Abstract
Southern elephant seals are important apex predators in a highly variable and unpredictable marine environment. In the presence of resource limitation, foraging behaviours evolve to reduce intra-specific competition increasing a species’ overall probability of successful foraging. We examined the diet of 141 (aged 1–3 years) juvenile southern elephant seals to test the hypotheses that differences between ages, sexes and seasons in diet structure occur. We described prey species composition for common squid and fish species and the mean size of cephalopod prey items for these age groups. Three cephalopod species dominated the stomach samples, Alluroteuthis antarcticus, Histioteuthis eltaninae and Slosarczykovia circumantarcticus. We found age-related differences in both species composition and size of larger prey species that probably relate to ontogenetic changes in diving ability and haul-out behaviour and prey availability. These changes in foraging behaviour and diet are hypothesised to reduce intra-specific food competition concomitant with the increase in foraging niche of growing juveniles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The distribution of biological resources within the Southern Ocean is highly variable, unpredictable and patchy at several spatial and temporal scales (El-Sayed 1988; Constable et al. 2003). Spatial and temporal variation in the physical oceanographic factors provide a diversity of habitats that influence the distribution, structure and abundance of ecological communities (Lutjeharms 1990; Rodhouse and White 1995; Arrigo et al. 1998; Constable et al. 2003). In recent years, our understanding of primary production and energy flow through the lower trophic levels of the marine food web in this region have improved greatly (Arrigo et al. 1998; Constable et al. 2003); however, there is still a significant lack of information regarding energy flow through the mid-order organisms (Rodhouse and White 1995) that are important prey sources for predators such as seabirds, pinnipeds and cetaceans.
As juveniles, individuals need to grow rapidly to maximise their lifetime reproductive fitness. This is more pronounced in polygamous species that show pronounced sexual dimorphism and where reproductive success in one sex (generally males) is highly variable (Trivers 1985; Clinton 1994). Phenotypic plasticity in growth patterns combined with ontogenetic differences in behaviour may reduce competition for resources and the negative effects of environmental variation (Pianka 1981; Polis 1984; Schoener 1986; Post and Parkinson 2001; Bolnick et al. 2003). The end result will be ontogenetic niche shifts (Woodward and Hildrew 2002), and ultimately resource partitioning of total niche width of the species attributed to the age/size structure of the population (Warren 1996; Williams and Martinez 2000; Bolnick et al. 2003). As such, a reduction in intra-specific competition by resource partitioning has been observed for many species (Polis 1984) over a range of spatial scales, especially where food resources may be limited due to intra- and inter-annual variation in productivity (Perry 1996; Kato et al. 2000; Wikelski and Wrege 2000; Bowen et al. 2002), though there have been few detailed studies of larger marine predators. Therefore, in an unpredictable environment it is likely that predator species will display ontogenetic niche shifts that will reduce competition and maximise foraging success for each age class (Van Valen 1965; Takimoto 2003; Field et al. 2005a).
The southern elephant seal (Mirounga leonina) is an apex predator of the pelagic open-ocean system. This species has a circumpolar distribution, is a wide-ranging, deep-diving predator that spends more than 80% of its annual cycle at sea (Le Boeuf and Laws 1994). They are major consumers of biomass, primarily squid and fish (Boyd et al. 1994; Bradshaw et al. 2003; Hindell et al. 2003). The population of M. leonina at Macquarie Island in the Pacific sector of the Southern Ocean has been declining since 1950 for reasons that are still unclear (Hindell et al. 1994), although it has been suggested that this species is susceptible to changes in the availability of prey (Hindell et al. 1994; Guinet et al. 1999; Slip and Burton 1999; McMahon et al. 2003, 2005). In particular, juvenile survival, which has been suggested to be influenced by ontogenetic changes in morphology, behaviour and foraging experience, appears to be one of the driving factors in the decline and population change in general (McMahon et al. 2003; Hindell et al. 1994).
Recent studies have demonstrated that there is an ontogenetic change in diving and foraging capacity in elephant seals, and though their complex physiology is not completely understood as the animals age and increase in body size, their ability to dive longer and deeper also increases (Le Boeuf et al. 1996; Slip 1997; Stewart 1997; Irvine et al. 2000; Le Boeuf et al. 2000; Field et al. 2004, 2005a). Elephant seals are also highly sexually dimorphic as adults (although less so as juveniles); however, gender differences in energy use by juveniles relating to the requirements for moulting and sexual development have been demonstrated (Field et al. 2005b). Thus, it is likely that juvenile elephant seals should demonstrate shifts in diet structure as they age towards adulthood.
Previous studies of southern elephant seal diet (Rodhouse et al. 1992; Green et al. 1998; Slip 1995; van den Hoff 2004) have identified that they are opportunistic generalist feeders with a broad foraging niche (Whitehead et al. 2003), with geographical (Green et al. 1998; Danieri et al. 2000) and seasonal (Piatkowski et al. 2002; Bradshaw et al. 2003) differences in diet composition. However, no one has addressed the hypothesised change in diet composition within the juvenile years.
