the colonies—the maximum and minimum space between the bark and the trunk where P. nidificator lives—with a digital caliper (centimeters), and weekly, we gathered all prey found in the plastic collectors using a wet, small brush. This survey was conducted from August 2015 to May 2016 and during this period, we studied all marked colonies. All the biological material was conserved in 70% alcohol, organized by colony. At the end of the study, we identified the taxonomic orders of the collected arthropods and counted and measured (length and width [centimeters]) the individuals that were found in one piece using a digital caliper. We considered length the longitudinal measure of prey, excluding antennae (if present) and other terminal appendages, whereas prey width was considered as the latero-lateral measurement from the largest part of prey. Once the prey-sampling period was complete, we collected the marked colonies and counted the number of individuals, both adults and nymphs, within each colony. We did not count the number of individuals at the beginning of this study as counting implies in damaging the bark structure of colonies.
Prey size preference
We raised 15 colonies of P. nidificator at the Laboratory of Behavioral Ecology and Interactions of the Federal University of Uberlândia. They were housed in Petri dishes (15 cm in diameter) with a fixed piece of bark, so we could see the colony through the glass (e.g., Tizo-Pedroso and Del-Claro 2014). All colonies were collected from both study sites, the Sabiá Municipal Park and the Caça e Pesca Itororó de Uberlândia Reserve. They were kept for approximately 3 months before we started this study, being fed weekly with ants (Camponotus mus and Camponotus crassus) and termites (Nasutitermes sp.). Then, we established two trials. First, we offered, simultaneously, workers of Tapinoma sessile ants of 2 mm length to each colony and observed the behavior for 1 h. This ant is commonly found under rough bark of C. peltophoroides trees where P. nidificator also lives. The number of ants offered was related to each colony size. We ensured that each pseudoscorpion had at least one ant to feed on and we observed one colony at a time, noting every individual (adult or nymph) that fed on the captured ants, but we only noted the individuals that fed for at least 2 min.
For the second trial, Camponotus mus ants were used to feed the colonies. As in T. sessile, this species is also commonly found on the trunk of C. peltophoroides trees and is consumed by P. nidificator. We offered one C. mus (0.6–1.0 cm) to each colony and observed its capture and the number of adults and nymphs that fed on it. However, previous observations showed that we needed to observe each colony for a longer period of time (2 h) for two reasons: first, the time P. nidificator took to subdue the prey until its death was greater due to the ant size; second, as we offered only one ant to the colony, some individuals took more time to find the prey. As in the first treatment, we noted every individual that fed for at least 2 min on the ant. In both tests, we did not distinguish whose individuals were responsible for prey captures.
Statistical analysis
A Student’s t test was used to analyze possible differences in prey length from each studied site. We used a logarithmic transformation and checked data normality both visually, using boxplots and histograms, and by a Lilliefors probability test (P = 0.15; n = 310). Mean prey width did not conform to a normal distribution, even with transformations, so we used a non-parametric Mann-Whitney test to verify whether this factor differed between the studied sites.
We used linear and nonlinear regressions associating the mean size of width and ∆ width (maximum width-minimum width) of prey collected from each colony with the mean and ∆ opening (maximum opening-minimum opening in bark) found in the bark openings of each tree. The bark opening size is associated to the potential prey that can be captured by P. nidificator—large bark openings should allow the capture of small and large prey, which means that prey’s ∆ width might be higher in these colonies. It is possible that prey’s mean width has the same pattern, as the capability of capturing large prey in colonies with large bark openings might increase the mean width of collected prey. We did not use length measures as we conjectured that it should not be a relevant variable to test against bark openings; moreover, animal’s length and width are usually correlated variables, so the best approach is to use just one of them. We also used linear regressions to examine the association between the number of P. nidificator individuals from each colony and the mean prey size (length and width) to ∆ width of the sampled prey. Other linear regressions were performed to test associations between the number of prey found and the number of pseudoscorpions per colony, as well as a comparison between the number of prey found and the bark opening size (mean bark opening size and ∆ bark opening size). In the laboratory study, we used chi-square tests to verify any differences in preference for small or large prey by adults and nymphs of P. nidificator, in which the final numbers of feeding individuals per colony were used to create a 2 × 2 contingency table.
