Abstract
Many acoustic animals exhibit temporally structured chorusing, and in some cases, groups of calling males display elaborate forms of synchrony and/or alternation. Such temporal structure has traditionally been explained as an adaptation by which chorusing males preserve critical call features, maximize the attractiveness of their local group to females, or improve their ability to detect, evaluate, and/or evade rival males or predators. However, an alternative possibility is that synchrony and alternation simply emerge as incidental by-products of basic pairwise signal interactions between male neighbors. Thus, females may not be influenced by synchrony and alternation, and males may not benefit per se from the very chorus that they collectively produce. We studied chorusing in the bushcricket Ephippiger diurnus, a species that sings in both synchrony and alternation, by presenting natural and modified chorus stimuli to females in a series of playback tests. We found that females responded readily to the various stimuli, but we did not observe an elevated response to the natural chorus stimuli in any experiment or in any of the several E. diurnus populations tested. Our results demonstrate for the first time how elaborate forms of synchrony and alternation can represent emergent properties of choruses as opposed to specialized group displays that afford particular advantages to the individual singers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acoustic animals commonly sing in the vicinity of conspecifics. Whether singers congregate in space passively owing to specific habitat requirements or because individuals are actively attracted to one another, the group that forms may generate a communal display (Gerhardt and Huber 2002; Bradbury and Vehrencamp 2011; Greenfield 2015). Such displays, normally termed “choruses,” are often noteworthy for the sheer number of participating individuals as well as their overall sound amplitude. In some cases, they also exhibit a striking temporal structure in which individuals coordinate their songs in synchrony or alternation.
Synchronous and alternating choruses occur in most taxa of acoustic animals, but they are best known in acoustic insects and anurans where males broadcast their advertisement songs with a regular rhythm controlled by a central pattern generator (Greenfield 2005). Analogous communal displays occur in other signaling modalities, notably bioluminescence (Buck and Buck 1968; Moiseff and Copeland 2010), reflected light (Aizawa 1998; Backwell et al. 1998; Pope 2005), and substrate vibration (Hunt and Morton 2001). More research has been directed toward understanding how these displays are controlled as opposed to why they occur. Thus, neuro-ethological studies relying on playback of song stimuli have shown that relatively simple “phase-delay mechanisms” are responsible for inter-individual signal interactions that ultimately generate choruses of song alternation or synchrony in some acoustic insect and anuran species: A male interrupts his song rhythm upon hearing a single song stimulus or call by a neighbor, resets his central pattern generator to a basal level, remains inhibited as such until the stimulus ends, and then rebounds from inhibition and resumes his previous, free-running rhythm (Greenfield 1994a, b). Observations of paired individuals and simulations show that two singers calling with similar rhythms, both adjusted by a phase-delay mechanism when interacting with a neighbor, will each reset his singing repeatedly and together call in alternation or synchrony. Alternation is typically found in cases where the rebound from inhibition is faster than the free-running call period; synchrony tends to arise where the rebound is approximately the same length as the call period (Greenfield et al. 1997). Congregations of singers in natural populations generally include more than two individuals, and choruses may comprise groups of neighbors that interact mutually while ignoring more distant singers (Minckley et al. 1995; Snedden et al. 1998; Greenfield and Rand 2000; Greenfield and Snedden 2003; also see Nityananda et al. 2007 on the role of active spacing by singers). For example, a singer may alternate with its nearest neighbor(s) and—by default—synchronize with its neighbor’s neighbors. In other species, signal interaction mechanisms combine phase delay with acceleration or deceleration of the free-running rhythm, and very precise synchrony may result (Hartbauer et al. 2005; Nityananda and Balakrishnan 2007).
The traditional explanations for temporally structured choruses invoke cooperation or conflict among male singers (Nityananda and Balakrishnan 2009). Synchrony has been explained as a means by which a local group of singers cooperatively reduces signal interference, thereby increasing the group’s attractiveness to females. Thus, males may achieve improved signal perception because synchrony (1) preserves a species-specific rhythm that females must hear before approaching a group or any one singer (Walker 1969), (2) preserves critical aspects of the call “envelope” which would otherwise be masked by neighbors’ calls (Greenfield and Schul 2008; cf. Moiseff and Copeland 2010), (3) or maximizes the group’s overall sound amplitude, a phenomenon known as the “beacon effect” (Buck and Buck 1968; e.g., Hartbauer et al. 2014). On the other hand, synchrony might serve to increase the confusion faced by natural enemies: Phonotactic predators and parasites may be unable to localize any one singer in a group if sound arrives from all directions at the same time (e.g., Tuttle and Ryan 1982). Conversely, males in a synchronous chorus may be better able to detect approaching enemies, as the silent lull in each call cycle affords unmasked hearing of environmental sounds (cf. Brunel-Pons et al. 2011). These advantages in avoiding and detecting natural enemies may also accrue to females in the vicinity of a chorus (Alem et al. 2011). Alternation has also been explained as a means for reducing interference, as a male who alternates with a neighbor(s) can clearly evaluate that rival and may then behave accordingly, e.g., matching or exceeding his neighbor’s song features (Greenfield and Minckley 1993; Grafe 1996). Moreover, by alternating a male ensures that his own signal can be clearly perceived and evaluated by local females. Thus, a “superior” male would reduce the risk that females confuse him with an inferior singer in the vicinity. But, cooperation may also be a driving factor behind alternation in choruses: Where the attractiveness of a chorus to females depends on acoustic power, i.e., energy integrated over a given interval of time, as opposed to peak amplitude, individual males would aid and abet their group by alternating such that its sound is broadcast over the maximum proportion of time. Female attention to the acoustic power of a chorus may be expected because this parameter could be a reliable proxy for the number of male singers (Alem et al. 2015). In general, females are likely to favor larger over smaller groups of advertising males, e.g., leks, as sites for encountering mates.