Therefore, in this study, we examine the diet of juvenile southern elephant seals for intra-specific and seasonal differences that may result from variation in at-sea behaviour. Furthermore, we address the complex question of whether the previously observed seasonal differences in metabolic rate within the juvenile age classes are a function of variation in prey species abundance or whether it is variation due to physiological limitations. We hypothesise that (1) as juvenile seals age and grow, they are able to dive deeper and travel farther from Macquarie Island, they may be able to exploit larger prey and/or increase number of species available to them as a function of prey spatial variation; (2) seasonal differences in at-sea behaviour and haul-out patterns may also affect prey availability and hence diet composition; and (3) there may be sexual differences in the diet selected due to the different metabolic requirements of males and females (Field et al. 2005b). Finally, where intra-specific differences have been found, we have calculated the minimum sample required to find a difference using a novel approach that can be used for future lavaging studies.
Methods and materials
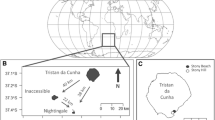
From September 1999 to September 2000, 141 known age juvenile southern elephant seals (McMahon et al. 2003) were stomach lavaged during their annual haul-out periods as they returned ashore at Macquarie Island (158°57′E, 54°30′S; Fig. 1). Juvenile seals were determined as being between 1 and 4 years old, and are referred to as 1-, 2- or 3-year-old seals. Seals caught between the start of September and the end of February were regarded as having been caught during summer and those caught between March–August represent those caught in winter.
The known foraging range of juvenile southern elephant seals from Macquarie Island shown as shaded in grey (adapted from Field et al. 2004), and the possible area the seal may have used within 7 days (700 km) of returning to haul-out shaded within the hatched circle, using 100 km day−1 as a daily rate of travel (adapted from Field et al. 2004)
Sample collection
The beaches on and near the northern isthmus of the island were searched daily for marked individuals returning ashore that day. As the seals returned to the island for either their mid-year (winter) or moult (summer) haul-out, they were caught by placing a canvas bag over the seal’s head (McMahon et al. 2000) and anaesthesia was administered intravenously using prescribed doses (Field et al. 2002) of a combined 1:1 mixture of tiletamine and zolazepam (Telazol®, Forte Dodge, Castle Hill, NSW, Australia).
Once anaesthetised, the seals were weighed (± 1 kg), measured (± 10 mm) and lavaged (Slip 1995). The regurgitant was filtered through a 1-mm sieve to retrieve the stomach contents. The lavage procedure was repeated three times to remove the bulk of the stomach contents. The filtered stomach contents were then placed into a storage jar and filled with 70% ethanol until the contents were sorted and the prey items identified.
To test for age and sex differences in size (mass) of seals that were lavaged, which may attribute to dietary differences, we used a two-way general linear models (GLM) and the ANOVA function in the R package (Ihaka and Gentleman 1996) to describe the relationships between age and sex on seal mass.
In preparation for sample sorting and identification, the stomach contents were flushed with fresh water and placed in a sorting tray. From the stomach samples, the presence of fish otoliths, eyes and bones, squid mouthparts (consisting of an upper and lower beak), penn and eyes, crustaceans and other invertebrates, parasitic worms, sediment and plastic particles were identified. Lower squid beaks were identified to the lower taxa possible, using voucher specimen collections (from Malcolm Clarke held at the Australian Antarctic Division) and descriptions in Clarke (1986), and the lower rostral lengths (LRL) measured to ± 0.01 mm. Slosarczykovia circumantarcticus (Cherel et al. 2004) was previously mis-identified as Brachioteuthis picta (Rodhouse et al. 1992), Mastigoteuthis sp. (Green et al. 1998) and Chiroteuthis sp. (Slip 1995) until correctly identified as a separate genus by Lipinski (2001). Fish otoliths were also identified to genus or species level where possible using a voucher collection (from Dick Williams held at the Australian Antarctic Division) and the descriptions in Williams and McEldowney (1990). Most otoliths showed significant erosion (Williams and McEldowney 1990) and only one pair could be measured.
Statistical analysis—general differences in the prey
To test for overall differences in general prey types (presence/absence), statistical comparisons were made between different sex, age and season groups using ANOSIM analyses on Bray–Curtis dissimilarity matrices (Primer-e, PML, Plymouth, UK) using 999 permutations. Where significant differences between the main effects were found, the differences in prey type (presence/absence) were described using similarity of percentages analyses, SIMPER (Primer-e, PML, Plymouth, UK).
Intra-specific differences in prey species abundance
Common prey species were defined as only those species that were found in (5% of the samples). To test for intra-specific and seasonal differences in the abundance of prey, we used non-parametric multivariate tests (ANOSIMs and SIMPER, as above) that allowed robust analysis of combined prey species data. These tests are limited in their ability to perform multiple interactions between group variables, so in order to test our main hypothesis (i.e. that there are age differences in the diet of juvenile seals) we needed to control for the potential effects of season and gender. Therefore, based on three a priori decisions, we used a hierarchical approach (Fig. 2), first testing for the effect of sex, then controlling for season and finally for age effects by removing any possible confounding interactions that may occur. Due to the limited number of individual seals lavaged in some of the groups, comparisons were only made where we had more than five individuals in each group.