Data availability
Data from this study is available at Figshare: https://figshare.com/articles/Manuscript_data/5863698
Results
Predation strategy and colony characterization
We found 310 prey items in the collectors, 237 in Sabiá Park, and 73 in the Caça e Pesca Reserve, from 37 colonies (18 from Sabiá Park and 19 from Caça e Pesca). We could not use part of the original 60 collectors as they were damaged or removed during the survey period. The arthropods collected belonged to four insect orders (Hymenoptera, Dermaptera, Hemiptera, and Coleoptera) plus two individuals from the order Araneae (spiders). The most predominant prey was ants, with 286 individuals (Table 1). They comprised 92.26% of all individuals found, and their body size ranged from 1.73 to 15.87 (x̄ = 6.84 ± 0.14 mm) mm in length and 0.49 to 5.69 mm in width (x̄ = 1.45 ± 0.04 mm). The second most abundant type of prey was Coleoptera, with 15 individuals (4.84%), followed by three Hemiptera (0.97%).
The Student’s t test showed that the mean length of prey items differed between the study areas, with prey found in the Sabiá Park being longer than those obtained from the Caça e Pesca Reserve (t1,308 = 3.74; P < 0.001; data transformed using logarithmic function), while our Mann-Whitney test showed no difference in prey width between the two study sites (U1 = 8302.50; P = 0.60; Table 1). The negative control collectors were empty most of the time during the study, indicating that our sampled arthropods were effectively captured and discarded by pseudoscorpion colonies.
In Sabiá Park, colonies contained 9–67 individuals in total, which consisted of 2–36 adults and 7–50 nymphs. At the Caça e Pesca Reserve, we found 5–32 individuals per colony, comprising 1–23 adults and 0–31 nymphs. We collected a total of 810 individuals in both areas combined (Table 2).
We did not find a relationship between the number of pseudoscorpions per colony and the mean width of captured prey, both using the total number of individuals (F1,32 = 0.78; P = 0.38) and the number of adults (F1,32 = 0.99; P = 0.33). However, we found a positive relationship between the number of individuals and the variation in the width of prey (∆ width), both using the total number of individuals (F1,31 = 15.55; R2 = 0.33; P < 0.001) and the number of adults (F1,31 = 17.40; R2 = 0.36; P < 0.001; Fig. 2). We did not find a relationship between the mean prey width and the mean bark opening size (F1,32 = 0.48; R2 = 0.02; P = 0.48) as well as for ∆ bark opening and the mean of prey width (F1,32 = 1.02; R2 = 0.03; P = 0.32); however, ∆ prey width was related to ∆ bark opening and we found that a quadratic model was the best fit (F2,27 = 5.61; R2 = 0.29; P = 0.009; Fig. 3).
Relationship between the total number of individuals and adults of Paratemnoides nidificator with the mean prey width and prey width variation. The total number of individuals and adults represent non-significant relationships at α = 0.05. All x and y variables were log10 transformed in order to normalize the data
There is a positive relation between the number of adult pseudoscorpions and the number of prey per colony (F1,32 = 29.91; R2 = 0.48; P < 0.001) as well as with the total number of individuals (F1,32 = 20.02; R2 = 0.36; P = 0.001) and the ∆ bark opening (F1,31 = 5.48; R2 = 0.15; P = 0.026). A multiple linear regression showed that the total of adults and ∆ bark opening were related to the number of captured prey per colony (F1,29 = 15.01; R2 = 0.51; P < 0.001); according to Akaike Information Criterion (AIC), they provided a better model (AIC = 11.58) in comparison to the total of adults (AIC = 12.10) and ∆ bark opening (AIC = 26.87) separately. There was no significant linear association between the mean bark opening and the number of captured prey per colony, although there was a positive tendency (F1,31 = 3.52; R2 = 0.10; P = 0.070).
Prey size preference
We used 109 nymphs and 136 adults for tests with small prey and 122 nymphs and 77 adults for trials with large prey (x̄ = 111 individuals). Nymphs showed no significant preference for any offered prey; 55.06% fed on small prey while 67.2% fed on large prey (χ2 = 3.59; P = 0.058). Among adults, only 25% fed on small prey, while 63.63% fed on large ants (χ2 = 30.86; P < 0.001). We found a difference between nymphs and adults regarding the small prey trials (χ2 = 23.09; P < 0.001), but no difference concerning the large prey tests (χ2 = 0.27; P = 0.60; Fig. 4).