An alternative explanation for temporally structured chorusing is that group synchrony and alternation simply emerge as by-products of basic signal interactions between neighbors (Greenfield and Schul 2008). Hearing in various acoustic species is subject to psychophysical “precedence effects” that may influence females to orient and move toward males producing leading calls while ignoring males whose calls follow a short interval later (Wallach et al. 1949; Zurek 1987; Litovsky et al. 1999; e.g., Wyttenbach and Hoy 1993; Snedden and Greenfield 1998; Marshall and Gerhardt 2010). Under these circumstances, any mechanism by which males increase their production of leading calls and decrease following ones would be selectively favored. A phase-delay mechanism as described above would satisfy these criteria, and it normally yields either synchrony or alternation at the level of the chorus (Greenfield et al. 1997). This proposition that chorusing originates in certain perceptual effects is not mutually exclusive with the traditional hypotheses that chorusing offers specific advantages in attracting females to a group, evaluating rival males, or detecting and evading phonotactic natural enemies. Nonetheless, it remains possible that a synchronous or alternating chorus is only an emergent property. That is, participating males would not benefit from synchrony or alternation per se, and attending females would not be influenced by these collective features when evaluating and orienting toward a chorus.
We addressed the question of whether temporally structured chorusing per se is a specific adaptation driven by female preference for a given form(s) of collective signal timing by studying female orientation and movement toward male song in the bushcricket Ephippiger diurnus (Orthoptera: Tettigoniidae: Bradyporinae). Individual E. diurnus males call rhythmically, and groups of males generate a chorus in which nearest neighbors generally alternate with one another (Greenfield and Snedden 2003). But, a considerable amount of synchrony also occurs, particularly when two or more males each alternate with the same centrally located individual and thereby call at the same time. Previous studies showed that females move toward male song whose rhythm leads that of a neighbor by a brief interval (Greenfield et al. 2004; Party et al. 2014). A phase-delay mechanism regulates signal interactions between neighboring males (Greenfield et al. 1997) and has probably evolved under the selection pressure imposed by female preference for leading calls. In a series of playback experiments, we tested the responses of female E. diurnus to natural chorus stimuli and to modified chorus stimuli in which the temporal structure had been altered. If chorusing structure is driven by female preference, we predict higher female responses toward natural as opposed to modified chorus stimuli. We also predict higher response levels to chorus stimuli in which the phase relationships between male song rhythms are characterized by synchrony or alternation, which do occur regularly in natural choruses, and relatively lower response levels to stimuli characterized by overlapped songs, which occur but rarely.
Females responded positively to playback of all chorus stimuli tested, and we did not observe heightened responses to natural stimuli or to synchronized and/or alternated calls in any experiment. Our results refute the several hypotheses proposing that the collective signal timing exhibited by chorusing males results from female preference for such group display. Rather, our results are most consistent with the alternative proposition that temporally structured chorusing in E. diurnus represents an emergent property of relatively simple, pairwise interactions.
Materials and methods
Populations studied and rearing
E. diurnus is a large, flightless bushcricket found in southern France and northeastern Spain. Its population genetic structure is characterized by geographically isolated populations between which relatively little gene flow occurs (Spooner and Ritchie 2006). The various clades differ morphologically and also acoustically (Duijm 1990). Populations are distinguished by the male song (number of syllables per call, call rhythm, carrier frequency), male song interactions (duration of inhibition in the phase-delay mechanism), and female response to male song (preference for number of syllables per call, extent of preference for leading calls) (Ritchie 1996; Brunel 2012). We tested five different E. diurnus populations that were predicted to represent two major clades (Table 1; Fig. 1; Spooner and Ritchie 2006). We obtained adults either by collecting late-instar nymphs or very young adults in the field and then rearing them in the laboratory or by rearing them from eggs laid in the laboratory by insects collected in the field during a previous year. All five populations were reared on cabbage, pollen, and fish flakes provided ad libitum while caged individually and kept in an environmental room maintained at 25 °C and a 16:8 L/D photoperiod. At the time of testing, all insects were at least 10-day post adult molt, the approximate age at which receptivity to males and male song begins. Adult females were kept in a room separate from males to minimize habituation to male song prior to their tests.
Map of southern France and northeastern Spain showing locations of E. diurnus populations (numerals 1–15a) used in experiments 1, 2, and 3 (circled numerals on map) and in the phylogeographic analysis. Dashed grey line represents the approximate separation between the two major clades inferred from analysis of COI data (see text and Fig. 4). Blue lines represent major rivers, and solid grey lines represent relief isolines for 1200 and 1800 m. Collecting sites: (1) Mireval, (2) Vias, (3) Peyriac de Mer, (4) Feuilla, (5) Font Romeu, (6) Latour de Carol, (7) Col de Puymorens (Pyrénées Orientales), (7a) Col de Puymorens (Ariège), (8) Col de Mantet, (9) Merens-les-Vals, (10) Col du Chioula, (10a) Espezel, (11) Carcanières, (12) Port de Lers, (13) Vilamòs, (14) Montauban de Luchon, (14a) Cigalère, (15) Le Lioran (Cantal), (15a) Pouzol (Cantal)
Phylogeography
To confirm phylogeographic relationships between the five populations studied, we applied a phylogenetic analysis to 16 populations sampled in southern France and northern Spain, including the five populations of the current study. We conducted our own analysis because the populations that we studied did not coincide with those in an earlier phylogeographic study of E. diurnus (Spooner and Ritchie 2006). By analyzing phylogeography, we could determine whether our findings on chorusing are widely distributed across clades or, alternatively, differ between the various clades.