The hierarchal statistical design used in this study to exclude potential confounding effects of sex and season. Comparisons were restricted to groups with >5 stomach samples and are indicated with double-headed arrows. The numbers below each of the tested groups indicate the number of samples and the connecting arrows show the tested comparisons. Asterisk indicates the level of significance (P) of each test where (* >0.05, ** >0.005 and *** >0.001)
Size of prey
Squid size is known to vary throughout the year. Therefore, we compared the mean LRL of the of those common prey species that occurred in all age and sex groups during a limited time (from 22 April 2000 to 19 May 2000) in winter to reduce the influence of variation prey size due to these temporal growth patterns of the individual squid species. Using a one-way linear model in the R package (Ihaka and Gentleman 1996) there were no significant difference in the mean LRL for any of the six common species during this winter observational period. To examine differences in the size of squid eaten by different aged and sex groups, we used two-way GLM and the ANOVA function in the R package (Ihaka and Gentleman 1996).
Minimum sample requirement
As a guide for future diet studies using stomach lavaging in this species, we determined the minimum number of samples required to detect significant age differences in the abundance of prey in their diet. We reasoned that after a number of samples have been compared for each age group, the addition of further samples will not increase the probability of finding a difference between age groups. We took two samples at random from each age group and compared the groups using an ANOSIM. We repeated this 1,000 times, and calculated the number of times that the ANOSIM found a significant difference. We then increased the number of samples taken from each age group by 1 and repeated the process. This was repeated until we reached maximum number of samples within an age group. We were then able to plot the probability of finding a difference at the 5% significance level (α = 0.05) between age groups against the number of samples required from each age group.
Results
During their annual moult, as the seals returned in the austral summer, the mean body mass \( (\ifmmode\expandafter\bar\else\expandafter\=\fi{X}\;{\text{ $ \pm $ \;SD)}} \) of juvenile male and female seals ranged from 200 ± 42 kg for 1-year olds, 256 ± 36 kg for 2-year olds and 350 ± 38 kg for 3-year olds. In winter, during their mid-year haul-out, each of the age groups had increased in mass, where mean masses were 210 ± 29 kg for 1-year olds, 316 ± 47 kg for 2-year olds and 438 ± 92 kg for 3-year olds. Overall, the mass of the seals increased significantly between each age group (F(3,123) = 87.063, P > 0.001) and though male seals were slightly larger than females in each age group they were not significantly different (F(1,123) = 3.796, P = 0.054).
Overall diet composition
Squid remains were found in all 141 samples and fish remains in 107 samples (76%). The remains of Gammarid sp. and Hyperid sp. Amphipods were also found in 17% of the stomach samples, although these were partially digested and could not be identified further. The occurrence (presence/absence) of squid and fish remains in the overall diet composition (Table 1) was unaffected by seal age and sex, and season [Age: Global R = 0.009, significance level of sample statistic (SSS) = 17.3%, number of permuted statistics (NPS) ≤ 0 = 172; Sex: Global R = −0.007, SSS = 85.7%, NPS ≤ 0 = 856; Season: Global R = −0.009, SSS = 63.6%, NPS ≤ 0 = 635; Table 1]. Also noteworthy was that no plastic particles were found in any of the samples.
Squid and fish taxa
Fifteen squid and 17 fish taxa were found, excluding combined genera groups where individual species were identified, within the diet samples (Table 2). The most abundant squid species found in the samples were A. antarcticus (∼60% of samples), Slosarczykovia circumantarctica (∼68%) and Histioteuthis eltaninae (∼80%). Of the fish taxa identified, two genera of Myctophidae were most common, Electrona and Gymnoscopelus species, and were found in ∼11 and ∼9% of the samples, respectively. All taxa are known to have either sub-Antarctic or Antarctic distributions (Clarke 1986; Rodhouse et al. 1992; Slip 1995; van den Hoff 2004).
Prey species abundance differences
In each of the four comparisons between sexes (controlling for sample size, season and age, Fig. 2), there were no significant differences in the abundance of prey species between male and female juvenile seals (1-year olds in winter: Global R = 0.069, SSS = 6.2%, NPS ≤ 0 = 61; 2-year olds in summer: Global R = 0.064, SSS = 18.8%, NPS ≤ 0 = 187; 2-year olds in winter: Global R = 0.077, SSS = 15.2%, NPS ≤ 0 = 151; 3-year olds in summer: Global R = −0.008, SSS = 48.4%, NPS ≤ 0 = 483). Because there was no sex effect, we pooled the data and tested for a difference between the seasons for each age group. Again, there were no significant seasonal differences within each age group (1-year olds: Global R = 0.102, SSS = 22.4%, NPS ≤ 0 = 223; 2-year olds: Global R = −0.014, SSS = 52.0%, NPS ≤ 0 = 519; 3-year olds: Global R = 0.054, SSS = 17.6%, NPS ≤ 0 = 175). Because there were no sex or season effects, we pooled all our data to determine if there were significant differences in prey abundance among 1-, 2- and 3-year-old seals. Our analysis showed that there were significant differences observed between the 1-year olds and 2- and 3-year olds (Global R = 0.148, SSS = 0.1%, NPS ≤ 0 = 0; Fig. 3). The three most common species in all the three age groups (accounting for ∼80% of the diet) were A. antarcticus, S. circumantarctica and H. eltaninae, although they occurred in different proportions (Table 3). From the SIMPER analyses, five species accounted for ∼70% of the dissimilarity between 1-year-old seals and the 2- and 3-year olds. These species in order of importance were H. eltaninae (>20%), S. circumantarctica (>20%), A. antarcticus (>10%), the combined lantern fish taxa of Electrona sp. (∼7%) and Psychroteuthis glacialis (∼6%), whereas 1-year-old seals had greater numbers of S. circumantarctica and Electrona sp., but less H. eltaninae and P. glacialis than 2- and 3-year olds.
