Abstract
Phoresy is a passive transportation behavior where one organism (phoront) disperses to a new location by attaching to another organism. Pseudoscorpions are arthropod predators that mainly live in soil, subterranean habitats, and under tree bark. Some species also live in animal nests and engage in phoresy on small mammals, suggesting close associations with these animals. However, the relationship between phoretic pseudoscorpions and hosts as well as the ecological significance of phoresy remain largely unexplored. Here, to understand the function of phoresy of Megachernes ryugadensis, phoretic on small mammals, their phoretic behavior was investigated in a deciduous forest in northern Japan; individual-level dynamics of phoresy were examined by over 3-year mark-recapture surveys that concurrently marked the host and phoront; and host characteristics, such as sex and age class, were analyzed based on a 2-year small mammal trapping survey. The primary host species was the abundant Japanese wood mouse Apodemus speciosus. Out of 132 pseudoscorpions marked, 5 were recaptured approximately 1 month later. No pseudoscorpions were recaptured within the same census period (3–4 days) when they were marked, indicating that phoresy events last less than one night, and pseudoscorpions are unlikely to engage in phoresy again within a few weeks of their initial engagement. Furthermore, analysis of host characteristics revealed a tendency for female mice and adult individuals to have a higher probability of being hosts compared with males and subadults, respectively. Based on the findings in this and previous studies, the function of phoresy in this species is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dispersal, the movement of an organism to a new habitat for reasons such as finding a mate, obtaining food, or avoiding competition and interbreeding, has ecological and evolutionary implications, influencing population dynamics, distribution patterns, and genetic connectivity (Bowler and Benton 2005). Phoresy, a type of passive transportation, involves one organism (phoront, usually small with limited mobility) dispersing to a new location by attaching to another organism (host) (Bartlow and Agosta 2021). Phoresy is employed by various animals, including nematodes, mollusks, insects, and arachnids (White et al. 2017; Bartlow and Agosta 2021). Among arachnids, phoresy is particularly common in mites (Barton et al. 2014; Keum et al. 2016) and pseudoscorpions (Muchmore 1971; Poinar et al. 1998).
Pseudoscorpions, belonging to the class Arachnida and order Pseudoscorpiones (Harvey 2013), are predacious arthropods with over 4000 described species found worldwide, except in Antarctica [World Pseudoscorpiones Catalog (WPC) 2022]. However, our understanding of pseudoscorpion biology is still limited (Murienne et al. 2008; Harvey et al. 2012; Tapia-Ramírez et al. 2022). Although pseudoscorpions primarily live in soil, subterranean habitats, and under tree bark, they have been found in the nests of small mammals, birds, bumblebees, and in bat guano in caves, indicating close associations with these animals (Levi 1953; Weygoldt 1969; Zeh and Zeh 1992a; Francke and Villegas-Guzmán 2006; Tizo-Pedroso and Del-Claro 2007; Hlebec et al. 2023a, b). Some pseudoscorpion species have been observed engaging in phoresy on small mammals and various arthropods including insects, hypothesized that such a relationship may provide the following benefits: prey opportunities, e.g., ectoparasites in host fur (phagophily); access to new habitats; avoidance of predation on offspring, intraspecific competition, and inbreeding; and finding mates and breeding sites (as reviewed by Poinar et al. 1998). However, the significance of phoresy for pseudoscorpions remains largely unknown owing to a lack of fundamental ecological information about their habits.
The genus Megachernes in the family Chernetidae includes several species suggested to be commensal and phoretic with small mammals (Durden 1991; Harvey et al. 2012). Megachernes ryugadensis Morikawa 1954, first collected from bat guano in a cave in Kochi, Japan, is one of the largest pseudoscorpions in Japan (Morikawa 1954). It has been observed performing phoresy on forest-dwelling rodents and moles. A recent study based on a 2-year small mammal trapping survey reported the phoretic behavior of M. ryugadensis as follows (Okabe et al. 2020): (1) the large Japanese wood mouse Apodemus speciosus was the most frequently observed host species, being the most abundant in the study site; (2) tritonymphs, as well as adult male and female pseudoscorpions, were observed as phoronts; (3) females with a brood sac were phoretic throughout the year, although the percentage fluctuated seasonally; and (4) little seasonality was observed in the phoretic ratio (number of individual mammals with attached pseudoscorpions divided by the number of individual mammals captured in each census). The primary function of phoresy in M. ryugadensis remains unclear, with Okabe et al. (2020) excluding the possibility of phagophily because M. ryugadensis clings to the host with pedipalps, making prey capture challenging. Instead, Okabe et al. (2020) suggested that the primary function of phoresy is to disperse to a new habitat. However, the specific demands driving the phoretic behavior of M. ryugadensis remain unknown.
