Abstract
Acoustic insects usually sing amidst conspecifics, thereby creating a social environment—the chorus—in which individuals communicate, find mates, and avoid predation. A temporal structure may arise in a chorus because of competitive and cooperative factors that favor certain signal interactions between neighbors. This temporal structure can generate significant acoustic interference among singers that pose problems for communication, mate finding, and predator detection. Acoustic insects can reduce interference by means of selective attention to only their nearest neighbors and by alternating calls with neighbors. Alternatively, they may synchronize, allowing them to preserve call rhythm and also to listen for predators during the silent intervals between calls. Moreover, males singing in choruses may benefit from reduced per capita predation risk as well as enhanced vigilance. They may also enjoy greater per capita attractiveness to females, particularly in the case of synchronous choruses. In many cases, however, the overall temporal structure of the chorus is only an emergent property of simple, pairwise interactions between neighbors. Nonetheless, the chorus that emerges can impose significant selection pressure on the singing of those individual males. Thus, feedback loops may occur and potentially influence traits at both individual and group levels in a chorus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Temporal interactions with conspecifics
Whether by accident or design, acoustic insects generally find themselves singing in the presence of conspecifics. In many cases, the signaling activity of acoustic neighbors elicits specialized temporal interactions between the calls of these individuals, phenomena that interest us as observers of natural history and also as students of neuro-ethology, behavior, and evolution. In this review, I describe the various factors that can lead acoustic insects to aggregate within earshot of one another, why they may then engage in certain signal interactions, what mechanisms may control these interactions, the acoustic environment that emerges from signal interactions within a population, and how this environment may ultimately influence the very insects who created it.
How and why groups form
In many, if not most, cases, the contiguity of singing males arises because of specific requirements for habitat or resources that are not evenly distributed in the landscape. This type of aggregation is ‘passive,’ but once in place it may nonetheless exert a strong influence on how neighbors behave (Greenfield 2005). For example, individuals singing in a passive aggregation may be under selection to adjust the timing of their calls such that the attraction of females to the group as a whole is improved or conspicuousness to phonotactic natural enemies is reduced. The potential effects of timing adjustments continue after females arrive at the group, as certain signal interactions may afford an individual male advantages in competition with his neighbors. Similarly, natural enemies who manage to locate a group of singing males may find it more difficult to localize some individual males because of the way they time their calls relative to neighbors.
But in other cases, individuals are mutually attracted to one another, behavior that may occur with or without critical resources or habitat being found in the vicinity. This type of aggregation is ‘active,’ and in theory it may reflect any of several fundamental selection pressures, e.g., thermoregulation; construction of burrows, webs, or other architecture; ability to find resources; and improved reproductive opportunities (Breed and Moore 2012). Because the calls of acoustic insects are generally male advertisements, it is most useful to consider the active aggregations of singing males as ‘leks,’ assemblages of sexually displaying males that females visit primarily for mating (Höglund and Alatalo 1995). Numerous explanations have been proposed to account for leks, including the hypotheses that these groups form where the home ranges of multiple females intersect (the hot-spot model; Bradbury and Gibson 1983), that they form near unusually attractive males (the hot-shot model; Beehler and Foster 1988), that males use information from conspecifics as a ‘short-cut’ to finding habitat at which they could display effectively (Muller 1998), that females prefer clusters of males per se (Kokko 1997), and that males can lower the risk of predation by displaying in a cluster (Wiley 1991). The last 2 hypotheses merit particular attention because they pertain specifically to the broadcast and perception of signals and to the signal interactions that may arise between males calling in a group. For example, females may be attracted to male groups because they are then afforded the opportunity to compare potential mates in a simultaneous, as opposed to sequential, manner (Alexander 1975; Bradbury 1981; Greenfield and Shaw 1983). This preference may be explained by simultaneous comparison being more accurate, and also economically advantageous in terms of time spent and distance traveled. Thus, selection pressure may influence males to aggregate regardless of their individual ‘quality.’ In terms of exposure to predation, both male signalers and the females who visit them may experience a lower per capita risk when signalers are grouped if the attraction of predators to groups does not increase commensurately with group size, an effect generally termed ‘dilution’ (Turchin and Kareiva 1989; e.g., Alem et al. 2011). Individuals in a group of signalers may also receive more warnings of approaching predators because of vigilance within the group (Gibson et al. 2002). In acoustic species, the vigilance effect may function in cases where males cease signaling upon detecting a nearby predator, and neighboring males—who did not directly detect the predator—follow suit: They respond to the silence response of the first male(s) with their own silence, and possibly other defensive behavior (Dapper et al. 2011).