Discussion
We concluded that colony size, habitat structure, and prey size preference have considerable impact on the predation ecology of a social predator. Using the pseudoscorpion P. nidificator as a model, we showed that (a) larger groups of this social predator manage to capture more prey items with a wider range of sizes; (b) the habitat structure, here expressed by the bark openings of colonies, influences the size of prey captured, and the relationship between bark openings and prey width showed an optimum range (Fig. 4); and (c) adults of this pseudoscorpion have distinct preferences for prey of different sizes, while nymphs do not present prey size preference.
Our field survey corroborated Tizo-Pedroso and Del-Claro’s (2007) findings. These authors found that P. nidificator feeds on prey of different sizes, including small insects (1–2 mm) and large Scarabaeidae beetles (13 mm), although we found even larger prey (an ant of 15.7 mm length). However, the variety of prey items we found was different from Tizo-Pedroso and Del-Claro (2007): they found that, in dry season, 70% of P. nidificator prey was represented by ants, while in wet season, ants comprised 28% of prey. Although we did not control the prey survey by season, the number of ants we found was clearly greater than that of Tizo-Pedroso and Del-Claro (2007), representing 92.8% of all prey items, and this pattern did not change between the two areas studied here.
For our first hypothesis, we expected that larger colonies (more individuals) would be able to capture larger prey, on average; however, we did not find this relationship. Instead, we observed that larger colonies were able to capture more prey items and these prey presented a greater variation on their size (prey ∆ width). These effects might occur simply because large colonies have a greater probability of capturing prey due to a higher number of predators foraging simultaneously. Furthermore, a high number of foraging individuals also increases the chance of cooperative hunting, which eases the process of capturing large prey. This also explains why the relationship was stronger when we compared prey ∆ width only with the number of adults in each colony, as they are the main force capable of capturing prey (Tizo-Pedroso and Del-Claro 2007).
It is known that some sit-and-wait predators have a tendency to capture large prey (Griffiths 1980; Greene 1986). This occurs because these predators are able to ambush prey while avoiding a direct fight which would raise their chance of being hurt, or even killed, by large prey (Forbes 1989; Dietl 2003; Rutten et al. 2006). Other studies about prey capture behavior in P. nidificator showed that larger colonies are able to capture large prey more frequently (see Tizo-Pedroso and Del-Claro 2007, 2011). However, these studies were conducted under laboratory conditions and did not measure the effects of bark openings on subduing prey. It is therefore possible that, because of heterogeneity in bark structure, some pseudoscorpion colonies experience physical restrictions in accessing some types and/or sizes of prey, which can limit the overall potential of large colonies to exploit the absolute prey availability in natural conditions. The preference for large prey is what we observed for P. nidificator, but as opportunistic predators, they also caught a reasonable range of prey sizes. We suggest that this range of prey sizes is the reason for the lack of relationship between the colony size and the mean prey size, as the high variety of prey items, particularly the small ones, might have reduced the mean size of captured prey. The positive relationship between the colony size and ∆ prey width probably reflects this argument.
We found a relationship between the bark openings’ variation and the observed prey size variation that partially corroborated our second hypothesis. It seems that colonies residing in trees with a great variation in the bark openings’ size (great heterogeneity) are able to capture a high number of prey items (although the size effect is modest) as well as prey of increased size variation; they might capture small prey in the smaller openings and large prey in the larger openings. However, this relationship is not linear; it appears that neither colonies with the smallest nor the greatest ∆ opening values are able to capture such a wide variety of prey. For example, colonies that live within small bark openings might be able to more often capture small individuals; therefore, the values of ∆ opening should be low. On the other hand, the extreme values of ∆ opening reflect the challenge of colonies in capturing either larger individuals or the smaller ones, as the openings are either too wide or too narrow to enable the capture of most prey.
We believe that the use of bark openings by P. nidificator works as a trap and eases the process of prey subduing. It is not common to observe P. nidificator attacking prey outside of tree bark, so we highlight the importance of the bark in the predation process (Tizo-Pedroso and Del-Claro 2007). Additionally, the structure of bark openings of C. peltophoroides not only provides a predation advantage to P. nidificator by easing the process of prey immobilization but also protects these animals from being attacked by their own predators, or by large and dangerous prey. Additional laboratory experiments showed that, when the bark opening is too wide, some ants like Camponotus mus are capable of turning and biting the attacking pseudoscorpion, which sometimes results in the mutilation of their pedipalps or even their death (unpublished data). Thus, the width of bark openings is critical and its heterogeneous distribution (openings of different sizes) should enhance P. nidificator survival, as is the case for many species living in heterogeneous habitats (Nelson and Bonsdorff 1990; Heck and Crowder 1991; Babbitt and Tanner 1998; reviewed by Kovalenko et al. 2012). In addition, bark openings may play a role in the evolution of P. nidificator social behavior by providing conditions for large prey capture, essential to the maintenance of social arthropods, according to the prey size hypothesis (Avilés et al. 2007; Powers and Avilés 2007; Purcell 2011).