Dispersal to a new habitat is likely to be triggered by reasons, such as escaping from habitat degradation, avoiding intraspecific competition or predation on offspring, searching for mates and breeding sites, and avoiding inbreeding. To evaluate the validity of these possibilities, a deeper understanding of the phoretic behavior is needed. For instance, it has not been clarified for M. ryugadensis whether the same individual performs phoresy multiple times in its lifetime, but this information is important in examining the purpose of phoresy in this species. Furthermore, if the same individual performs phoresy multiple times, how often or at what intervals does it initiate phoresy? If dispersal by phoresy of this species is due to habitat degradation or avoiding competition or predation, phoresy may not be repeated in a short period of time. These questions have not been investigated at all in pseudoscorpions phoretic with small mammals. To answer them, it is necessary to examine the phoretic behavior of this species at the individual level.
In the present study, to elucidate the phoretic behavior of M. ryugadensis at the individual level, we conducted a mark-recapture survey that concurrently marked hosts and phoronts. Additionally, host characteristics, i.e., sex and age class (adult or subadult), were analyzed using data obtained from a 2-year small mammal trapping survey (Okabe et al. 2020) to identify specific tendencies in host-phoront associations under natural conditions. Using these assessments, we aimed to provide insights into the function of phoretic behavior in pseudoscorpions associated with small mammals.
Materials and methods
Study site and species selection
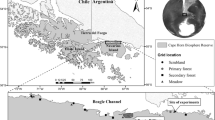
Field surveys were conducted at the Takizawa Research Forest of Iwate University, Morioka, Iwate, Japan (39° 47′ N, 141° 09′ E, approximately 200 m a.s.l.), a secondary deciduous forest dominated by Quercus serrata (Fagaceae). Five small mammal species were recorded at this site (Okabe et al. 2020): the large Japanese wood mouse (A. speciosus), the small Japanese wood mouse (Apodemus argenteus), Anderson’s red-backed vole (Eothenomys andersoni), the Japanese white-toothed shrew (Crocidura dsinezumi), and the Japanese shrew mole (Urotrichus talpoides). Among these species, A. speciosus was the most abundant, with densities of 24.1–248.1 individuals/ha during the continuous trapping survey period in 2016–2017.
The field surveys consisted of two parts. First, a continuous trapping survey was conducted in 2016 and 2017. Some results from this survey, including the phoretic ratio and phoront characteristics, were previously published by Okabe et al. (2020). However, in the present study, a new analysis of host characteristics was performed using the same dataset. Given that the majority of hosts observed in this survey were A. speciosus, probably due to its high density compared to other species (373 out of 381 phoretic events; Okabe et al. 2020), our analyses focused only on cases where A. speciosus was the host. The second part was a mark-recapture survey conducted on four occasions between 2019 and 2021.
Megachernes ryugadensis (Fig. 1) is a relatively large pseudoscorpion species, having an adult body length of approximately 3–5 mm (Morikawa 1954). Limited field ecological information is available, but the species is believed to primarily feed on small arthropods (Harvey et al. 2012). The presence of M. ryugadensis in the nests and fur of small mammals, as well as its predation on mammalian ectoparasites (such as ticks and mites) in captivity, suggests a potential mutualistic relationship with host animals (Okabe et al. 2018). In a preliminary survey, more than 50 M. ryugadensis individuals were found in a nest of A. speciosus at the study site (T. Shimada, personal observation), indicating that small mammal nests may serve as the primary habitat for this species.