We analyzed two to four adult individuals per locality, and two additional specimens of Sorapagus catalaunicus (Tettigoniidae: Bradyporinae) were used as outgroups. Genomic DNA was extracted from one leg of each specimen with the DNeasy Blood and Tissue Kit (Qiagen; Hilden, Germany). A DNA fragment consisting of 551 bp of the mitochondrial cytochrome oxidase I subunit (COI) gene was amplified using C1-J-1718 and C1-N-2191 primers as specified in Simon et al. (1994). The PCR products were directly sequenced from both directions by Eurofins Genomics and then edited, cleaned, and assembled manually using Geneious version Pro 5.6.6 (Biomatters). We employed jModelTest 2.1.3 (Posada 2008) to select the best-fitting nucleotide substitution model, and further, Bayesian phylogenetic analyses were performed using two independent MCMC chains of 10,000,000 generations in MrBayes 3.2.1 (Ronquist and Huelsenbeck 2003) sampled each 1000 generations. The first 25 % of the sampled trees were discarded as “burnin”
Chorus recording
To evaluate the incidence of alternation and synchrony in E. diurnus choruses, we measured the patterns of group calling in two of the five populations in the current study, Peyriac de Mer and Vilamòs. These two populations represented two different clades and modes of singing, polysyllabic (Peyriac de Mer) and mono- or bi-syllabic (Vilamòs) (Table 1; Fig. 1). For each population, we assembled choruses of four males held in individual screen cages (10-cm height, 10-cm diameter) arranged at the corners of a 40-cm square within a laboratory arena surrounded by acoustic insulation foam (cf. Party et al. 2014). A condenser microphone (model CM16/CMPA; Avisoft Bioacoustics, Berlin, Germany; frequency response ± 3 dB, 10–150 kHz) suspended above each male recorded his singing; a tube of acoustic insulation placed around the microphone reduced cross talk from neighboring males. We recorded four and six four-male choruses from the Peyriac de Mer and Vilamòs populations, respectively, using different individuals in each chorus. To examine potential differences in calling patterns as the number of males in a group changes, we also recorded two-male choruses from both populations. Individuals recorded in two-male choruses came from the same pool of insects used for the four-male choruses. Microphone outputs of a given chorus were sent to a multichannel recorder (TASCAM DR-680, Teac Corporation), digitized, and saved as a sound file for later analysis with acoustic signal processing software (BatSound Pro 4.0; Pettersson Elektronik, Uppsala, Sweden).
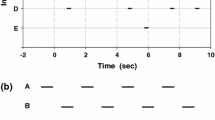
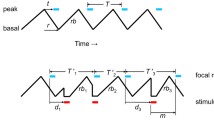
From each chorus recording, we selected a 1-min sample during which all males called regularly. We analyzed the temporal pattern of a chorus by tabulating the number of incidents of call synchrony, overlapping of calls, and isolated calls wherein an individual sang in alternation with his neighbor(s) during the 1-min interval. These three temporal categories are defined as a delay (d) ≤ 100 ms between the onsets of the first and last male to call, 100 < d ≤ 400 ms, and d > 400 ms (Fig. 2). We chose these criteria because previous studies (Brunel 2012) indicated that E. diurnus males typically do not call during a 300-ms interval beginning 100 ms after the onset of a song stimulus; this critical interval reflects the insect’s phase-delay mechanism. Current observations indicate that the interval in the Mireval and Vilamòs populations approximates these values, whereas in the Font Romeu and Peyriac de Mer populations, it is prolonged. For each incident of synchrony and overlapping of calls, we noted the number of males who sang.
Categories of call timing in a four-male chorus (individuals A–D): I synchrony between all four males (delay between onsets of first and last calls in a group of calls, d, <100 ms); II and III isolated calls by males A and C (d > 400 ms); IV and V overlapping of calls by males B and D (100 ms < d < 400 ms)
Responses to chorus stimuli
Our basic protocol consisted of broadcasting natural and modified chorus stimuli to individual females walking on a 30-cm diameter locomotion compensation sphere (LC-300, SYNTECH; Hilversum, Netherlands) and comparing their orientation and movement toward these stimuli. We conducted two experiments (1 and 2) using a no-choice protocol in which the various stimuli were presented in separate trials and one choice experiment (3).
Experiment 1
We tested the responses of females to representative samples of a two-male chorus from their population and to a modification of these samples. We prepared our chorus stimuli by using two condenser microphones (model CM16/CMPA; Avisoft Bioacoustics) to record pairs of males from a given population while singing 40 cm apart in a laboratory room maintained at 25 °C. We digitized (UltraSoundGate 416-200; Avisoft Bioacoustics, Berlin, Germany) and saved these recordings to stereo sound files on a computer and later analyzed these files with BatSound Pro 4.0 to select typical samples of matched chorusing, i.e., two males calling at similar rhythms, an approximately 180° phase relationship, and with calls having average features for the population. The two males in the selected pairs differed in sound amplitude, though, and the orientations of the males relative to the microphones increased this amplitude difference to approximately 2–4 dB in the recording. A 30-s segment of the recording represented the natural chorus stimulus, one that might be heard by a female approaching a pair of singing males, one closer to her than the other, in the field. Using BatSound Pro 4.0, we then modified inter-call intervals in the natural chorus stimuli to have each male produce two consecutive calls one to three times during the 30-s segment, maintaining a minimum 1.0 s between such consecutive calls, and to alter the phase relationship during the remaining incidents of alternation from approximately 180° to a full range of phase angles (Table 2(A); Fig. 3a). Thus, females were exposed to exactly the same calls and a similar amount of sound energy in both the natural and modified chorus stimuli.
a Example of a natural chorus stimulus and its modification used in experiment 1 with females from the Merens-les-Vals population. b Five chorus stimuli used in experiment 2 with females from the Font Romeu population. For each stimulus, a two-channel oscillogram shows the relative timing of broadcasts of the left and right loudspeakers over a 10-s interval
We tested 13 and 19 females from the Vilamòs and Merens-les-Vals populations, respectively (Table 2(A)). Each female was tested with three natural chorus stimuli recorded from her population and with a modification of these three stimuli. We randomized the sequence with which the six stimuli were presented to each female, and we allowed a minimum 15 min to elapse between a female’s consecutive trials. Stimulus broadcasts lasted 60 s in each trial. All testing was conducted during morning hours, the general period of mating activity in E. diurnus. Temperature was held at 25 °C, as in rearing and song recording, and the testing apparatus was illuminated from above by diffuse incandescent light.