According to our third hypothesis, we wanted to verify whether nymphs of P. nidificator have distinct interest in small prey items in comparison to adults, as smaller prey offer proportionally more energy to nymphs than to adults. Our results revealed that, in laboratorial conditions, both nymphs and adults fed on small ants, although adults preferred larger prey. P. nidificator is known for collectively capturing large prey and sharing it among the colony; however, as revealed by Tizo-Pedroso and Del-Claro’s (2007) surveys, P. nidificator feeds on a variety of items but the small prey represented only a little fraction of its diet.
As Yip et al. (2008) stated, large colonies of Anelosimus spiders tend to capture less prey per spider than in small colonies. Still, this system is sustained because these colonies are able to capture larger prey. Considering this, it was expected that P. nidificator adults would prefer larger ants as a diet option. Its preference for large ants possibly indicates a relationship with the higher energy intake these prey provide. Even considering the increase in time and energy that is needed to capture and subdue them, small differences in prey size can be enough to substantially change the amount of energy a predator obtains (Conway et al. 1999).
In trials using small ants (T. sessile), we observed that they easily sneak into the colonies, do not hesitate to approach the pseudoscorpions, and can be killed instantly by one individual (except very small nymphs). Hunting small prey is considered an alternative strategy for smaller and less competitive individuals, as they do not need the participation of others to perform the capture (Gese et al. 1996; Ebert 1998). For nymphs, the benefits might be greater, as they have a limited hunt capacity and presumably have less energetic needs than adults.
In this study, we demonstrated that P. nidificator is an opportunistic predator and manages to capture a wide range of prey size and items including large prey—which appears to be their food preference—using the bark openings to ease the predation process. According to prey size hypothesis, social species require a higher total amount of energy; thus, we propose that the bark openings represent an important factor regarding the evolution of P. nidificator social behavior, as it potentially allows the capture of larger and more nutritious prey.
References
Alonso H, Almeida A, Granadeiro JP, Catry P (2015) Temporal and age-related dietary variations in a large population of yellow-legged gulls Larus michahellis: implications for management and conservation. Eur J Wildl Res 61:819–829
Avilés L, Agnarsson I, Salazar PA, Purcell J, Iturralde G, Yip EC, Powers KS, Bukowski TC (2007) Altitudinal patterns of spider sociality and the biology of a new midelevation social Anelosimus species in Ecuador. Am Nat 170:783–792
Babbitt KJ, Tanner GW (1998) Effects of cover and predator size on survival and development of Ranautricularia tadpoles. Oecologia 114:258–262
Barnard CJ (2004) Animal behavior: mechanism, development, function, and evolution. Pearson Education, Canada
Belleggia M, Figueroa DE, Irusta G, Bremec C (2014) Spatio-temporal and ontogenetic changes in the diet of the Argentine hake Merluccius hubbsi. J Mar Biol Assoc UK 94:1701–1171
Boggs CL (2009) Understanding insect life histories and senescence through a resource allocation lens. Funct Ecol 23:27–37
Brechbühl R, Casas J, Bacher S (2011) Diet choice of a predator in the wild: overabundance of prey and missed opportunities along the prey capture sequence. Ecosphere 2:1–15
Byk J, Del-Claro K (2011) Ant-plant interaction in the Neotropical savanna: direct beneficial effects of extrafloral nectar on ant colony fitness. Popul Ecol 53:327–332
Chase JM, Abrams PA, Grover JP, Diehl S, Chesson P, Holt RD, Richards SA, Nisbet RM, Case TJ (2002) The interaction between predation and competition: a review and synthesis. Ecol Lett 5:302–315