Size of squid prey
Lower rostral lengths of the seven squid species found in the diet of all 97 juvenile seals in winter were compared among the three age groups. There were significant differences for only two of the prey species (Fig. 4) in the size of beaks found, with older seals taking larger Martialia hyadesi (ANOVA; F(2,38) = 3.24, P = 0.050). Sex differences (Fig. 5) in size of prey between male and female seals were found for only one prey species, M. hyadesi, where males had larger beaks in their samples (ANOVA; F(1,39) = 6.63, P = 0.014).
Minimum sample required
There was a clear asymptote in the curve for the number of samples needed to find a significant difference (randomised ANOSIM; Fig. 6). After including 13 random samples from each age group, the probability of finding a significant difference (SSS < 0.05) between the groups was 95%. By comparing 15 samples there was a 99% probability of finding a significant difference.
Discussion
Southern elephant seals are deep-diving opportunistic, generalist feeders, and this particular foraging strategy may have evolved as a result of the dynamic and unpredictable seasonal and spatial distribution and abundance of target prey within the Southern Ocean. This species shows a high degree of dimorphism, with adult females and males being at least 10 and 100 times greater in mass than newborns. Assuming that resources are limiting, however, one might expect that behavioural shifts among different ‘ecological’ species or sub-groups within the species would lead to a reduction in intra-specific competition for food resources (Polis 1984; Bolnick et al. 2003; Takimoto 2003; Field et al. 2005a). There are clear differences in diet among the 1-, 2- and 3-year-old seals, in terms of both species abundance and prey size, but no sex or season effect. These differences are most likely due to an increased diving ability, with increased body size and foraging range as the seals grow. The similarity in body size between same age male and females may be the primary reason for no sexual differences in the diet. Furthermore, a lack of a sex difference indicates that these dietary changes are not driven by differing metabolic requirements between the sexes (Field et al. 2005b). Therefore, dietary changes appear to be a function of ontogenetic changes in foraging capacity and range, regulated by physiological limitations and seasonal haul-out patterns (Field et al. 2005a). However, with a lack of information on prey distribution, the real reasons for age differences remain unclear.
As with many other species (Adams 1996; Wikelski and Wrege 2000; Spina 2000), an increase in size with age influences the ability of an individual to obtain prey and expand its foraging niche (Polis 1984; Radloff and Du Toit 2004). As seals grow, there are changes in the physiological diving abilities that allow older, larger individuals to dive deeper and longer (Le Boeuf et al. 1996; Burns 1999; Hindell et al. 1999; Irvine et al. 2000). Thus, older individuals that can remain at deeper depths longer have greater access to deeper-dwelling species. Our results show a clear change in the composition of diet among juvenile seals. The older seals have greater proportions of the larger squid in their samples (Kondakovia longimana, M. hyadesi, Moroteuthis ingens and Moroteuthis knipovitchi), which may not have been available to the smaller individuals. Indeed, there is some evidence for pelagic cephalopods that older and larger individuals are capable of more extensive vertical migrations (Jackson 1993; Arkhipkin and Bjørke 1999).
However, there was no difference in the size of most other prey species ingested. Therefore, differences in prey composition among age groups could also be due to variation in foraging range that appears to be regulated, in part, by variation in seasonal haul-out patterns according to age (Field et al. 2005a). This regulation appears to occur independently of dispersal capacity, because different age classes demonstrate similar rates of travel (Field et al. 2005a). Although our results only represent the diet as the seals return to the island, it appears that variation in foraging and haul-out behaviour in conjunction with modification of diet composition all contribute to a general reduction in intra-specific competition for this wide-ranging Antarctic marine predator.
Other studies of elephant seal diet have shown differences between juveniles and adults (Green et al. 1998; Slip 1995), although the nature of these differences were inconsistent. Slip (1995) found that juveniles were different from both male and female adults, while Green et al. (1998) found only differences between juveniles and adult females that could not be readily explained. Only one other study has specifically tested for differences among size/age classes, but the results were inconsistent and no trends were found (Rodhouse et al. 1992).