Experiment 2
We followed an overall procedure similar to that in experiment 1 and tested five different synthetic chorus stimuli representing two males calling at identical rhythms, the average value in the respective population. Two of these were “natural” stimuli, one with the two males calling in perfect alternation, i.e., a 180° phase angle between the males, and a second with calling in near synchrony, a 10° phase angle. Pairs of males calling with a 20°, 30°, and 40° phase angle, representing song overlap, served as three modified stimuli (Table 2(B); Fig. 3b). We used a single, selected male call as a fundamental signal in our chorus stimuli so that other acoustic variables, e.g., number of syllables per call or carrier frequency, which vary within and between individuals would not influence our experiments. Using a signal processing program (Audacity 2.0.5, Free Software Foundation; Boston, USA), we repeatedly copied the selected call to the two channels of a stereo playback file and adjusted the rhythm and phase accordingly. To avoid any consequences of one channel always representing the leader, we alternated between positive and negative phase angles in consecutive call cycles of the 10°, 20°, 30°, and 40° stimuli (Fig. 3b).
We tested 22–24 females from each of four populations, Font Romeu, Mireval, Peyriac de Mer, and Vilamòs. Each female was tested with all five stimuli representing the two natural and three modified phase relationships. The sequences of stimulus presentation and stimulus duration were as applied in experiment 1.
In both experiments 1 and 2, we began each trial by placing the female on top of the locomotion compensation sphere and simultaneously starting the sphere control unit. The chorus stimulus and recordings of the walking path started simultaneously, about 30 s after placing the insect on top of the sphere. The control unit rotated the sphere whenever the female moved, and it effected this rotation in a compensatory manner such that the insect always remained at the sphere’s highest point, a fixed distance from the loudspeakers broadcasting the stimulus. Thus, the female was subject to an “open loop” test.
We presented a chorus stimulus by playing its stereo sound file on a computer, converting this digital playback signal to analogue (UltraSoundGate Player 216H, Avisoft Bioacoustics, Berlin, Germany), and sending the analogue output to two adjacent loudspeakers (Ultrasonic Dynamic Speaker Vifa, Avisoft Bioacoustics, Berlin, Germany) positioned at the level of the top of the sphere, 20 cm from the test female. The broadcasts were amplified (UltraSoundGate Player 216H, Avisoft Bioacoustics, Berlin, Germany) to 80 dB sound pressure level (SPL) (0 dB = 20 μPa), thereby representing males singing 2 m distant; in experiment 1, this amplification was referenced to the louder of the two males. Silent loudspeakers placed on the opposite side of the sphere served to balance the visual stimuli surrounding the test insect.
The compensatory rotations effected by the sphere control unit were transmitted to another computer, and a software program (TrackSphere, SYNTECH) processed these data in real time to depict the virtual trajectory of the female during her trial. Via analysis of the trajectory, we determined the female’s total distance moved and her distance moved toward the stimulus. The directionality of a female’s trajectory in a trial was calculated as the distance moved toward the stimulus divided by the total distance moved. Directionality was measured to distinguish females who moved the same distance toward the stimulus but at different speeds, faster individuals exhibiting more transverse turning. We then compared the distances and directionalities observed in response to the natural and modified chorus stimuli and thus developed a preference function for these several stimuli. Females normally moved 1–3 m toward the stimulus during a 60-s trial. Consequently, we judged that a female who moved less than 30 cm in the direction of the stimulus during all trials in experiment 1 or in experiment 2 was non-receptive and she was eliminated from analysis.
We conducted experiments 1 and 2 with a no-choice protocol because females moving among and toward choruses in the field are likely to hear only one chorus at a time: In most cases, groups of singing males are separated by 10–15 m or more. Choice tests are generally more likely than single-stimulus presentations to reveal a preference between two stimuli, but such preference can be a laboratory artifact where the opportunity to effect such choice seldom arises in a natural population (Dougherty and Shuker 2015). In addition, the no-choice protocol afforded us the opportunity to evaluate a large array of stimuli in the restricted time intervals dictated by the experimental design.
Experiment 3
Notwithstanding the justification offered above for our use of no-choice experiments, we conducted a further series of choice tests to determine whether simultaneous preference does exist for some chorus stimuli over others. Should such preference occur in cases where differential responses to single stimuli are not observed, they could be critical in the event that male groups are relatively close. We used pairs of natural and modified stimuli from experiment 2 and tested the preference for near synchrony (10° phase angle) vs song overlap (30° phase angle) and the preference for song overlap (40° phase angle) vs perfect alternation (180° phase angle) (Table 2(C)). The natural and modified stimuli tested in a trial were themselves broadcast in alternation. We decreased the rates of each stimulus accordingly so that leader-follower relationships between them did not occur (Table 2(C)).
We conducted our choice tests with two pairs of loudspeakers placed on opposite sides of the locomotion compensation sphere. Each pair broadcasts a particular chorus stimulus, and we adjusted the stimulus amplitude to 70-dB SPL. This lower amplitude was used to reduce the possibility of “confusing” the test females with excessive levels of male song and to represent sound amplitudes that might be experienced between two choruses in the field. The four loudspeakers were driven by a TASCAM DR-680 multichannel recorder. We tested 13–19 females from each of three populations, Merens-les-Vals, Mireval, and Vilamòs. Each female was tested in two 60-s trials representing the two categories of choice tests described above. All other aspects of the playback protocol were the same as used in experiments 1 and 2.
We used circular statistics to analyze the trajectories of females walking on the sphere. Thus, in every trial, we determined the average direction of the female over the 60-s duration of playback and her directionality, calculated as the distance she moved along the axis of that average direction divided by the total distance of her trajectory. For each population, we also calculated the mean vector angle and the dispersion. Polar plots are used to portray each female’s vector angle and the mean vector angle of the population.
In all three experiments, we tested stimuli representing two-male choruses in order to avoid the many complex interactions that would arise in larger choruses and render our analyses difficult. We also note that choruses begin with pairs of singing males, and any female preferences that exist for particular phase relationships within groups of singers are expected to occur in pairs.