Chen X, Jiang Y (2006) Diet of Chinese skink, Eumeces chinensis: is prey size important? Integr Zool 1:59–66
Conway DVP, Coombs SH, Lindley JA, Llewellyn CA (1999) Diet of mackerel (Scomber scombrus) larvae at the shelf-edge to the south-west of the British Isles and the incidence of piscivory and coprophagy. Vie Milieu 49:213–220
Del-Claro K & Tizo-Pedroso E (2009) Ecological and evolutionary pathways of social behavior in Pseudoscorpions (Arachnida: Pseudoscorpiones). Acta Ethologica, 12:13–22
Dietl GP (2003) Coevolution of a marine gastropod predator and its dangerous bivalve prey. Biol J Linn Soc 80:409–436
Dugatkin LA (2014) Principles of animal behavior. W. W. Norton & Company, New York
Ebert D (1998) Behavioral asymmetry in relation to body weight and hunger in the tropical social spider Anelosimus eximius (Araneae, Theridiidae). J Arachnol 26:70–80
Field IC, Bradshaw CJA, van den Hoff J, Burton HR, Hindell MA (2007) Age-related shifts in the diet composition of southern elephant seals expand overall foraging niche. Mar Biol 150:1441–1452
Folsom TC, Collins NC (1984) The diet and foraging behavior of the larval dragonfly Anax junius (Aeshnidae), with an assessment of the role of refuges and prey activity. Oikos 42:105–113
Forbes LS (1989) Prey defences and predator handling behaviour: the dangerous prey hypothesis. Oikos 55:155–158
Fossette S, Gleiss AC, Casey JP, Lewis AR, Hays GC (2011) Does prey size matter? Novel observations of feeding in the leatherback turtle (Dermochelys coriacea) allow a test of predator-prey size relationships. Biol Lett rsbl20110965
Gese EM, Ruff RL, Crabtree RL (1996) Intrinsic and extrinsic factors influencing coyote predation of small mammals in Yellowstone National Park. Can J Zool 74:784–797
Gonzaga MDO, Vasconcellos-Neto J (2002) Influence of collective feeding on weight gain and size variability of Anelosimus jabaquara Levi 1956 (Araneae: Theridiidae). Behaviour 139:1431–1442
González-Bernal E, Brown GP, Cabrera-Guzmán E, Shine R (2011) Foraging tactics of an ambush predator: the effects of substrate attributes on prey availability and predator feeding success. Behav Ecol Sociobiol 65:1367–1375
Greene CH (1986) Patterns of prey selection: implications of predator foraging tactics. Am Nat 128:824–839
Griffiths D (1980) Foraging costs and relative prey size. Am Nat 116:743–752
Hamilton IM, Barclay RMR (1998) Diets of juvenile, yearling, and adult big brown bats (Eptesicus fuscus) in southeastern Alberta. J Mammal 79:764–771
Harper DG, Blake RW (1988) Energetics of piscivorous predator-prey interactions. J Theor Biol 134:59–76
Heck K Jr, Crowder LB (1991) Habitat structure and predator: prey interactions in vegetated aquatic systems. In: Bell SS, McCoy ED, Mushinsky HR (eds) Habitat structure: the physical arrangement of objects in space. Springer, London, pp 281–299
Higgins LE, Buskirk RE (1992) A trap-building predator exhibits different tactics for different aspects of foraging behaviour. Anim Behav 44:485–499
Houston AI, McNamara JM (1999) Models of adaptive behaviour: an approach based on state. Cambridge University Press, UK
Judson ML (2016) Pseudoscorpions (Arachnida, Chelonethi) in Mexican amber, with a list of extant species associated with mangrove and Hymenaea trees in Chiapas. Bol Soc Geol Mex 68:57–79
Kamil AC, Krebs JR, Pulliam HR (1987) Foraging behavior. Plenum Press, New York
Kovalenko KE, Thomaz SM, Warfe DM (2012) Habitat complexity: approaches and future directions. Hydrobiologia 685:1–17
Krebs JR, Davies NB (1993) An introduction to behavioural ecology. Blackwell Scientific Publications, Oxford
Křivan V (1996) Optimal foraging and predator-prey dynamics. Theor Popul Biol 49:265–290
Manatunge J, Asaeda T, Priyadarshana T (2000) The influence of structural complexity on fish-zooplankton interactions: a study using artificial submerged macrophytes. Environ Biol Fish 58:425–438
McCoy ED, Bell SS (1991) Habitat structure: the evolution and diversification of a complex topic. In: Bell SS, McCoy ED, Mushinsky HR (eds) Habitat structure: the physical arrangement of objects in space. Springer, London, pp 3–27