Special features of the acoustic modality
Signal interactions between males advertising in a group may arise in any modality, but they are very well developed where the signals are acoustic. Sound broadcasts are particularly susceptible to neighbor–neighbor interactions because of several physical factors. First, sound spreads spherically from a monopole source, and even where the geometry of the sound radiator imparts some directionality to the broadcast, as is the case with most acoustic insects, the sound field is broad (Thiele and Bailey 1980; Greenfield 2002; cf. Fletcher 1992). Consequently, a female (or male) receiver is likely to be situated where the sound fields of two or more male signalers intersect. While binaural hearing may allow her to discriminate the amplitude of a male to the left from that of a male to the right, she will nonetheless remain within a combined sound field that is formed from the linear superposition of sound waves broadcast by the several males. And even when the males’ calls do not overlap in time to create this combined sound field, she may still be influenced by temporal relationships between their songs (Greenfield 1994a). These issues are less likely in olfaction and vision: Odor molecules generally spread from their sources by convection, and pheromone plumes drifting downwind in air or downstream in water from separated signalers may remain as distinct filaments over considerable distances (Liu and Haynes 1992; Baker et al. 1998). Similarly, animals that have image-forming eyes may experience little difficulty resolving the visual stimuli from 2 or more neighbors even when they are closely spaced.
Second, sound travels over relatively long distances despite being attenuated by obstacles in the natural environment, atmospheric absorption, and spherical spreading (Römer and Lewald 1992). Long-range transmission increases the likelihood that a given individual’s calls interact with those of multiple neighbors in the manner described above. The long-range aspect of sound communication may be contrasted with other mechanical signals such as substrate vibrations, which are very common among insects and other arthropods. Most vibration signals dampen markedly over short distances (Greenfield 2002), and their transmission is usually restricted to a plane (surface of the ground or water) or a linear configuration (stems or branches of vegetation; structure of a social insect nest) (Hill 2008). Unless a receiving individual is in the middle of a dense cluster of signalers transmitting vibrations and all rest on the same substrate, that receiver may seldom perceive the vibrations of more than one signaler (but see Eriksson et al. 2011; Virant-Doberlet et al. 2011 who indicate that vibration signals may travel between adjacent plants in some cases).
Third, acoustic signalers usually have the ability to control the timing of a call in a regular and highly precise manner. In insects, such control generally imparts elements of rhythm to a call, and these elements may experience relatively little degradation over distance unless the signal is transmitted through a complex environment, e.g., dense vegetation, and suffers heavily from reverberation (Richards and Wiley 1980; cf. Römer and Lewald 1992). Both females and neighboring males normally perceive the rhythm and other temporal features of a focal male’s call. In the case of males, their perception may allow them to effect precise signal interactions with neighbors’ calls. For example, individuals may increase call length or call rhythm in response to a neighbor, or they may align their rhythm with that of a neighbor by a particular phase angle. These interactions may be perceived and evaluated by the signalers themselves, and also by other males and females in the vicinity. Information from these interactions has the potential to influence the behavior of all receiving individuals. Interactions also occur in visual signals, notably in bioluminescent ones (Buck 1988), as well as in substrate vibration (Hunt and Morton 2001). They are largely absent in chemical signals except at the crudest level (Lim and Greenfield 2007). While some insects exhibit a rhythmic pulsing of pheromone release (Conner et al. 1980), atmospheric turbulence is expected to degrade the rhythmic features of the plume within a short distance such that the odor pulses coalesce into a single, continuous filament (cf. Dusenbery 1992).
What types of call interactions occur in acoustic insects?
Morning and evening choruses
The simplest, and perhaps most common, form of signal interaction in acoustic insects is the concentration of song at a particular time of the day or night (Walker 1983). In nocturnal insects, this concentration often occurs at the very beginning of the night, whereas in diurnal species the activity may peak several hours after dawn. These daily schedules of calling may be explained by a ‘gating’ phenomenon in which maturing females become newly receptive each day but would not move toward males until the appropriate hour of the photoperiod. For nocturnal species, this hour may occur precisely at dusk, and declining temperatures later at night lead to the concentration of activity during a relatively brief time interval while it is still warm. A similar phenomenon may regulate diurnal species except that temperatures at dawn may be too cold for activity, whereas temperatures usually remain favorable for many hours during the day. Thus, diurnal species typically begin calling at mid-morning and continue for several hours afterward (e.g., Greenfield 1992). Other factors that potentially constrain the daily singing schedules of acoustic insects include atmospheric conditions unfavorable to sound transmission at certain times and signal interference from other species (e.g., Greenfield 1988; Römer et al. 1989).
While a morning or evening chorus may simply arise from a shared response to photoperiodic cues without any direct interaction between singers, the occurrence of such mutual responses can greatly increase the precision of the chorus, largely by sharpening the activity peak. When a particularly ‘motivated’ singer initiates calling at the start of the daily advertisement period, neighbors may begin singing earlier than they might in isolation, and they may also continue singing for a longer duration and with more regularity than otherwise. Conceivably, these mutual responses may spread via a ‘chain-reaction effect’ from that motivated initiator to individuals who are quite distant: an insect fugue. Thus, waves of singing activity would arrive at most of the contiguous parts of a population and synchronize the onset of the chorus over a large area, even among individuals who would not be able to hear one another directly (Greenfield and Shaw 1983).
Presumably, the temporal clustering of song, whether by indirect responses to a common environmental cue or by direct interaction between neighbors, represents a form of signal competition: When one or more male neighbors are calling, a given male is under selection pressure to at least match his neighbors’ broadcasts if he is to achieve any mating success. This form of intra-sexual competition for mates has been designated a ‘spree,’ the temporal equivalent of a lek (Walker 1983).