Results
Phylogeography
Our analysis of COI data primarily shows that the 16 E. diurnus populations sampled from southern France and northern Spain can be resolved into two major clades (Fig. 4). Clade 1 includes populations along the Mediterranean coast south of Narbonne, Dept. Aude, France, and extends west on southern slopes of the Pyrénées Mountains. Clade 2 includes populations along the Mediterranean coast north of Narbonne and extends west in valleys and on northern slopes of the Pyrénées as well as north into the Massif Central. Phenotypically, these clades are best distinguished by temporal features of the male song: three or more syllables per call in clade 1 and one or two syllables per call in clade 2 (Table 1; cf. Duijm 1990). In the current study, Font Romeu and Peyriac de Mer represent clade 1, and Merens-les-Vals, Mireval, and Vilamòs represent clade 2. Our overall phylogeography (Figs. 1 and 4) is largely comparable to that presented in Spooner and Ritchie (2006). Differences mainly reflect our reduced sampling near the Mediterranean Sea and our more extensive sampling to the west in the departments of Ariège and Haute Garonne.
Phylogeny of E. diurnus from southern France and northeastern Spain generated by sampling cytochrome oxidase subunit I data from 46 individuals distributed among 16 populations (see Fig. 1); the outgroup is represented by Sorapagus catalaunicus samples. Two major clades are designated according to this analysis. Red asterisks indicate samples from populations tested in the current study. Horizontal scale at bottom indicates 0.03 nucleotide substitutions per site; values over branches represent posterior probabilities for branches with a probability >50 %
Chorusing
In both the Peyriac de Mer and Vilamòs populations, two-male choruses generally exhibited alternation, a small percentage of synchrony, and very rarely an overlapping of calls (Fig. 5a). Calling patterns in the four-male choruses were quantitatively different. In both populations, synchrony was more frequent than in the two-male choruses (Fig. 5b). Synchronous calling generally included two males, but some incidents including three and even four males were also found (e.g., Fig. 6). Again, incidents of overlapping of calls were rare (Fig. 5b). These results confirm that the 180° and 10° phase angle playbacks in experiment 2 represent natural chorus stimuli, for these populations (cf. Table 2(B)), and that the 20°, 30°, and 40° playbacks represent modified stimuli.
Analyses of relative call timing in E. diurnus choruses in the Peyriac de Mer and Vilamòs populations. a Proportion of call cycles during a 1-min recording of a two-male chorus during which the males’ calls overlapped, i.e., delay between the onsets of the males’ calls was 100–400 ms, or were synchronized, i.e., delay between the onsets of the males’ calls was <100 ms (see Fig. 2). b Proportion of call cycles during a 1-min recording of a four-male chorus during which calls of two or more males overlapped or were synchronized. In both a, b, values show the median proportion and the 25–75 % range of proportions determined from all choruses recorded in the respective population. In all cases, isolated calls produced in alternation represent the remaining proportion
Responses to chorus stimuli
Experiment 1
In both the Merens-les-Vals and Vilamòs populations, we compared the responses of a given female to each of the three pairs of natural and modified chorus stimuli tested. Tabulations of these responses revealed no significant differences in distance moved to the stimulus for all three stimulus pairs tested in the Merens-les-Vals population (paired t tests; −0.27 < t < 0. 28; p > 0.75), but one significant difference among the three stimulus pairs tested in the Vilamòs population (t = −3.02; p = 0.01). Importantly, however, the significant difference entailed a higher level of response to the modified chorus stimulus. We observed no significant differences in either population in directionality of the trajectory (−1.89 < t < 1.21; p > 0.09) (Fig. 7).
Responses of females from the Merens-les-Vals and Vilamòs populations to three different natural chorus stimuli (N1–N3) and to the modifications of each of those stimuli (M1–M3) presented in experiment 1. Values show median distance moved in direction of the stimulus during a 60-s trial, 25–75 % range of distances, and 10–90 % range
Three females from Merens-les-Vals were unreceptive and were not used in analysis. Among retained insects, the percentage of trials in which the female moved less than 30 cm toward the stimulus did not differ between the natural and modified chorus stimuli for any stimulus pair in either population (Fisher’s exact test; p > 0.38).
Experiment 2
In three of the four populations examined, we found no significant differences in distance moved to the stimulus among all five chorus stimuli tested (Table 3). However, an overall significant difference among chorus stimuli was observed in the Vilamòs population, and analyses of all pairwise combinations of stimuli showed that this difference was due to comparisons of the 20° phase angle stimulus with both the 40° and 180° stimuli. Here, the 40° and 180° stimuli elicited higher response levels (Table 3). Analysis of data from all populations pooled (n = 83 retained individuals) did not reveal an overall significant difference in response level among the five stimuli (one-way repeated measures ANOVA; SigmaStat 3.5, Systat Software Inc., San Jose, CA, USA; F = 1.67; p = 0.16). Further analysis of this overall result showed that acceptance of the null hypothesis was supported by substantial power (post hoc power estimate = 0.82; G*Power version 3.1.9.2; Kiel, Germany; effect size was determined from means and standard deviations of responses to each of the five stimuli) (Fig.8).
Between one and three females in each of the four populations were unreceptive and were not used in analysis. As before, the percentage of trials in which the retained females moved less than 30 cm toward the stimulus did not differ between the natural (10° and 180° phase angle) and modified (20°, 30°, and 40°) stimuli in any population (Χ 2 test with Yates correction or Fisher’s exact test; p > 0.19 in all populations).
Our analysis of the directionality of female response in experiment 2 revealed similar results. In three of the four populations, there were no significant differences in directionality of the trajectory among all five chorus stimuli tested (Table 3). In the Peyriac de Mer population, however, an overall significant difference was found, and analyses of all pairwise combinations of stimuli showed that this result was attributed to comparison of the 30° and 180° stimuli. Importantly, responses to the 30° (modified) stimulus were more directional. Analyzing all populations pooled indicated an overall significant difference in directionality (one-way repeated measures ANOVA; F = 2.58, p = 0.04), which was attributed to comparison of the 40° (modified) and 180° (natural) stimuli; the 40° stimulus elicited more directional responses.