Nelson WG, Bonsdorff E (1990) Fish predation and habitat complexity: are complexity thresholds real? J Exp Mar Biol Ecol 141:183–194
O’Brien WJ, Browman HI, Evans BI (1990) Search strategies of foraging animals. Am Sci 78:152–160
Powers KS, Avilés L (2007) The role of prey size and abundance in the geographical distribution of spider sociality. J Anim Ecol 76:995–1003
Purcell J (2011) Geographic patterns in the distribution of social systems in terrestrial arthropods. Biol Rev 86:475–491
Rovero F, Hughes RN, Chelazzi G (2000) When time is of the essence: choosing a currency for prey-handling costs. J Anim Ecol 69:683–689
Rutten AL, Oosterbeek K, Ens BJ, Verhulst S (2006) Optimal foraging on perilous prey: risk of bill damage reduces optimal prey size in oystercatchers. Behav Ecol 17:297–302
Scharf I, Lubin Y, Ovadia O (2011) Foraging decisions and behavioural flexibility in trap-building predators: a review. Biol Rev 86:626–639
Shochat E, Stefanov WL, Whitehouse MEA, Faeth SH (2004) Urbanization and spider diversity: influences of human modification of habitat structure and productivity. Ecol Appl 14:268–280
Stephens DW (2008) Decision ecology: foraging and the ecology of animal decision making. Cogn Affect Behav Neurosci 8:475–484
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, UK
Tizo-Pedroso E, Del-Claro K (2005) Matriphagy in the neotropical pseudoscorpion Paratemnoides nidificator (Balzan 1888) (Atemnidae). J Arachnol 33:873–877
Tizo-Pedroso E, Del-Claro K (2007) Cooperation in the neotropical pseudoscorpion, Paratemnoides nidificator (Balzan, 1888): feeding and dispersal behavior. Insect Soc 54:124–131
Tizo-Pedroso E, Del-Claro K (2011) Is there division of labor in cooperative pseudoscorpions? An analysis of the behavioral repertoire of a tropical species. Ethology 117:498–507
Tizo-Pedroso E, Del-Claro K (2014) Social parasitism: emergence of the cuckoo strategy between pseudoscorpions. Behav Ecol 25:335–343
Tizo-Pedroso E, Del-Claro K (2018) Capture of large prey and feeding priority in the cooperative pseudoscorpion Paratemnoides nidificator. Acta Ethol 21:109–117. https://doi.org/10.1007/s10211-018-0288-5
Velasque M, Del-Claro K (2016) Host plant phenology may determine the abundance of an ecosystem engineering herbivore in a tropical savanna. Ecol Entomol 41:421–430
Vilela AA, Torezan-Silingardi HM, Del-Claro K (2014) Conditional outcomes in ant-plant-herbivore interactions influenced by sequential flowering. Flora 209:359–366
Vincent TLS, Scheel D, Brown JS, Vincent TL (1996) Trade-offs and coexistence in consumer-resource models: it all depends on what and where you eat. Am Nat 148:1038–1058
Ward A, Webster M (2016) Social foraging and predator-prey interactions. In: Ward A, Webster M (eds) Sociality: the behaviour of group-living animals, 1st edn. Springer, Switzerland, pp 1–8
Winemiller KO (1989) Ontogenetic diet shifts and resource partitioning among piscivorous fishes in the Venezuelan ilanos. Environ Biol Fish 26:177–199
Yip EC, Powers KS, Avilés L (2008) Cooperative capture of large prey solves scaling challenge faced by spider societies. Proc Natl Acad Sci 105:11818–11822
Acknowledgments
We thank Eduardo Novaes Ramires, Vanessa Stefani, Egon Vilela, and three anonymous reviewers for corrections and suggestions in this manuscript.
Funding
RFM thanks CAPES for a master fellowship. KDC thanks CNPq and FAPEMIG for research grants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. C. Choe
Electronic supplementary material
ESM 1
(XLSX 15 kb)
Rights and permissions
About this article
Cite this article
Moura, R.F., Tizo-Pedroso, E. & Del-Claro, K. Colony size, habitat structure, and prey size shape the predation ecology of a social pseudoscorpion from a tropical savanna. Behav Ecol Sociobiol 72, 103 (2018). https://doi.org/10.1007/s00265-018-2518-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2518-2