Three species dominated the diet, although there were distinct differences between the different age groups. The diet of 1-year-old seals was dominated by S. circumantarctus, a small muscular squid (to around 150 mm; Lipinsky 2001) with a broad Southern Ocean distribution (Cherel et al. 2004). In contrast, the 2- and 3-year olds’ diets were dominated by the slightly smaller H. eltaninae (to around 100 mm; Voss et al. 1998), which has a sub-Antarctic distribution (Cherel et al. 2004). Furthermore, the diet of 2-year olds, though not statistically significantly different from that of 3-year olds, was intermediate between the younger and older seals. Although little is known about the distribution of the main prey species, there is the potential that temporal and spatial movements of the prey species could influence the diet of the seals. All common prey species were found in the diets of all age groups throughout the year, and there were no seasonal differences in prey species abundance from our ANOSIM and SIMPER analyses. Therefore, although we do not discredit that there may be some variation in prey species abundance, it would seem likely that the differences we have observed are due to the different feeding strategies of the juvenile seals through differences in prey availability. Availability could be influenced by whether they are a solitary or schooling species, or by the relative costs of catching prey (for example, a slow swimming species verses a cryptic fast swimming species). For the larger squid species commonly found in the diet of the different age groups in winter, M. hyadesi (around 400 mm; Roper et al. 1984), the size of prey also increased with age. Only the larger seals may have been able to forage deeper and catch these larger prey, thereby increasing the range of prey sizes available and their foraging niche (Jackson et al. 2004; Radloff and Du Toit 2004). However, also important is the increase in the abundance of H. eltaninae as the seals get older. The reasons for this increase are unclear but, as with the increase in larger squid, are most likely due to the distribution of the prey species, which at present is unknown but could be inferred from fine scale studies of the seals diving and foraging behaviour.
There is a suite of methods available for the determination of diet, including direct and indirect observation, genetic sampling (Symondson 2002; Jarman et al. 2002), fatty acid signature analyses (Brown et al. 1999; Bradshaw et al. 2003; Iverson et al. 2004), stable isotope analyses (Iverson et al. 2004; Hooker et al. 2001), and through the study of remains in faecal and stomach contents (Santos et al. 2001). All these methods have advantages and disadvantages, but it is only through the direct analysis of prey remains that can we determine both species identity and ecological information (such as size structure) about the prey. Furthermore, our use of a novel approach to determine the minimum sample required to find a difference has given greater confidence to our results and provides some guidance for future studies using this technique to minimise disturbance and the impact of dietary studies.
In our study, we lavaged the stomachs of the seals as soon after their return as possible, however, these samples are only representative of their foraging as they return to Macquarie Island (Fig. 1) and not their entire foraging areas. Elephant seals have a rapid rate of digestion (∼13 h; Krockenberger and Bryden 1994), although hard parts of the prey may be retained in the stomach for over 7 days (Tollit et al. 1997). Therefore, our samples are likely to have come from within 700 km of the island, using 100 km day−1 as the rate of travel by juvenile seals (Field et al. 2005a). This was reflected in the reduced abundance of Antarctic species in their diets. The results may also over-estimate the presence of prey with larger hard parts due to differential digestive rates (Daneri and Carlini 2002). Furthermore, some of the prey remains in our samples may have been from secondary ingestion (Arnett and Whelan 2001). From the few diet studies of squid found in the Southern Ocean (Phillips et al. 2003), it is clear that they are voracious predators that eat fish and squid and show seasonal differences in their diet.
Squid and fish, including some commercially taken species (Burton and van den Hoff 2002), are the main prey of southern elephant seals (Rodhouse et al. 1992; Green et al. 1998; Slip 1995; Danieri et al. 2000; Piatkowski et al. 2002; Bradshaw et al. 2003; van den Hoff et al. 2003; van den Hoff 2004), although the variation in their relative proportions is less well-known (Santos et al. 2001) for ecological and methodological reasons (Bradshaw et al. 2003). As in other diet studies of Macquarie Island juvenile elephant seals (1–4 years old, Green et al. 1998; 1-year olds, van den Hoff 2004), squid are the primary prey, however, the occurrence of fish remains found in our study (all age groups ∼76%) were higher than in previous studies where fish were found in only 10% of samples (Green et al. 1998). This difference is likely to be due to the inter-annual variability in the availability of fish prey as suggested by Danieri et al. (2000).
Compared to previous studies (Green et al. 1998; van den Hoff 2004), the fish component of our juvenile diet sample contained many more fish species from pelagic, demersal and benthic habitats, including the Patagonian Toothfish (Dissostichus eleginoides) and the Ebinania macquariensis, an endemic benthic species only found around Macquarie Island (Williams 1988). The main difference, however, is the dominance of the squid S. circumantarcticus, which contributed ∼55% of the total number of beaks in our samples, but only ∼6% in Green et al. (1998). Other differences include a reduction in H. eltaninae (∼12% compared to ∼20% in our study) and A. antarcticus and M. knipovitchi (∼30 and 12%, respectively, compared to 9 and 1.6%, respectively, in our study). These species are all commonly found south of the Polar Front (except H. eltaninae), therefore, these differences may result from a change in prey species availability among years due to inter-annual variation in the position or strength of water mixing at the Polar Front (Antonelis et al. 1994).
Although our data only represent the end of the foraging trip, there were clear age-related differences in diet, though it still remains unclear as to the proportions of fish and squid that are eaten while farther away at sea. As the stomach contents are likely to have been collected in broadly similar geographic regions (and there was no evidence of seasonal changes in diet composition), these differences must relate to some intrinsic difference in the seals (i.e. size; Radloff and Du Toit 2004). The intra-specific differences in diet composition linked with the increased foraging ranges with age (Field et al. 2005a) provide further evidence to support the hypothesis that ontogenetic niche expansion acts to reduce intra-specific competition. However, the diet composition varies with age and spatially, which needs to be addressed using the suite of dietary tools currently available to gain a better understanding of the dynamic ecological niche of this apex predator.