Unison bout calling
The next level of signal interaction among acoustic insects would be the concentration of song during repeated ‘bouts’ of calling throughout the daily activity period (Greenfield 1983; Schwartz 1991; Jones et al. 2014). A bout may be initiated by a particular individual who is then joined by a majority of calling males in the local population. Calling typically continues for several minutes after which a silent interval—during which very few or no males sing—of varying length ensues. Thus, the dynamics of a calling bout could resemble a morning or evening chorus except that it is shorter and is not released by an environmental cue (e.g., photoperiodic or temperature trigger).
Why acoustic insects might call in repeated bouts rather than for a continuous period? This question has been posed for anurans, many of whom also exhibit repeated calling bouts during the evening. In anurans, three hypotheses have been considered: Females prefer males who call cyclically; males are inhibited from calling when the overall sound amplitude from the chorus becomes too high; and males are limited by energy (Schwartz et al. 1995). Several tests refuted the first two and supported the last one. It was proposed that by calling in numerous bouts over the entire daily activity period, a male retains the possibility of encountering females who arrive or become receptive at any time during that period while not exhausting himself early, which would occur were he to call continuously. Because acoustic insects who sing with a regular rhythm for an extended part of the day or night are likewise limited by energy (Reinhold et al. 1998; Ketola et al. 2009; Hartbauer et al. 2012), the same explanation may apply. But unison bout calling may also represent a fine-scale means of avoiding acoustic interference from heterospecific singers in the vicinity, a mechanism analogous to that described above for morning and evening choruses.
Modification of signal characters
More complex signal interactions entail the precise modification of song elements in response to neighbors’ calls. These modifications range from increasing the value of a particular signal character in the presence of singing neighbors to more elaborate alignment of rhythm phase with neighbors. This latter adjustment can impart an overall temporal structure to the chorus broadcast by a local population.
The usual way in which males modify their signal characters when singing in the presence of neighbors is by accelerating call rhythm (Jia et al. 2001; cf. Milner et al. 2012). This change may be an extension of the advancement of call initiation at the beginning of a morning or evening chorus and the more regular and longer duration of calling in these assemblages: A general increase in the level of sexual advertisement is stimulated by potential competitors for arriving females. The specific modification, acceleration of call rhythm, might occur for several reasons: First, in many acoustic insect species, the male call rhythm is not a fixed value—at a given temperature—that serves in species recognition but rather is subject to directional selection imposed by female choice (Greenfield 2002). Here, males who sing faster, within a certain range that extends well beyond the mean call rhythm in the population, are preferred. Thus, males accelerate their rhythm because they must at least match their neighbors in order to attract females in the vicinity. Second, among the various song parameters that might influence female preference, call rhythm can often be controlled. Rhythm generally reflects the stroke rate of appendages, legs, or wings in most cases and this rate may be increased for short periods. Carrier frequency and song amplitude, however, may reflect body structure and size, rendering them relatively fixed parameters that are less easily modified for signal competition with neighbors.
Several temporal song parameters other than call rhythm may also influence female preference, and singing males can modify some of these. In various acoustic insects, females prefer longer calls (Ritchie 1996; Greenfield 2002), and males can vary their call length by adding or subtracting call elements. Such modification, however, is not likely to occur independently of other song parameters such as rhythm (Greenfield and Minckley 1993). Owing to energy limitations, a male may be constrained to produce a certain number of leg or wing strokes per day or per period between feeding sessions. Consequently, if call length is increased, call rhythm is likely to decrease. Do males then favor maintaining one parameter over another? The outcome may depend on how females integrate and weigh the several temporal parameters in their overall evaluation of male song (Jang and Greenfield 1998, 2000; Trobe et al. 2011), and also on how males interact with their neighbors and mutually assess their calls.
In the above explanation for call acceleration in the presence of singing neighbors, it was implicitly assumed that calling males had some information on their relative call rhythms. In many cases, this information may not be available, and a male simply accelerates his rhythm to the extent possible and with the expectation that he may then match or exceed his neighbors’ rhythms (Jia et al. 2001; Lafaille et al. 2010). But in some cases, where a male interacts in pairwise fashion with a specific neighbor, a precise determination of relative rhythm is achieved via ‘call matching’: The two males alternate calls, possibly increasing their rhythms as the interaction progresses, until one can, or will, no longer match the other (Greenfield and Minckley 1993; cf. Gerhardt et al. 2000 on matching call length). The male who has dropped out of the interaction usually departs for another calling site, and a physical encounter—which would ensue should the two males be perfectly matched—is thereby avoided. Under such circumstances males would be expected to emphasize call rhythm over call length when modifying their signals. The following section presents additional factors, involving the way females perceive male signal interactions in a chorus that may also lead males to maintain their rhythm at the expense of other song parameters.