Apodemus speciosus is a small rodent with an adult body mass of approximately 20–50 g (Ohdachi et al. 2015) that is widely distributed in forests and grasslands in Japan. It occupies the ground surface and shallow underground areas, using tunnels created by itself and other animals. For breeding, the species constructs underground ball-shaped nests made of fallen leaves. The emergence of young mice in this region is most common in spring (April–May) and fall (October–November). Apodemus speciosus is primarily omnivorous or granivorous, with foraging items varying seasonally (Tatsukawa and Murakami 1976; Sato et al. 2018, 2019). The species is known to hoard seeds in nests or temporary caches for future use (Shimada 2001; Shimada et al. 2015; Yoshikawa 2023). All procedures involving live animals followed the guidelines for obtaining mammal specimens approved by the Mammal Society of Japan.
Continuous trapping survey
We conducted censuses of small mammals, mainly targeting wood mice (A. speciosus and A. argenteus), using Sherman-type live traps. The trapping took place during alternate weeks between April and November in 2016 and 2017. Each census involved setting 106 traps on the ground, spaced at 10 m intervals in a grid pattern, for three consecutive nights over a fixed study site measuring 0.54 ha (90 × 60 m). Captured animals were individually identified by toe-clipping, and species, sex, and body weight were recorded. Age class (adult or subadult) of each individual was determined based on its body weight as follows. Individuals born in the most recent breeding season that had not yet reached sexual maturity were defined as subadults. To classify these individuals, we used a weight threshold of 25 g following Murakami (1974), with those weighing ≥ 25 g classified as adults and those weighing < 25 g classified as subadults. During the 2016 censuses, when phoretic pseudoscorpions were observed gripping the hair of host mammals with their chelae on the first or second day of sampling, we recorded the identification (ID) of the host animal and the number of pseudoscorpions on each host. The host mice were then released, leaving the phoronts attached to them. On the third and final day of sampling, we carefully removed the pseudoscorpions from the hosts using forceps. In the 2017 censuses, when phoretic pseudoscorpions were found, we recorded the ID of the host animal and the number of phoronts on each host, removing them from the hosts daily. The phoretic ratio was calculated as the number of individual mice found with attached pseudoscorpions at least once within each census relative to the total number of individual mice captured in each census.
Mark-recapture survey
To examine the phoretic behavior of M. ryugadensis at the individual level, we conducted mark-recapture surveys. Small mammals were individually identified upon capture, as in the continuous trapping survey. Phoretic pseudoscorpions were marked with 11 different colored permanent markers, of which one or two colors were used for a single individual (Magic Ink Paint, Teranishi Chemical Industry Co., Ltd., Osaka, Japan): a spot of single color was applied on the posterior part of dorsal surface for the first 11 individuals for each of the survey periods (Fig. 1), while two spots of different colors were applied side by side for the 12th and later ones. When applying two spots, we selected as different hues as possible among the 11 colors. Paint was applied using the head of an insect pin with the target individual keeping attached to the host. Photographs of the marked pseudoscorpions were taken immediately after marking to use for individual identification. The colors and shapes of the markings allowed us to distinguish them when they were recaptured. The paint spots were tiny and dried quickly, and the preliminary test on three captive individuals confirmed that the spots did not have negative effects on the behavior or survival of marked pseudoscorpions, as they seemingly moved without any difficulty and were confirmed to have survived at least 3 months. Although their markings had worn off a little, they remained distinguishable at least 4 weeks.
The mark-recapture surveys were conducted four times between 2019 and 2021 (Table 1). These surveys were conducted during periods of high mouse density and phoretic ratios according to past observations, with the aim of obtaining as many marked pseudoscorpions as possible. The trapping procedures for each census were the same as those used in the continuous trapping surveys. However, each census of this survey lasted 3–4 consecutive nights, with intervals between censuses of 25 or 26 days for the first three censuses and 12 days for the last one. We identified the developmental stage of the marked pseudoscorpions but could not determine the sex in the field. If a marked pseudoscorpion was found, the host ID and the capture location were recorded. The recaptured pseudoscorpion was then removed from the host using forceps and brought back to the laboratory for ID verification by checking against photographs and for sex identification under a stereomicroscope.