We then divided the 60-s trials into initial and final 30-s segments and analyzed the distance moved to the stimulus in each time segment separately. For the initial 30-s segment, we found significant differences only in the Font Romeu (10° vs 180° stimuli; higher response to 180°) and Vilamòs (20° vs 40° stimuli and 20° vs 180° stimuli; higher response to 40° and 180° stimuli, respectively) populations (Table 3). Analysis of data from all populations pooled revealed an overall significant difference in response level (one-way repeated measures ANOVA; F = 3.29, p = 0.01), which was attributable to comparison of the 20° and 40° stimuli. Here, the 40° stimulus elicited a higher response. Examining the final 30-s segment revealed significant differences only in the Vilamòs population (20° vs 40° stimuli and 20° vs 180° stimuli; higher response to 40° and 180° stimuli, respectively). In analyzing all populations pooled, we found no significant difference in responses during the final 30-s segment to the five chorus stimuli (F = 0.99, p = 0.41).
Experiment 3
In two of the three populations subjected to choice tests of natural (10° phase angle or perfect alternation) vs. modified chorus stimuli (overlapping phase angles of 30° or 40°), we found no preference for the natural stimuli. In the Vilamòs population, mean vector angles of individual female trajectories were uniformly distributed in the two tests conducted (Rayleigh test; p > 0.10; Fig. 9). In the Mireval population, the mean vector angles of individual female trajectories were not uniformly distributed in the two tests (Rayleigh test; p = 0.02), but the vectors are not clustered around the predicted direction, the loudspeaker broadcasting the natural chorus stimuli (V test; p > 0.25). However, a significant preference was found in the Merens-les-Vals population for the natural chorus stimulus in the choice test presenting a 10° phase angle vs. a 30° phase angle (Rayleigh test; p = 0.02 and V test; p = 0.0025). In the second choice test (40° phase angle vs. perfect alternation), Merens-les-Vals females exhibited a uniform distribution of mean vector angles (Rayleigh test; p > 0.50).
Responses of females from three E. diurnus populations in two different choice tests of synthetic chorus stimuli (10° vs. 30° phase angle; 40° vs. 180° phase angle) presented in experiment 3. Polar plots show the mean vector angles of the trajectories of individual females tested during 60-s trials on the locomotion compensator. Vector lengths represent directionality (distance along the axis of the mean direction divided by the total distance of the trajectory); longer vectors represent straighter trajectories. In each polar plot, the red arrow shows the mean vector angle for the population
Discussion
Despite singing with markedly different numbers of syllables per call and call rhythms (Table 1), the two E. diurnus populations that we recorded (Peyriac de Mer, Vilamòs) exhibited similar patterns of chorusing (Fig. 5). Moreover, these patterns were consistent with those reported earlier (Greenfield and Snedden 2003) in a monosyllabic population recorded in the field at St. Jean de Buèges, Dept. Hérault, France. This latter population may be phylogeographically close to the Mireval population tested in the current study (Spooner and Ritchie 2006). In general, we observed alternation and synchrony of the calls broadcast by neighbors. Most incidents of synchrony probably occurred when each of two males alternated with a third male situated centrally. But, our observations that synchrony also occurs in two-male choruses indicate that some incidents arose when two neighbors happened to call at the same time and each one inhibited and reset his call rhythm in response to the other. This mutual resetting then resulted in both males again singing at approximately the same time during the next call cycle, an event that was sometimes repeated several times until between-male differences in call rhythm broke the pattern of synchrony, and alternation was regained (cf. Sismondo 1990). The very low incidence of overlapping of calls in choruses probably reflects how a male’s phase-delay mechanism inhibits him from singing during a critical interval following the onset of calls of close neighbors. Thus, a male’s calls either occur in alternation with a neighbor(s)—following the end of the critical interval or occur in synchrony—before inhibition by the neighbor(s) can operate. That is, a brief (∼100 ms) effector delay occurs between the central triggering of a focal male’s call and the actual onset of that call, and a stimulus or neighbor’s call that begins <100 ms before the moment when the onset of that focal male’s call is set to occur will not effect inhibition: That call has already been triggered and will be broadcast in synchrony with the stimulus or neighbor (Greenfield et al. 1997).
Females tested on the locomotion compensation sphere usually walked several meters in the direction toward the chorus stimulus during their trial, and directionalities of these trajectories were generally >0.5. Thus, we did not observe any widespread difficulty experienced by females in responding toward chorus stimuli or in moving on the test apparatus. Nonetheless, we observed little preference for any one of the various stimuli tested over another in any of the three experiments or in either of the two clades. In experiment 1, test females generally responded equivalently to a natural chorus of two males and to a modification of that chorus in which the inter-call intervals of the males were rearranged such that the regular pattern of alternation was not present. The only exception to this pattern entailed higher responses to one of the modified chorus stimuli in one population. Similarly, in experiment 2, females mostly exhibited equivalent responses to all five stimuli, which included two natural and three modified (overlapping) phase relationships between male songs. The few cases where females exhibited a preference for one or more stimuli over another in terms of distance moved to the stimulus or directionality of the trajectory did not reveal an overall reduction in response to stimuli in which the male songs overlapped one another. Moreover, power analysis confirms the null hypothesis that response levels did not differ among stimuli. Finally, in experiment 3, females preferred the natural chorus stimulus over the modified one in only one of six choice tests conducted. Overall, these results clearly show that E. diurnus females did not exhibit an elevated response to stimuli that represented the temporal relationships typically found in natural choruses.
Results from experiments 1, 2, and 3 indicate that the temporal structure in E. diurnus choruses does not serve to increase the number of females that a group of males attracts collectively. Moreover, our chorus recordings suggest that other functions traditionally ascribed to call alternation between neighbors are equally unlikely: Whereas males generally alternate with their nearest neighbors, by default, they also synchronize with their second, and third, nearest neighbors. Consequently, in most circumstances, females who have arrived at a chorus would not be afforded the opportunity to compare two nearby focal males whose alternating calls are not at least partially masked by the singing of other local males. Similarly, a male may not clearly hear and evaluate the singing of a nearby focal neighbor with whom he is alternating calls. Observations of the spatial distribution of singing males in the field (Greenfield and Snedden 2003) suggest that the calls of these local surrounding males would often be only 3–6 dB lower in amplitude, as perceived by a receiver situated at a point in the vicinity, than the calls of the focal individuals. Given such small differences in relative amplitude, these surrounding males would probably not be ignored by females or males that are evaluating their nearest neighbor(s). Experiments addressing selective attention in E. diurnus choruses suggest that a male typically responds to, i.e., is inhibited and reset by, his nearest neighbor and those whose calls are within 6 dB in amplitude of that nearest individual (Greenfield and Snedden 2003).