Phase alignment: mechanisms
In most acoustic insects, song originates from the repeated contraction of muscles that are otherwise involved in body movement, or that originally evolved in that context and has been co-opted for communication. Typically, these are muscles that directly or indirectly control the movement of legs or wings (Walker et al. 1970; von Helversen and Elsner 1977). The activity of these muscles appears to be controlled by a central pattern generator (Gerhardt and Huber 2002; cf. Schöneich and Hedwig 2011 for localization of the generator) that establishes a rhythmic movement of the appendages or other body parts, and, in turn, a rhythm with which song elements such as calls, chirps, phrases, syllables, or pulses are repeated. Thus, rhythm is rather ubiquitous in insect song, and the possibility of signal interactions between the rhythms of neighbors then arises. In most cases, these rhythm interactions are alignments at a particular phase angle.
Phase alignment falls into two broad categories, synchrony (e.g., Walker 1969), in which the phase angle between neighbors’ rhythms approximates 0°, and alternation, in which the phase angle approximates 180° (e.g., Shaw 1968). Both synchrony and alternation can only take place when the repetition rates of the interacting rhythms are similar, which may occur by chance or because one or more of the singers adjust their rates. Synchrony may involve a great many singers, whereas alternation in its pure form would be restricted to a single pair. However, choruses that include alternation among 3 or more singers can arise when n singers each maintain phase angles of 360°/n with a different neighbor (Fig. 1a), or when singer A alternates with singer B, who alternates with singer C, who then synchronizes with A (Fig. 1b). Choruses representing this latter situation can include more than 3 interacting singers (Greenfield and Snedden 2003), and they generally involve a form of selective attention among the males. In the example illustrated in Fig. 1b, A and C pay attention to and alternate with B, their nearest neighbor, while ignoring each other. On the other hand, B alternates with both A and C and is able to do so because these 2 neighbors synchronize by default as a result of their alternation with B.
a Singing by 5 male Tungará frogs (Physalaemus pustulosus) recorded at a small pond in Gamboa, Panama (redrawn from Greenfield 2005). Horizontal lines in each of the 5 traces indicate the timing of each male’s calls. b Hypothetical chorus of 3 males in which A and C each alternate with B, their closer neighbor. By default, the calls of A and C are approximately synchronous
How do neighboring singers achieve phase alignment of their rhythms? The mechanisms also appear to fall into 2 basic categories, although there are many variations of each. In many acoustic insect species, a focal singer will reset his rhythm upon hearing a single call of a neighbor and then immediately return to his previous, free-running rhythm following that momentary adjustment (Greenfield 1994a, b). Playback trials in which synthetic stimuli were broadcast suggest the operation of an ‘inhibitory-resetting mechanism’ in which a focal singer’s central rhythm generator is reset to its basal level by perception of a conspecific call and held at this level until the end of the stimulus (Fig. 2). The generator is then released from inhibition and ascends toward its excitatory level, at which point it triggers a call. Thus, a stimulus that arrives while the focal singer’s rhythm generator is ascending typically results in a delay of his subsequent call, whereas a stimulus arriving, while the generator is descending may result in an advance. The specific outcome will depend on the duration of the stimulus and the relative durations of the descending and ascending parts of the central rhythm generator. For example, where the descending part is relatively long, a short stimulus arriving immediately after the generator reached its excitatory level is likely to advance the singer’s call.
Mechanism in which the central pattern generator (oscillator) that regulates a male’s call rhythm is inhibited by a song stimulus or a neighbor’s call, reset to its basal level, and held there until the end of the stimulus. At that point the oscillator is released from inhibition and rebounds toward its peak level, at which the next call is triggered and is broadcast after a short delay (t). Thus, a stimulus or neighbor’s call (a) normally delays a focal male’s phase relative to the neighbor’s rhythm, but a stimulus (b) that appears just after the oscillator has reached its peak level may also advance the focal male’s phase, particularly if the stimulus is short and the descending part (r) of the oscillator is long. The rebound following release from inhibition may be more rapid than that found when the oscillator is free-running
Simulation modeling shows that when single, isolated playback stimuli are replaced by a neighboring male who sings with a call rhythm and an inhibitory-resetting mechanism similar to those of the focal singer, mutual phase alignments emerge (Greenfield et al. 1997). The nature of these alignments reflects several parameters, the most critical being the way in which a singer’s rhythm generator resumes following inhibition (Fig. 2). Where the generator rebounds quickly after inhibition by the neighbor, the 2 singers alternate calls. But where the generator rebounds with approximately the same slope exhibited during solo calling, the 2 singers may synchronize. Here, synchrony is only approximate, as one singer will normally lead his neighbor by a brief interval during each call cycle, the role of leader switching randomly over successive cycles. Moreover, a given singer may occasionally omit a call cycle and then re-enter the chorus, in approximate synchrony, in the next cycle. If the singers differ considerably in their free-running rhythms, the faster singer is predicted to assume the role of leader during most call cycles, while the slower one will skip calls during many cycles (e.g., Hartbauer et al. 2005).