Host characteristics
To analyze the host characteristics determining which types of individual mice were used as hosts by pseudoscorpions, a generalized linear mixed model (GLMM) was employed. The analysis used data obtained from the continuous trapping survey. The response variable was a binary variable indicating whether or not each wood mouse became a host during each census. A binomial error and logit-link function were applied. The explanatory variables included the sex and age class of each mouse and the month of each census. The survey year was also included as a random effect. The significance of the model was assessed using a likelihood ratio test, with the model lacking explanatory variables serving as the null hypothesis (α = 0.05). In this analysis, events in each census were treated as independent, even for wood mice captured multiple times throughout the study period. All statistical analyses were conducted using the glmmML package implemented in R version 4.2.2 (R Core Team 2022).
Results
The average number of phoretic pseudoscorpions per host was 1.76 ± 2.03 S.D. during the mark-recapture surveys. A total of 132 pseudoscorpions, including 3 tritonymphs, were marked during the surveys. Out of these, 125 were found clinging to 70 A. speciosus, 6 to 4 A. argenteus, and 1 to 1 U. talpoides. Five of the marked pseudoscorpions were recaptured, all of which were found on adult A. speciosus; 4 out of 5 recaptured pseudoscorpions were adult female, and the rest was adult male (Table 2). Among these hosts, 36 A. speciosus and 2 A. argenteus were recaptured within the same census period (3–4 days), but no pseudoscorpions were recaptured within the period in which they had been marked. In the first three census periods (Table 1), with intervals of 25 or 26 days between censuses, 82 pseudoscorpions were marked, and 5 were recaptured. Conversely, in the last census period, with a 12-day interval between censuses, 50 pseudoscorpions were marked, but none were recaptured. Of the five recaptured pseudoscorpions, one was found on the same host (male A. speciosus), whereas the other four were discovered on different host individuals. Among these four, three were marked on male mice and recovered from female mice, and the remaining pseudoscorpion was marked and recaptured from different male mice. The distance between the initial capture and recapture locations was 0–44.7 m.
During the continuous trapping survey, 1982 A. speciosus were captured, with 373 individuals carrying pseudoscorpions (mean phoretic ratio: 18.8%). Table 3 shows the captured A. speciosus count, the number of mice carrying pseudoscorpions (hosts), and the phoretic ratio for each month. The phoretic ratio remained relatively stable throughout the study period (April–November) but showed a tendency to decrease in August and November. However, there were no significant differences in phoretic ratios between months or years (two-way ANOVA; month, F(7, 7) = 1.14, P = 0.433; year, F(1, 7) = 2.30, P = 0.173). Female mice exhibited a higher mean phoretic ratio (21.8%; 200 out of 919) compared with males (16.3%; 173 out of 1063; Fig. 2), and adults had a higher mean phoretic ratio (20.4%; 352 out of 1723) compared with subadults (8.1%; 21 out of 259; Fig. 3). The results of the GLMM analysis of host characteristics supported these observations (Table 4). The probability of being a host was higher for female and adult mice compared male and subadult mice, respectively. Seasonal comparisons revealed a lower likelihood of phoresy occurring in August compared with other months. The likelihood ratio test rejected the null hypothesis; hence, the proposed model was adopted (difference in deviance = 61.8, df = 9, p < 0.001).
Discussion
Recapture probability of M. ryugadensis
The recapture probability of M. ryugadensis was low, with only 5 out of 132 individuals being recaptured. This could be due to three factors: (1) a high mortality rate among pseudoscorpions, (2) infrequent phoretic behavior resulting from long intervals between phoresies or a small proportion of individuals engaging in repeated phoresy, and (3) low detection probability of phoretic pseudoscorpions despite repeated phoresy. Considering that the recapture probability of A. speciosus within the same census period was high (> 50%) and M. ryugadensis is easily visible due to its large size, it is unlikely that the low detection probability of phoretic pseudoscorpions is the main reason. Additionally, if the low recapture probability was due to high mortality, it would not explain why recaptured individuals were found only in the mark-recapture surveys conducted with 1-month intervals, and none were recaptured in the surveys with shorter intervals. Therefore, the most likely explanation is the low frequency of phoresy.