The temporal structure of choruses has also been explained as a means of avoiding or reducing attacks by acoustic predators or parasites. Again, our chorus recordings indicate that this is improbable in E. diurnus: Natural choruses include both synchronous and alternating phase relationships between male songs, and the incidence of synchrony increases and approaches that of alternation as the number of singing males increases to as few as four individuals. Whereas a given form of chorusing, particularly synchrony, may conceivably afford some protection from natural enemies, the collective broadcast of a second form of chorusing would, in most cases, greatly reduce any benefit that might be obtained from the first.
The findings and interpretations of experiments 1, 2, and 3 and the chorus recordings support the hypothesis that the elaborate chorus structure observed in various E. diurnus populations is largely an emergent property. It arises when neighboring males respond to one another by mutually applying their phase-delay mechanisms. This signal interaction mechanism has probably evolved under the sexual selection pressure imposed by females, who generally prefer male calls that lead their neighbors by a brief interval (cf. Greenfield and Roizen 1993). The alternation between neighboring males is most regular when the two interacting individuals sing with similar call rhythms that they modify with the same form of phase-delay mechanism (Greenfield 1994a). In this situation, females may not pay attention to the specific chorus structure that a group of males generates collectively; likewise, males may not benefit per se from the very chorus structure that they themselves produce. Whereas chorusing males certainly gain an advantage when they do not sing during the brief interval following a nearby neighbor during which females will not orient and move toward a call, a male’s attractiveness or status within the chorus is not enhanced by the specific collective pattern typically generated.
Studies of collective signaling displays suggest that various examples are emergent phenomena as described here for E. diurnus choruses. Such suggestions are reported in diverse animal taxa (Greenfield 1994b; Kahn et al. 2014); temporally, they entail both synchrony and alternation, and they involve different signaling modalities, including acoustic and visual displays. They may occur in species whose close relatives effect similar collective displays that do represent specific adaptations. For example, synchronous chorusing in the bushcricket genus Neoconocephalus includes cases that are seemingly emergent and other cases wherein synchrony, by providing a “modulation depth” between calls broadcast by local males, satisfies the requirement of females to hear a male call envelope clearly (Greenfield and Schul 2008).
Human perceptual and aesthetic sensibilities are drawn to patterns in nature, and we may be naturally inclined to attribute purpose and value to phenomena that are structured and that please our senses (Strogatz 2003). Temporally structured choruses broadcast by acoustic animals certainly attract our attention, and it may be difficult to accept the possibility that they do not necessarily fulfill a specific function for the singers and for the conspecifics who listen. Nonetheless, thorough analyses reveal that some chorusing emerges as an incidental by-product of simple neighbor-neighbor interactions. These emergent phenomena serve as a reminder that not all behavioral features that we observe, regardless of their complexity, have arisen as specialized adaptations to particular selection pressures.
References
Aizawa N (1998) Synchronous waving in an ocypodid crab, Ilyoplax pusilla: analyses of response patterns to video and real crabs. Mar Biol 131:523–532
Alem S, Koselj K, Siemers BM, Greenfield MD (2011) Bat predation and the evolution of leks in acoustic moths. Behav Ecol Sociobiol 65:2105–2116
Alem S, Clanet C, Dixsaut A, Party V, Greenfield MD (2015) What determines lek size? Cognitive constraints and per capita attraction of females limit male aggregation in an acoustic moth. Anim Behav 100:106–115
Backwell P, Jennions M, Passmore N, Christy J (1998) Synchronized courtship in fiddler crabs. Nature 391:31–32
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication, 2nd edn. Sinauer Associates, Sunderland, 697 pp
Brunel O (2012) ‘De la communication acoustique au sein du groupe: contraintes et mécanismes’. Ph.D. thesis, Université François Rabelais, Tours, France
Brunel-Pons O, Alem S, Greenfield MD (2011) The complex auditory scene at leks: balancing anti-predatory behaviour and competitive signalling in an acoustic moth. Anim Behav 81:231–239
Buck J, Buck E (1968) Mechanism of rhythmic synchronous flashing of fireflies. Science 159:1319–1327
Dougherty LR, Shuker DM (2015) The effect of experimental design on the measurement of mate choice: a meta-analysis. Behav Ecol 26:311–319
Duijm M (1990) On some song characteristics in Ephippiger (Orthoptera, Tettigonioidea) and their geographic variation. Neth J Zool 40:428–453
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans: common problems and diverse solutions. Univ. of Chicago Press, Chicago, 531 pp
Grafe T-U (1996) The function of call alternation in the African reed frog Hyperolius marmoratus: precise call alternation prevents auditory masking. Behav Ecol Sociobiol 38:149–158
Greenfield MD (1994a) Cooperation and conflict in the evolution of signal interactions. Annu Rev Ecol Syst 25:97–126
Greenfield MD (1994b) Synchronous and alternating choruses in insects and anurans: common mechanisms and diverse functions. Am Zool 34:605–615
Greenfield MD (2005) Mechanisms and evolution of communal sexual displays in arthropods and anurans. Adv Study Behav 35:1–61
Greenfield MD (2015) Signal interactions and interference in insect choruses: singing and listening in the social environment. J Comp Physiol A 201:143–154, Special issue: insect hearing: from physics to ecology
Greenfield MD, Minckley RL (1993) Acoustic dueling in tarbush grasshoppers: settlement of territorial contests via alternation of reliable signals. Ethology 95:309–326