Rhythm acceleration in groups of singing males can mitigate some of the potential effects resulting from differences in free-running rhythms between males, as slower singers are likely to approach the rhythms of the faster ones. Thus, some synchrony can be maintained even when free-running rhythms differ. However, call alternation is generally more precise than the synchrony that arises from inhibitory resetting. Call alternation is typically found in species with slower free-running rhythms (≤1 call s−1), while synchrony is the common alignment in species and populations with faster rhythms (>1 call s−1) (Greenfield 1994a). Phase alignments by inhibitory resetting are not observed for rhythms faster than 5 s−1, a limit that may reflect temporal constraints in chirping insects. For example, a minimum delay exists between the peak level of a singer’s central rhythm generator and the onset of the triggered call (Fig. 2).
In other acoustic insects, a singer does not appear to effect a major call delay or advance in response to each of a neighbor’s calls, but rather to modify the rate and phase of his rhythm such that it gradually aligns with the neighbor’s (Hanson 1978; Sismondo 1990; cf. Hartbauer et al. 2005; Nityananda and Balakrishnan 2007). This type of mutual phase alignment yields synchrony that is more precise than the synchrony arising from inhibitory-resetting mechanisms, although in some cases an individual that habitually leads his neighbor(s) by a brief delay is present (Hartbauer et al. 2005; Nityananda and Balakrishnan 2008). In general, a neighbor’s song influences the timing of a train of successive calls in a focal male’s song, not a single call cycle as in inhibitory resetting. Via small adjustments in phase and rhythm in each call cycle, slow singers accelerate and fast ones decelerate, and a single collective rhythm, with all singers aligned at a phase angle ≈ 0°, is achieved in the chorus. But critical playback experiments with synthetic stimuli are often lacking, and in many acoustic insects we cannot specify the details of the phase alignment mechanism(s) producing precise synchrony. Several species of fireflies also exhibit very precise collective synchrony, and similar mechanisms are proposed for phase alignment of the male flash rhythms (Strogatz and Mirollo 1990; Ermentrout 1991).
Phase alignment: evolution
Why do specific phase alignments occur in various acoustic insect species? This question is more difficult to answer than determining how alignment mechanisms work, and there are more hypotheses than thorough empirical tests. Explanations for alternation may be more straightforward than for synchrony, and they are generally based on the importance of reducing acoustic interference that the focal male, rival males in the vicinity, and attracted females might experience. It is assumed that a singer hears less clearly during the precise interval when his sound is broadcast because of either masking or physiological mechanisms that reduce acoustic sensitivity and thereby prevent injury to the singer’s ears or inappropriate self-directed behavior (Hedwig 1990; Greenfield and Minckley 1993; Poulet and Hedwig 2002; cf. Narins 1992). Thus, alternation ensures that a singer can clearly evaluate a neighboring singer’s calls and adjust his own singing and location if necessary, as described in the previous section under the rubric of call matching. Alternation would also ensure that the singing neighbor clearly perceives the focal singer and makes his own adjustments, which can benefit both males. Local females would also more clearly perceive the acoustic characteristics of calling males when they alternate, discrimination which could benefit the superior singer: Females would be less likely to mistake an inferior neighbor as having broadcast the more attractive song. Because both the attractive male and the attracted female would benefit from call alternation, sexual conflict is unlikely to arise and interrupt the selection favoring this chorusing trait.
Several explanations for call synchrony in acoustic insects also focus on acoustic interference. One hypothesis proposes that singers who align their rhythms in synchrony are less vulnerable to attack by certain natural enemies, phonotactic predators, and parasites who may have difficulty localizing any one singer once they enter the chorus: The broadcast of sound from all directions at the same instant might pose significant cognitive problems for acoustic localization (cf. Tuttle and Ryan 1982). Presumably, conspecific females do not suffer from this bewilderment; otherwise, the synchrony would be self-defeating. Another hypothesis focuses on the call features that females need to hear for species recognition and how these features may be masked if neighboring singers do not synchronize. This problem would arise in those cases where male singers broadcast a specific call rhythm, at a given temperature, and females rely on hearing this rhythm as the first step in recognizing conspecific males. Were males in a local chorus to sing without aligning their rhythms at a phase angle fairly close to 0°, females might not discern this critical song character and would not move toward the chorus or toward any one singer (Walker 1969). In other cases the simple nature of discontinuous calling may be the critical character that females must recognize, and where discrete calls, possibly with a particular call ‘envelope,’ clearly separated by inter-call intervals cannot be discerned, attraction would not occur. This situation is more likely to arise in species with high ‘duty cycles,’ as neighbors who alternate call rhythms would mask one another’s inter-call intervals, and females would hear nearly continuous sound of rather constant amplitude unless they are very close to one of the males. Some evidence for this effect occurs in the North American bushcricket Neoconocephalus nebrascensis (Greenfield and Schul 2008; cf. Moiseff and Copeland 2010 for an analogous situation in bioluminescent signaling).