The mark-recapture method with individual identification has been applied to various animal groups, including insects, fish, birds, and mammals, to study individual behavior and estimate population size. In the case of pseudoscorpions, Zeh and Zeh (1992a, b, c, d) used this method on Cordylochernes scorpioides, a pseudoscorpion species that attaches to the elytra of harlequin beetles (Acrocinus longimanus), and revealed their phoretic and reproductive behaviors. Although there have been no other studies using the mark-recapture method on pseudoscorpions, the present study demonstrates the effectiveness of marking both the host and phoront simultaneously as a method for understanding phoretic behavior.
Characteristics of M. ryugadensis phoresy
Using the mark-recapture method, this study revealed new aspects of the phoretic behavior of M. ryugadensis. First, it was observed that individual pseudoscorpions can engage in phoresy more than once in their lifetime, although this may not be true for all individuals. Second, all recaptured pseudoscorpions were found on A. speciosus approximately 1 month later, and none were recaptured within the same census period in which they were marked. Although the mark-recapture surveys were conducted at different intervals between censuses (Table 1), no pseudoscorpions were recaptured in the census with the 12-day interval. These findings suggest that a single phoretic event lasts for a short duration, possibly within a night, and it is unlikely that a pseudoscorpion will engage in phoresy again within a few weeks of its first phoresy event. Wood mice are nocturnal and typically leave their nests or roosts at dusk, returning at dawn, although not always to the same location (Ohdachi et al. 2015; Oishi et al. 2018). Therefore, it can be inferred that pseudoscorpions may attach to mice in their nests or roosts and detach during the mice’s nocturnal activities or when they reach their next location.
Among the five recaptured pseudoscorpions, four were found on different host individuals, suggesting that M. ryugadensis can change its habitat through phoresy, traveling to the nest or roost of a different individual mouse. It is unclear whether host switching occurs through direct contact between mice or through sharing of nests and roosts. Nonetheless, host switching increases the probability of pseudoscorpions reaching new habitats.
Certain tendencies were observed regarding wood mice as hosts of M. ryugadensis. Female mice had a higher probability of being hosts compared with males, and adults had a higher probability of being hosts compared with subadults. However, as M. ryugadensis exhibits phoretic behavior in response to even a piece of fur placed before them (Okabe et al. 2020), it is unlikely that they have a specific preference for individual mice. Instead, these tendencies may reflect differences in nest usage among individual mice, as nests are assumed to be the primary habitat of M. ryugadensis. Female mice, which raise their offspring, spend more time in nests than males or subadults, providing more opportunities for pseudoscorpions to engage in phoresy.
The phoretic ratio remained relatively stable throughout the study period but significantly decreased in August, which is the dormant period for wood mouse reproduction in the region. It is unclear whether the population size of pseudoscorpions declined during this period or if phoretic behavior decreased. However, the reduced use of nests by wood mice for reproduction during this period may have reduced the chances of contact between the mice and pseudoscorpions.
Possible reasons for phoresy in M. ryugadensis
Based on the findings of this study and Okabe et al. (2020), we can consider the possible reasons for phoresy in M. ryugadensis. Two main reasons have been proposed for pseudoscorpions engaging in phoresy: predation during phoresy (phagophily) and dispersal (Poinar et al. 1998). However, the possibility of phagophily was ruled out by Okabe et al. (2020) because pseudoscorpions could not engage in both predatory and phoretic behaviors simultaneously. The present study supports this conclusion, as the interval between phoresies was found to be at least a few weeks; it seems too long, considering the species’ active foraging behavior (Okabe et al. 2018). Therefore, it is likely that the primary function of M. ryugadensis riding on small mammals is dispersal to new habitats.
Dispersal through phoresy can be motivated by various factors, such as relocating to better patches due to habitat degradation or competition, avoiding predation on offspring, searching for mates and breeding sites, and avoiding inbreeding (Bowler and Benton 2005). For example, some pseudoscorpion species that attach to insects selectively engage in phoresy with newly emerged host individuals ready to migrate to different habitats, escaping from degraded habitats (Zeh and Zeh 1992b, c, d). In contrast, a study on pseudoscorpion phoresy on rodents in Central America revealed that only female pseudoscorpions engaged in phoresy, with > 50% of these females found to be carrying eggs (Tapia-Ramírez et al. 2022), indicating that phoresy in this case serves to protect offspring from predation.