References
Adams RA (1996) Size-specific resource use in juvenile little brown bats, Myotis lucifugus (Chiroptera: Vespertilionidae): is there an ontogenetic shift? Can J Zool 74:1204–1210
Antonelis GA, Lowry MS, Fiscus CH, Stewart BS, DeLong RL (1994) Diet of the northern elephant seal. In: Le Boeuf BJ, Laws RM (eds) Elephant seals: population ecology, behaviour, and physiology. University of California Press, Berkeley, pp 211–226
Arkhipkin AI, Bjørke H (1999) Ontogenetic changes in morphometric and reproductive indices of the squid Gonatus fabricii (Oegopsida Gonatidae) in the Norwegian Sea. Polar Biol 22:357–365
Arnett RTP, Whelan J (2001) Comparing the diet of cod (Gadus morhua) and grey seals (Halichoerus grypus): an investigation of secondary ingestion. J Mar Biol Assoc UK 81:365–366
Arrigo KR, Worthen D, Schnell A, Lizotte MP (1998) Primary production in Southern Ocean waters. J Geophys Res 103(C8):15587–15600
Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28
Bowen WD, Tully D, Boness DJ, Bulheier BM, Marshall GJ (2002) Prey dependent foraging tactics and prey profitability in a marine mammal. MEPS 244:235–245
Boyd IL, Arnbom TA, Fedak MA (1994) Biomass and energy consumption of the South Georgia population of the southern elephant seals. In: Le Boeuf BJ, Laws RM (eds) Elephant seals: population ecology, behaviour, and physiology. University of California Press, Berkeley, pp 98–120
Bradshaw CJA, Hindell MA, Best NJ, Phillips KL, Wilson G, Nichols PD (2003) You are what you eat: describing the foraging ecology of southern elephant seals (Mirounga leonina) using blubber fatty acids. Proc R Soc Lond B 270:1283–1292
Brown DJ, Boyd IL, Cripps GC (1999) Fatty acid signature analysis from the milk of Antarctic fur seals and southern elephant seals from South Georgia: implications for diet determination. Mar Ecol Prog Ser 187:251–263
Burton HR, van den Hoff J (2002) Humans and the southern elephant seal Mirounga leonina. Aust Mamm 24:127–139
Cherel Y, Duhamel G, Gasco N (2004) Cephalopod fauna of subantarctic islands: new information from predators. Mar Ecol Prog Ser 266:143–156
Clarke MR (1986) A handbook for the identification of cephalopod beaks. Clarendon, Oxford
Clinton WL (1994) Sexual selection and growth in male northern elephant seals. In: Le Boeuf BJ, Laws RM (eds) Elephant seals: population ecology, behaviour, and physiology. University of California Press, Berkeley, pp 154–168
Constable AJ, Nicol S, Strutton PG (2003) Southern Ocean productivity in relation to spatial and temporal variation in the physical environment. J Geophys Res 108:8079–9000
Daneri GA, Carlini AR (2002) Fish prey of southern elephant seals, Mirounga leonina, at King George Island. Polar Biol 25(10):739–743
Danieri GA, Carlini AR, Rodhouse PGK (2000) Cephalopod diet of the southern elephant seal, Mirounga leonina, at King George Island, South Shetland Islands. Antarct Sci 12:16–19
El-Sayed SZ (1988) Seasonal and inter-annual variabilities in Antarctic phytoplankton with reference to krill distribution. In: Sahrhage D (ed) Antarctic ocean and resource variability. Springer, Berlin Heidelberg New York, pp 101–119
Field IC, Bradshaw CJA, McMahon CR, Harrington J, Burton HR (2002) Intravenous anaesthesia of elephant seals (Mirounga leonina) using tiletamine and zolazepam: effects of age, size, condition and function of haul-out. Vet Rec 151:235–240
Field IC, Bradshaw CJA, Burton HR, Hindell MA (2004) Seasonal use of oceanographic and fisheries management zones by juvenile southern elephant seals (Mirounga leonina) from Macquarie Island. Polar Biol 27:432–440
Field IC, Bradshaw CJA, Burton HR, Sumner MD, Hindell MA (2005a) Resource partitioning through oceanic segregation of foraging juvenile southern elephant seals. Oecologia 142:127–135
Field IC, Bradshaw CJA, Burton HR, Hindell MA (2005b) Patterns of onshore mass change and metabolism in juvenile southern elephant seals. Physiol Biochem Zool 78(4):491–504
Green K, Slip DJ, Moore GJ (1998) The take of fish species by seabirds and marine mammals in the Australian Fisheries Zone around Heard Island—the potential for competition with a commercial fishery. Polar Biol 20:273–280
Guinet C, Jouventin P, Weimerskirch H (1999) Recent population change of the southern elephant seal at Iles Crozet and Iles Kerguelen: the end of the decrease? Antarct Sci 11(2):193–197
Hindell MA, Slip DJ, Burton HR (1994) Possible causes of the decline of southern elephant seal populations in the southern Pacific and southern Indian Oceans. In: Le Boeuf BJ, Laws RM (eds) Elephant seals: population ecology, behavior, and physiology. University of California Press, Berkeley, pp 66–84