Synchrony might also arise due to group-level competition for attracting females in populations, where calling males are dispersed in small, discrete aggregations (cf. Buck and Buck 1968; Buck 1988). By synchronizing call rhythms, group members can maximize the peak sound amplitude broadcast collectively by the aggregation (Nityananda and Balakrishnan 2009). Thus, they would be a more attractive ‘beacon’ than neighboring groups that do not align their rhythms in this way. Here, as well as in the hypotheses invoking acoustic interference in the previous paragraph, synchronizing males would be behaving cooperatively, and mechanisms that yield precise synchrony by adjusting both the repetition rate and phase of call rhythms may be favored by selection.
Understanding choruses whose temporal structure is only characterized by approximate synchrony can be more problematic than those structured by precise phase alignment. These weakly structured choruses may often arise from inhibitory-resetting mechanisms, which can yield either synchrony or alternation, depending on temporal features of the mechanism and the call rhythm. The variable nature of this outcome, combined with the imprecision of synchrony where it does occur, suggests that approximate synchrony is not a cooperative event that benefits those who produce it but rather a property that emerges from simpler interactions (Greenfield and Roizen 1993). This view is supported by tests of female perception and attraction in several acoustic insects, which indicate that psychoacoustic effects can favor certain temporal interactions among neighboring male callers that collectively yield group synchrony in some cases but alternation in others. Inhibitory-resetting mechanisms readily generate these favored interactions.
In various acoustic insects and other acoustic animals, perception of 2 or more spatially separated sounds that occur during a short-time interval is dominated by the leading sound (Wyttenbach and Hoy 1993; Minckley and Greenfield 1995; Greenfield et al. 1997). This phenomenon may occur even when the several sounds do not overlap, suggesting that these species are subject to psychoacoustic ‘precedence effects’ (Litovsky et al. 1999) rather than simple physical masking of the following sound(s) by the leading one. To date, tests with acoustic insects have not eliminated the potential role of neurophysiological ‘forward masking’ in behavioral responses to leading sounds. However, a recent anuran study (Marshall and Gerhardt 2010) has done so and confirmed that female receivers effectively fuse a leading and following sound into a single stimulus but localize the leading one, a stricter definition of precedence. In the context of chorusing males in acoustic insects, playback experiments show that females generally orient and move toward the leading caller and ignore the other(s) (Greenfield 1994b). Moreover, precedence may be more critical than signal characters based on acoustic energy, such as call length or call rhythm, in attracting females (Greenfield and Roizen 1993; Party et al. 2014; cf. Höbel 2010). Leading calls may also be more attractive than following calls perceived at higher amplitude until an SPL (sound pressure level) difference of 6–8 dB is reached (Snedden and Greenfield 1998; cf. Dyson and Passmore 1988). These perceptual features would impose strong selection pressure on males to avoid calling immediately after a neighbor and to increase the number of calls immediately before a neighbor (Fig. 3). When 2 or more males sing with comparable rhythms, adjustments to rhythm phase afforded by inhibitory-resetting mechanisms satisfy these demands on relative call timing: Simulations show that a male employing inhibitory resetting will produce more leading calls and fewer following ones than neighbors who do not make phase adjustments (Greenfield et al. 1997). That inhibitory resetting in males has co-evolved with precedence effects in females is indicated by the correlation among species, and among populations within highly variable species, between (1) the interval following onset of a neighbor’s call during which a focal male will not initiate his own call and (2) the maximum interval between 2 calls that elicit female preference for the leading one. That is, inhibitory resetting keeps males from singing during the precise intervals when their calls would be rather ineffective in their specific population.
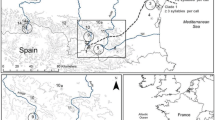
Coevolution between (1) precedence effect in female response to male song and (2) male phase adjustments to song rhythm in 6 populations (filled triangle) of Ephippiger diurnus (Orthoptera: Tettigoniidae) in southern France and northern Spain. Precedence effect index is the population mean of the maximum delay between the onset of a leading and following male call where females move toward the leader and ignore the follower. Phase adjustment index is the population mean of the minimum delay following a neighbor’s call, or a synthetic stimulus, before a focal male resumes calling. Spearman rank order correlation = 0.926 (p = 0.0167). Figure adapted from data in Brunel (2012)
According to the above information, a proportion of synchronous—and alternating—choruses are ‘epiphenomena’ that originate in psychoacoustic precedence effects (Greenfield et al. 1997). When expressed by females engaged in mate choice, these effects result in preferences for males who produce leading calls, which, in turn, select for certain mechanisms of phase adjustment, notably inhibitory resetting. Where several males using inhibitory resetting sing together, a structured chorus emerges, but the collective structure per se may be selectively neutral. Barring the possibility that alternation reduces acoustic interference or synchrony improves a group’s competitiveness, neither singing males nor attracted females may benefit from the overall chorus structure.
Studies of animal species that use reflected light and bioluminescent signals show that the precedence effect also occurs in these other modalities and can influence female preference (Backwell et al. 1998; Vencl and Carlson 1998). These findings suggest that the dominance of leading signals in perception may be a fundamental property of nervous systems (Römer et al. 2002; Siegert et al. 2011). Possibly, it forms part of a mechanism(s) that improves localization of the source of signals in certain species. It remains to be determined whether some group signaling events in modalities other than sound fit the scenario presented above.