Greenfield MD, Rand AS (2000) Frogs have rules: selective attention algorithms regulate chorusing in Physalaemus pustulosus (Leptodactylidae). Ethology 106:331–347
Greenfield MD, Roizen I (1993) Katydid synchronous chorusing is an evolutionarily stable outcome of female choice. Nature 364:618–620
Greenfield MD, Schul J (2008) Mechanisms and evolution of synchronous chorusing: emergent properties and adaptive functions in Neoconocephalus katydids (Orthoptera: Tettigoniidae). J Comp Psychol 122:289–297
Greenfield MD, Snedden WA (2003) Selective attention and the spatio-temporal structure of orthopteran choruses. Behaviour 140:1–26
Greenfield MD, Tourtellot MK, Snedden WA (1997) Precedence effects and the evolution of chorusing. Proc R Soc Lond B 264:1355–1361
Greenfield MD, Siegfreid E, Snedden WA (2004) Variation and repeatability of female choice in a chorusing katydid, Ephippiger ephippiger: an experimental exploration of the precedence effect. Ethology 110:287–299
Hartbauer M, Kratzer S, Steiner K, Römer H (2005) Mechanisms for synchrony and alternation in song interactions of the bushcricket Mecopoda elongata (Tettigoniidae: Orthoptera). J Comp Physiol A 191:175–188
Hartbauer M, Haitzinger L, Kainz M, Römer H (2014) Competition and cooperation in a synchronous bushcricket chorus. Roy Soc Open Sci 1:140167. doi:10.1098/rsos.140167
Hunt RE, Morton TL (2001) Regulation of chorusing in the vibrational communication system of the leafhopper Graminella nigrifrons. Am Zool 41:1222–1228
Kahn AT, Holman L, Backwell PRY (2014) Female preferences for timing in a fiddler crab with synchronous courtship waving displays. Anim Behav 98:35–39
Litovsky RY, Colburn HS, Yost WA, Guzman SJ (1999) The precedence effect. J Acoust Soc Am 106:1633–1654
Marshall VT, Gerhardt HC (2010) A precedence effect underlies preferences for calls with leading pulses in the grey treefrog, Hyla versicolor. Anim Behav 80:139–145
Minckley RL, Greenfield MD, Tourtellot MK (1995) Chorus structure in tarbush grasshoppers: inhibition, selective phonoresponse, and signal competition. Anim Behav 50:579–594
Moiseff A, Copeland J (2010) Firefly synchrony: a behavioral strategy to minimize visual clutter. Science 329:181
Nityananda V, Balakrishnan R (2007) Synchrony during acoustic interactions in the bushcricket Mecopoda ‘chirper’ (Tettigoniidae : Orthoptera) is generated by a combination of chirp-by-chirp resetting and change in intrinsic chirp rate. J Comp Physiol A 193:51–65
Nityananda V, Balakrishnan R (2009) Modeling the role of competition and cooperation in the evolution of katydid acoustic synchrony. Behav Ecol 20:484–489
Nityananda V, Stradner J, Balakrishnan R, Römer H (2007) Selective attention in a synchronising bushcricket: physiology, behaviour and ecology. J Comp Physiol A 193:983–991
Party V, Brunel-Pons O, Greenfield MD (2014) Priority of precedence: receiver psychology, female preference for leading calls and sexual selection in insect choruses. Anim Behav 87:175–185
Pope DS (2005) Waving in a crowd: fiddler crabs signal in networks. In: McGregor PK (ed) Animal communication networks. Cambridge Univ. Press, Cambridge, pp 252–276
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Ecol Evol 25:1253–1256
Ritchie MG (1996) The shape of female mating preferences. Proc Natl Acad Sci U S A 93:14628–14631
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P (1994) Evolution, weighting, phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am 87:651–701
Sismondo E (1990) Synchronous, alternating, and phase-locked stridulation by a tropical katydid. Science 249:55–58
Snedden WA, Greenfield MD (1998) Females prefer leading males: relative call timing and sexual selection in katydid choruses. Anim Behav 56:1091–1098
Snedden WA, Greenfield MD, Jang Y (1998) Mechanisms of selective attention in grasshopper choruses: who listens to whom? Behav Ecol Sociobiol 43:59–66
Spooner LJ, Ritchie MG (2006) An unusual phylogeography in the bushcricketEphippiger ephippiger from southern France. Heredity 97:398–408
Strogatz S (2003) Sync: how order emerges from chaos in the universe, nature, and daily life. Hyperion, New York, 338 pp
Tuttle MD, Ryan MJ (1982) The role of synchronized calling, ambient light, and ambient noise in anti-bat-predator behavior of a treefrog. Behav Ecol Sociobiol 11:125–131
Walker TJ (1969) Acoustic synchrony: two mechanisms in the snowy tree cricket. Science 166:891–894
Wallach H, Newman EB, Rosenzweig MR (1949) The precedence effect in sound localization. Am J Psychol 62:315–336
Wyttenbach RA, Hoy RR (1993) Demonstration of the precedence effect in an insect. J Acoust Soc Am 94:777–784
Zurek PM (1987) The precedence effect. In: Yost WA, Gourevitch G (eds) Directional hearing. Springer-Verlag, New York, pp 85–105
Acknowledgments
We thank Guy Bourdais, Séverine Devers, and Justine Penin for technical assistance in the laboratory and the Agence Nationale de la Recherche de France (contract ANR-11-BSV7-025-01; EVOLCHOR), the Centre National de la Recherche Scientifique (CNRS), and the Université François Rabelais de Tours for their financial support. Guillaume Baudouin offered his expertise in making the map of Ephippiger diurnus populations, and Darren Rebar and several anonymous referees provided valuable criticisms of earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards
This study was funded by grant ANR-11-BSV7-025-01 from the Agence Nationale de la Recherche de France. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare that they have no conflicts of interest pertaining to the research reported in this manuscript.
Additional information
Communicated by D. Gwynne
Rights and permissions
About this article
Cite this article
Party, V., Streiff, R., Marin-Cudraz, T. et al. Group synchrony and alternation as an emergent property: elaborate chorus structure in a bushcricket is an incidental by-product of female preference for leading calls. Behav Ecol Sociobiol 69, 1957–1973 (2015). https://doi.org/10.1007/s00265-015-2008-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-2008-8