Hindell MA, McConnell BJ, Fedak MA, Slip DJ, Burton HR, Reijnders PJH, McMahon CR (1999) Environmental and physiological determinants of successful foraging by naive southern elephant seal pups during their first trip to sea. Can J Zool 77:1807–1821
Hindell MA, Bradshaw CJA, Sumner MD, Michael KJ, Burton HR (2003) Dispersal of female southern elephant seals and their prey consumption during the austral summer: relevance to management and oceanographic zones. J Appl Ecol 40:703–715
van den Hoff J (2004) A comparative study of the cephalopod prey of Patagonian toothfish (Dissostichus eleginoides) and southern elephant seals (Mirounga leonina) near Macquarie Island. Polar Biol 27:604–612
van den Hoff J, Burton HR, Davies R (2003) Diet of male southern elephant seals (Mirounga leonina) hauled out at Vincennes Bay, East Antarctica. Polar Biol 26:27–31
Hooker SK, Iverson SJ, Ostrom P, Smith SC (2001) Diet of northern bottlenose whales inferred from fatty-acid and stable isotope analysis of biopsy samples. Can J Zool 75:188–197
Ihaka R, Gentleman R (1996) R: a language for data analysis and graphics. J Comput Graph Stat 5:299–314
Irvine LG, Hindell MA, van den Hoff J, Burton HR (2000) The influence of body size on dive duration of under-yearling southern elephant seals (Mirounga leonina). J Zool 251:463–471
Iverson SJ, Field C, Bowen WD, Blanchard W (2004) Quantitative fatty acid signature analysis: a new method of estimating predator diets. Ecol Monogr 74:211–235
Jackson GD (1993) Growth zone within the statolith microstructure of the deepwater squid Moroteuthis ingens (Cephalopoda: Onychoteuthidae): evidence for a habitat shift? Can J Fish Aquat Sci 50:2366–2374
Jackson AC, Rundle SD, Attrill MJ, Cotton PA (2004) Ontogenetic changes in metabolism may determine shifts for a sit and wait predator. J Anim Ecol 73:536–545
Jarman SN, Gales NJ, Tierney M, Gill PC, Elliot NG (2002) A DNA-based method for identification of krill species and its application to analysing the diet of marine vertebrate predators. Mol Ecol 11:2679–2690
Kato A, Watanuki Y, Nishiumi I, Kuroki M, Shaughnessy P, Naito Y (2000) Variation in foraging and parental behaviour of king cormorants. Auk 117:718–730
Krockenberger MB, Bryden MM (1994) Rate of passage of digesta through the alimentary tract of southern elephant seals (Mirounga leonina) (Carnivora: Phicidae). J Zool (Lond) 234:229–237
Le Boeuf BJ, Laws RM (1994) Elephant seals: an introduction to the genus. In: Le Boeuf BJ, Laws RM (eds) Elephant seals: population ecology, behavior, and physiology. University of California Press, Berkeley, pp 1–28
Le Boeuf BJ, Morris PA, Blackwell SB, Crocker DE, Costa DP (1996) Diving behaviour of juvenile northern elephant seals. Can J Zool 74:1632–1644
Le Boeuf BJ, Crocker DE, Costa DP, Blackwell SB, Webb PM, Houser DS (2000) Foraging ecology of northern elephant seals. Ecol Monogr 70:353–382
Lipinski MR (2001) Preliminary description of two new species of Cephalopods (Cephalopoda: Brachioteuthidae) from the South Atlantic and Antarctic waters. Bull Sea Fish Inst 1:3–14
Lutjeharms JHE (1990) The oceanography and fish distribution of the Southern Ocean. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. Institute of Ichthyology, Grahamstown, pp 6–28
McMahon CR, Burton H, McLean S, Slip D, Bester M (2000) Field immobilisation of southern elephant seals with intravenous tiletamine and zolazepam. Vet Rec 146:251–254
McMahon CR, Burton HR, Bester MN (2003) A demographic comparison of two southern elephant seals populations. J Anim Ecol 72:61–74
McMahon CR, Bester MN, Burton HR, Hindell MA, Bradshaw CJA (2005) Population status and trends of a wide-ranging marine mammal predator, the southern elephant seal: re-examining hypotheses to explain the decline. Mammal Rev 35:82–100
Perry G (1996) The evolution of sexual dimorphism in the lizard Anolis polylepis (Iguania): evidence from intraspecific variation in foraging behaviour and diet. Can J Zool 74:1238–1245
Phillips KL, Nichols PD, Jackson GD (2003) Size-related dietary changes observed in the squid Moroteuthis ingens at the Falkland Islands: stomach contents and fatty-acid analyses. Polar Biol 26:474–485
Pianka ER (1981) Competition and niche theory. In: May RM (ed) Theoretical ecology: principles and applications. Blackwell Scientific Publications, Oxford, pp 114–141
Piatkowski U, Vergani DF, Stanganelli ZB (2002) Changes in cephalopod diet of the southern elephant seal females a King George Island, during El Niño-La Niña events. J Mar Biol Assoc UK 82:913–916
Polis A (1984) Age structure component of niche width and intraspecific resource partitioning: can age groups function as ecological species? Am Nat 123:541–564