The acoustic environment of a chorus
Whenever insects call in groups, they create an acoustic environment in which they sing and listen. This effect occurs regardless of the complexity of the chorus structure, and whether it is a cooperative phenomenon providing mutual benefits or is an epiphenomenon and selectively neutral. We now ask whether the acoustic environment of the chorus poses problems for the participants, those who sing and listen in it. How do the males who sing in the chorus and the females—and the males—who listen to and evaluate conspecific advertisement calls cope with what is potentially a significant amount of acoustic interference? Do certain cognitive limitations constrain their abilities to sing and listen? Are they forced to compromise different behavioral functions because of such limitations?
The balancing of mating and defensive behavior is perhaps the major problem that chorusing insects face because of acoustic interference. Theoretically, acoustic insects might detect the approach of natural enemies by hearing their signals, e.g., echolocation signals of insectivorous bats, or the sounds they make inadvertently during movement (Bradbury and Vehrencamp 2011). But whereas these telltale cues may be readily perceived in the absence of noise, the background sound level of a chorus can render such perception difficult if not impossible (Brunel-Pons et al. 2011), particularly where the cues of enemies occur in the same frequency band as the chorus, or frequency tuning in hearing of the chorusing insects is poor. This difficulty may be somewhat reduced in a synchronous chorus, as the general lull between calls could afford males and females the opportunity to hear predator cues and behave appropriately (cf. Faure and Hoy 2000 on the role of silent pauses during calling). Consequently, being able to maintain defensive behavior during singing, as well as during the evaluation of song, may be yet another factor selecting for synchrony of call rhythms.
In addition to the problem of background noise, the chorus environment might hinder anti-predator behavior because signal competition would have priority over defense, and cognitive limitations could reduce performance when several functions or tasks are attempted simultaneously (Dukas 2004; Dukas and Ratcliffe 2009). When males engage in signal competition with neighbors, which they often do within choruses, they may reduce attention to predators and be more prone to risk, even when acoustic interference does not exist and impair perception of predator cues. Similarly, general cognitive limitation may result in decreased attention to predators when individuals are busy evaluating singing neighbors. In general, few studies have addressed the problem of maintaining defensive behavior while singing and listening in a chorus, and we do not know the extent to which these potential difficulties offset the advantages in vigilance and dilution of predation risk that choruses may afford.
Even the very function of mate evaluation may suffer within the acoustic environment of a chorus. While choruses do allow females to make simultaneous comparisons of potential mates, a factor that may have favored the evolution of active aggregation of singing males, the concentration of an excessive number of singers in a small area could actually be counterproductive. It has been proposed that such ‘sensory overload’ may be responsible for limiting the size of lekking aggregations in animal species (Hutchinson 2005), and this limitation should be most applicable in choruses. However, a more common manifestation of the problem created by a multitude of neighboring singers may be masking of individual acoustic signals by chorus background noise, an effect that can severely limit the number of males a female can hear and evaluate (see Gerhardt and Klump 1988 on this issue in anurans).
Selective attention and feedback loops
A fundamental way of coping with acoustic interference and other cognitive problems in choruses is selective attention to only a proportion of the singing neighbors that an insect is physiologically capable of hearing (Minckley et al. 1995; Snedden et al. 1998; Greenfield and Snedden 2003; cf. Greenfield and Rand 2000). This attention is normally achieved by sensory adaptation to the background level of the chorus such that the focal individual only perceives its nearest and/or most intense neighbors (Pollack 1988; Römer and Krusch 2000; cf. Nityananda et al. 2007 for other considerations). Presumably, these would be a female’s most eligible mates without further searching, and a male’s most important rivals for local females. But in some cases additional rules might be applied to modify the set of attended neighbors; e.g., a fixed number algorithm by which a focal individual pays attention to only his 2 most intense neighbors, provided that their calls exceed the threshold intensity level set by sensory adaptation to the background chorus (cf. Greenfield and Rand 2000). Selective attention may be particularly critical in choruses generated by inhibitory-resetting mechanisms: In the absence of such attention, males would be repeatedly inhibited by their many neighbors and would seldom if ever call. This outcome may be less preferable than forgoing inhibitory resetting entirely and broadcasting a great many following calls.
While choruses of alternation and approximate synchrony generated by inhibitory resetting may only be epiphenomena, they nonetheless represent acoustic environments that can impose selection on the participants’ behavior (Greenfield 2005). For example, males may exhibit strategic spacing and signaling during time intervals when competition is low (Nityananda et al. 2007; Nityananda and Balakrishnan 2008). These possibilities illustrate how feedback loops may occur in which a group-level effect that arises as an emergent property can yet influence the individual-level behavior that produced it (Fig. 4). Such feedback loops might accelerate evolution of the collective chorus, but they also have the potential to temper its development.