The primary reason for M. ryugadensis dispersal through phoresy is unlikely to be searching for mates and breeding sites, as many egg-bearing individuals and some tritonymphs have been observed engaging in phoresy (Okabe et al. 2020). Among the abovementioned possibilities, relocating to better patches seems consistent with the characteristics of M. ryugadensis phoresy, as individual pseudoscorpions can engage in phoresy repeatedly with intervals of several weeks. Moving to a new habitat when food availability decreases and competition intensifies is a common behavior among animals that move independently without phoresy (Bowler and Benton 2005; Szymkowiak et al. 2007). Therefore, M. ryugadensis may disperse in search of higher-quality habitats through phoresy, using food availability as an indicator of habitat quality.
Additionally, the hypothesis of avoiding predation may be promising to explain phoresy in adult females and tritonymphs. Phoresy of egg-bearing females has been frequently observed in M. ryugadensis (Okabe et al. 2020). Given that the risk of cannibalism and predation by other animals is expected to be high in small mammal nests, dispersing from such environments before hatching may be an important adaptation to increase offspring survival. This explanation could also apply to nymphs. Phoresy in tritonymphs, which are still vulnerable to predation, may serve to avoid predation pressure. However, younger nymphs may be functionally immature in terms of performing phoresy as their chela might be too small to grasp host hairs. To avoid predation, the location of disembarkation is particularly important. Unfortunately, no information regarding disembarkation in M. ryugadensis or other pseudoscorpions that engage in phoresy with small mammals is available. We speculate that egg-bearing individuals of M. ryugadensis that embark on mice at their nests might disperse to other nests or different types of habitats with lower predator densities.
Phoresy generally tends to develop in animals inhabiting ephemeral and patchy habitats, such as carrion, dung, rotting wood, and animal nests (White et al. 2017; Muster et al. 2021). The nests of wood mice may fulfill the criteria for M. ryugadensis, as their lifespan, with an estimated complete life cycle of over 1 year (Okabe et al. 2020), is longer than that of wood mice (Ohdachi et al. 2015). Although the process from nest construction to abandonment in A. speciosus is not fully understood, nests are believed to be constructed based on seasonal reproduction, resulting in an increase in parasites and invertebrates that use leaf litter and animal excrement. These factors create suitable habitats for various animals, including pseudoscorpions, that prey on small nidicolous animals. However, once mice have completed their reproduction process, prey availability in these habitats is likely to decline. In response to such nest phenology, M. ryugadensis may engage in phoresy with the nest owner to relocate to new habitats or avoid predation on their offspring. To gain a comprehensive understanding of the function of phoresy, further research is required to investigate the timing and direct cues for embarkation and disembarkation of M. ryugadensis, as well as the dynamics of their primary habitat (small mammal nests) and the faunal communities inhabiting them.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bartlow AW, Agosta SJ (2021) Phoresy in animals: review and synthesis of a common but understudied mode of dispersal. Biol Rev 96:223–246. https://doi.org/10.1111/brv.12654
Barton PS, Weaver HJ, Manning AD (2014) Contrasting diversity dynamics of phoretic mites and beetles associated with vertebrate carrion. Exp Appl Acarol 63:1–13. https://doi.org/10.1007/s10493-013-9758-7
Bowler DE, Benton TG (2005) Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev 80:205–225