Radloff FGT, Du Toit JT (2004) Large predators and their prey in a southern African savanna: a predator’s size determines its prey size. J Anim Ecol 73:410–423
Rodhouse PG, White MG (1995) Cephalopods occupy the ecological niche of epipelagic fish in the Antarctic Polar Frontal zone. Biol Bull 189:77–80
Rodhouse PG, Arnbom TR, Fedak MA, Yeatman J, Murray AWA (1992) Cephalopod prey of the southern elephant seal Mirounga leonina L. Can J Zool 70:1007–1015
Roper CFE, Sweeney MJ, Nauen CE (1984) Cephalopods of the world, vol 3. Food and Agriculture Organization, Rome, Italy, pp 277
Santos MB, Clarke MR, Pierce GJ (2001) Assessing the importance of cephalopods in the diets of marine mammals and other top predators: problems and solutions. Fish Res 52:121–139
Schoener TW (1986) Resource partitioning. In: Kikkawa J, Anderson DJ (eds) Community ecology pattern and process. Blackwell Scientific Publications, Carlton, pp 91–126
Slip DJ (1995) The diet of southern elephant seals (Mirounga leonina) from Heard Island. Can J Zool 63:1519–1528
Slip DJ (1997) Diving and foraging behaviour of juvenile southern elephant seals from Heard Island. In: Hindell M, Kemper C (eds) Marine mammal research in the Southern Hemisphere: status, ecology and medicine, vol 1. Beatty and Sons, Chipping Norton, pp 114–124
Slip DJ, Burton HR (1999) Population status and seasonal haulout patterns of the southern elephant seal (Mirounga leonina) at Heard Island. Antarct Sci 11(1):38–47
Spina AP (2000) Habitat partitioning in patchy environment: considering the role of intraspecific competition. Environ Biol Fish 57:393–400
Stewart BS (1997) Ontogeny of differential migration and sexual segregation in northern elephant seals. J Mammal 78:1101–1116
Symondson WOC (2002) Molecular identification of prey in predator diets. Mol Ecol 11:627–641
Takimoto G (2003) Adaptive plasticity in ontogenetic niche shifts stabilises consumer-resource dynamics. Am Nat 162:93–109
Tollit DM, Steward MJ, Thompson PM, Pierce GJ, Santos MB, Hughes S (1997) Species and size differences in the digestion of otoliths and beaks: implications for estimates of pinniped diet composition. Can J Fish Aquat Sci 54:105–119
Trivers RL (1985) Social evolution. Benjamin/Cummings Publishing Company, Menlo Park
Van Valen L (1965) Morphological variation and width of the ecological niche. Am Nat 99:377–390
Voss NA, Nesis KN, Rodhouse PG (1998) The cephalopod family Histioteuthidae (Oegopsida): systematics, biology, and biogeography. Smithson Contrib Zool 586(2):293–372
Warren PH (1996) Structural constraints on food web assembly. In: Hochberg ME, Clobert J, Barbault R (eds) Aspects of the genesis and maintenance of biological diversity. Oxford University Press, Oxford, pp 142–161
Whitehead H, MacLeod CD, Rodhouse P (2003) Differences in niche breadth among some teuthivorous mesopelagic marine mammals. Mar Mamm Sci 19:400–405
Wikelski M, Wrege PH (2000) Niche expansion, body size and survival in Galápagos marine iguanas. Oecologia 124:107–115
Williams R (1988) The nearshore fishes of Macquarie Island. Pap Proc R Soc Tasman 122:233–245
Williams RJ, Martinez ND (2000) Simple rules yield complex food web. Nature 404:180–183
Williams R, McEldowney A (1990) A guide to the fish otoliths from the waters off the Australian Antarctic Territory, Heard and Macquarie Islands. ANARE Research Notes 75, Australian Antarctic Division, Kingston
Woodward G, Hildrew AG (2002) Body-size determinants of niche overlap and intraguild predation within a complex food web. Ecology 71:1063–1074
Acknowledgments
The data were collected with the approval of the Australian Antarctic Animal Ethics Committee and permits from the Tasmanian Parks and Wildlife Service . We thank M. Biuw, J. Harrington, C. McKinley, N. Milius, R. Munro, M. Pauza and K. Wheatley and members of the 51st–53rd ANARE to Macquarie Island for their assistance during fieldwork. We thank D. Williams for the identification of the otoliths collection and Y. Cherel and M. Imber for reviewing our identifications of squid beaks. We thank M. Sumner for helping with R programming. Funding was provided by the Antarctic Science Advisory Committee and Sea World Research and Rescue Foundation Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M.S. Johnson, Crawley
Rights and permissions
About this article
Cite this article
Field, I.C., Bradshaw, C.J.A., van den Hoff, J. et al. Age-related shifts in the diet composition of southern elephant seals expand overall foraging niche. Mar Biol 150, 1441–1452 (2007). https://doi.org/10.1007/s00227-006-0417-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0417-y