Durden LA (1991) Pseudoscorpions associated with mammals in Papua New Guinea. Biotropica 23:204–206. https://doi.org/10.2307/2388309
Francke O, Villegas-Guzmán GA (2006) Symbiotic relationships between pseudoscorpions (Arachnida) and packrats (Rodentia). J Arachnol 34:289–298. https://doi.org/10.1636/04-36.1
Harvey MS (2013) Pseudoscorpions of the World, version 3.0. Western Australian Museum, Perth. http://www.museum.wa.gov.au/catalogues/pseudoscorpions. Accessed 21 June 2023
Harvey MS, Ratnaweera PB, Udagama PV, Wijesinghe MR (2012) A new species of the pseudoscorpion genus Megachernes (Pseudoscorpiones: Chernetidae) associated with a threatened Sri Lankan rainforest rodent, with a review of host associations of Megachernes. J Nat Hist 46:2519–2535. https://doi.org/10.1080/00222933.2012.707251
Hlebec D, Harms D, Kučinić M, Harvey MS (2023a) Integrative taxonomy of the pseudoscorpion family Chernetidae (Pseudoscorpiones: Cheliferoidea): evidence for new range-restricted species in the Dinaric Karst. Zool J Linn Soc zlad083. https://doi.org/10.1093/zoolinnean/zlad083
Hlebec D, Podnar M, Kučinić M, Harms D (2023b) Molecular analyses of pseudoscorpions in a subterranean biodiversity hotspot reveal cryptic diversity and microendemism. Sci Rep 13:430. https://doi.org/10.1038/s41598-022-26298-5
Keum E, Takaku G, Lee K, Jung C (2016) New records of phoretic mites (Acari: Mesostigmata) associated with dung beetles (Coleoptera: Scarabaeidae) in Korea and their ecological implication. J Asia-Pac Entomol 19:353–357. https://doi.org/10.1016/j.aspen.2016.04.002
Levi HW (1953) Observations on two species of pseudoscorpions. Can Entomol 85:55–62. https://doi.org/10.4039/Ent8555-2
Morikawa K (1954) On some pseudoscorpions in Japanese limegrottoes. Memoirs of the Ehime University Sect II Ser B 2:79–87
Muchmore WB (1971) Phoresy by north and central American pseudoscorpions. Proc Rochester Acad Sci 12:79–97
Murakami O (1974) Growth and development of the Japanese wood mouse (Apodemus speciosus) I. The breeding season in the field. Jap J Ecol 24:194–206
Murienne J, Harvey MS, Giribet G (2008) First molecular phylogeny of the major clades of Pseudoscorpiones (Arthropoda: Chelicerata). Mol Phylogenet Evol 49:170–184. https://doi.org/10.1016/j.ympev.2008.06.002
Muster C, Spelda J, Rulik B et al (2021) The dark side of pseudoscorpion diversity: the German Barcode of Life campaign reveals high levels of undocumented diversity in European false scorpions. Ecol Evol 11:13815–13829. https://doi.org/10.1002/ece3.8088
Ohdachi SD, Ishibashi Y, Fukui D, Saitoh T (eds) (2015) The wild mammals of Japan, 2nd edn. Shokado, Kyoto
Oishi K, Arakaki T, Nakamura M et al (2018) Spatial arrangement and size of home ranges of Apodemus speciosus inhabiting evergreen broad-leaved forest and adjacent cedar plantation, and migration between these stands. Mamm Sci 58:23–31
Okabe K, Makino S, Shimada T et al (2018) Tick predation by the pseudoscorpion Megachernes ryugadensis (Pseudoscorpiones: Chernetidae), associated with small mammals in Japan. J Acarol Soc Japan 27:1–11. https://doi.org/10.2300/acari.27.1
Okabe K, Shimada T, Makino S (2020) Preliminary life history observations of the pseudoscorpion Megachernes ryugadensis (Pseudoscorpiones: Chernetidae) phoretic on wood mice in Japan. J Arachnol 48:155–160. https://doi.org/10.1636/0161-8202-48.2.155
Poinar GO, Curcuc BPM, Cokendolpher JC (1998) Arthropod phoresy involving pseudoscorpions in the past and present. Acta Arachnol 47:79–96. https://doi.org/10.2476/asjaa.47.79
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 21 June 2023
Sato JJ, Kyogoku D, Komura T et al (2019) Potential and pitfalls of the DNA metabarcoding analyses for the dietary study of the large Japanese wood mouse Apodemus speciosus on Seto Inland Sea islands. Mamm Study 44:221–231. https://doi.org/10.3106/ms2018-0067
Sato JJ, Shimada T, Kyogoku D et al (2018) Dietary niche partitioning between sympatric wood mouse species (Muridae: Apodemus) revealed by DNA meta-barcoding analysis. J Mammal 99:952–964. https://doi.org/10.1093/jmammal/gyy063
Shimada T (2001) Hoarding behaviors of two wood mouse species: different preference for acorns of two Fagaceae species. Ecol Res 16:127–133
Shimada T, Takahashi A, Shibata M, Yagihashi T (2015) Effects of within-plant variability in seed weight and tannin content on foraging behaviour of seed consumers. Funct Ecol 29:1513–1521. https://doi.org/10.1111/1365-2435.12464
Szymkowiak P, Górski G, Bajerlein D (2007) Passive dispersal in arachnids. Biol Lett 44:75–101
Tapia-Ramírez G, Villegas-Guzmán GA, Lorenzo C, Hernández-Núñez A (2022) Phoretic relationship between rodents and pseudoscorpions (Arachnida) in Chiapas, México. Therya Notes 3:46–50. https://doi.org/10.12933/therya_notes-22-68
Tatsukawa K, Murakami O (1976) On the food utilization of the Japanese wood mouse Apodemus speciosus (Mammalia: Muridae). Physiol Ecol Jpn 17:133–144
Tizo-Pedroso E, Del-Claro K (2007) Cooperation in the neotropical pseudoscorpion, Paratemnoides nidificator (Balzan, 1888): feeding and dispersal behavior. Insect Soc 54:124–131. https://doi.org/10.1007/s00040-007-0931-z
Weygoldt P (1969) The biology of pseudoscorpions. Harvard University Press, Cambridge, Massachusetts
White PS, Morran L, de Roode J (2017) Phoresy. Curr Biol 27:R578–R580. https://doi.org/10.1016/j.cub.2017.03.073
World Pseudoscorpiones Catalog (WPC) (2022) World Pseudoscorpiones Catalog, Version 2022. Natural History Museum Bern. http://wac.nmbe.ch. Accessed 21 June 2023
Yoshikawa T (2023) The large japanese field mouse (Apodemus speciosus) as a consumer and potential disperser of seeds of the neurotoxic Japanese star anise (Illicium anisatum). Mamm Study 48:131–135. https://doi.org/10.3106/ms2022-0042
Zeh DW, Zeh JA (1992a) On the function of harlequin beetle-riding in the pseudoscorpion, Cordylochernes scorpioides (Pseudoscorpionida: Chernetidae). J Arachnol 20:47–51
Zeh DW, Zeh JA (1992b) Dispersal-generated sexual selection in a beetle-riding pseudoscorpion. Behav Ecol Sociobiol 30:135–142. https://doi.org/10.1007/BF00173949
Zeh DW, Zeh JA (1992c) Emergence of a giant fly triggers phoretic dispersal in the neotropical pseudoscorpion, Semeiochernes armiger (Balzan)(Pseudoscorpionida: Chernetidae). Bull Br Aracnol Soc 9:43–46
Zeh DW, Zeh JA (1992d) Failed predation or transportation? Causes and consequences of phoretic behavior in the pseudoscorpion Dinocheirus arizonensis (Pseudoscorpionida: Chernetidae). J Insect Behav 5:37–49
Acknowledgements
We are grateful to H. Sato (Tokyo Kasei University) for his valuable comments on our study. We also thank the staff of the Takizawa Research Forest of Iwate University for providing the study site and two anonymous reviewers for their constructive comments on the manuscript.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI grants (nos. 16K07794, 19H03005, 22K19882 for TS; nos. 17H00807 and 20H00652 for KO).
Author information
Authors and Affiliations
Contributions
This study was conceived and designed by TS, KO, and SM. All authors contributed to the field surveys. Data analyses were conducted by TS. The first draft of the manuscript was written by TS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The methods applied in the field were approved by the local government (Iwate Prefecture, Japan).
Competing interests
The authors declare no competing interests.
Additional information
Communicated by: Matjaž Gregorič
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimada, T., Okabe, K., Makino, S. et al. Phoretic behavior of the pseudoscorpion Megachernes ryugadensis on the Japanese wood mouse Apodemus speciosus. Sci Nat 110, 51 (2023). https://doi.org/10.1007/s00114-023-01881-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-023-01881-6