Emergent properties and feedback loops in insect choruses. Central neural mechanisms found in both males and females influence localization of leading sounds. In species that produce rhythmic calls such ‘precedence effects’ select for male timing adjustments (inhibitory-resetting mechanisms) by which the incidence of relatively ineffective following calls is reduced. When all males in a local group apply these timing adjustments to their calling a temporally structured chorus emerges: synchrony emerges if the adjustment mechanism includes a slow rebound from inhibition; alternation emerges if the rebound is fast. Dashed arrows indicate potential feedback in which the emergent chorus influences aspects of individual behavior of the males who created the chorus and of the females who listen to and evaluate the chorusing males. Figure adapted from Greenfield (2005)
Overall prospects
At present, a substantial amount of information exists on the mechanisms controlling signal interactions in choruses of acoustic insects, although questions remain on the control of precise synchrony. On the other hand, the ecological and evolutionary factors leading acoustic insects to chorus in particular ways are mostly unresolved. More importantly, very few studies have considered the acoustic environment created by chorusing insects, how the behavior of individual males and females may have adapted to this milieu, and that these adaptations may have further influenced the chorus. Understanding these effects operating between individual and group levels will demand that the approaches traditionally used in insect hearing and communication, animal behavior, and comparative physiology, be supplemented by other methodologies from sensory and cognitive ecology.
References
Alem S, Koselj K, Siemers BM, Greenfield MD (2011) Bat predation and the evolution of leks in acoustic moths. Behav Ecol Sociobiol 65:2105–2116
Alexander RD (1975) Natural selection and specialized chorusing behavior in acoustical insects. In: Pimentel D (ed) Insects, science and society. Academic Press, New York, pp 35–77
Backwell P, Jennions M, Passmore N, Christy J (1998) Synchronized courtship in fiddler crabs. Nature 391:31–32
Baker TC, Fadamiro HY, Cosse AA (1998) Moth uses fine tuning for odour resolution. Nature 393:530
Beehler BM, Foster MS (1988) Hotshots, hotspots and female preference in the organization of lek mating systems. Am Nat 131:203–219
Bradbury JW (1981) The evolution of leks. In: Alexander RD, Tinkle DW (eds) Natural selection and social behavior. Chiron Press, New York, pp 138–169
Bradbury JW, Gibson RM (1983) Leks and mate choice. In: Bateson P (ed) Mate choice. Cambridge University Press, Cambridge, pp 109–138
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication, 2nd edn. Sinauer Associates, Sunderland, Massachusetts
Breed MD, Moore J (2012) Animal behavior. Academic Press, Burlington, Massachusetts
Brunel O (2012) De la communication acoustique au sein du groupe: contraintes et mécanismes. Ph.D. thesis, Université François Rabelais, Tours
Brunel-Pons O, Alem S, Greenfield MD (2011) The complex auditory scene at leks: balancing anti-predatory behaviour and competitive signalling in an acoustic moth. Anim Behav 81:231–239
Buck J (1988) Synchronous rhythmic flashing in fireflies. II. Q Rev Biol 63:265–289
Buck J, Buck E (1968) Mechanism of rhythmic synchronous flashing of fireflies. Science 159:1319–1327
Conner WE, Eisner T, Vander Meer RK, Guerrero A, Ghiringelli D, Meinwald J (1980) Sex attractant of an arctiid moth (Utetheisa ornatrix): a pulsed chemical signal. Behav Ecol Sociobiol 7:55–63
Dapper AL, Baugh AT, Ryan MJ (2011) The sounds of silence as an alarm cue in Tungara frogs, Physalaemus pustulosus. Biotropica 43:380–385
Dukas R (2004) Causes and consequences of limited attention. Brain Behav Evol 63:197–210
Dukas R, Ratcliffe JM (2009) Cognitive ecology II. University of Chicago Press, Chicago
Dusenbery DB (1992) Sensory ecology: how organisms acquire and respond to information. W.H. Freeman and Company, New York
Dyson ML, Passmore NI (1988) The combined effect of intensity and the temporal relationship of stimuli on phonotaxis in female painted reed frogs Hyperolius marmoratus. Anim Behav 36:1555–1556
Eriksson A, Anfora G, Lucchi A, Virant-Doberlet M, Mazzoni V (2011) Inter-plant vibrational communication in a leafhopper insect. PLoS ONE 6:e19692
Ermentrout GB (1991) An adaptive model for synchrony in the firefly Pteroptyx malaccae. J Math Biol 29:571–585
Faure PA, Hoy RR (2000) The sounds of silence: cessation of singing and song pausing are ultrasound-induced acoustic startle behaviors in the katydid Neoconocephalus ensiger (Orthoptera: Tettigoniidae). J Comp Physiol A 186:129–142
Fletcher NJC (1992) Acoustic systems in biology. Oxford University Press, Oxford
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans: common problems and diverse solutions. University of Chicago Press, Chicago
Gerhardt HC, Klump GM (1988) Masking of acoustic signals by the chorus background noise in the green tree frog: a limitation on mate choice. Anim Behav 36:1247–1249
Gerhardt HC, Roberts JD, Bee MA, Schwartz JJ (2000) Call matching in the quacking frog (Crinia georgiana). Behav Ecol Sociobiol 48:243–251
Gibson RM, Aspbury AS, McDaniel L (2002) Active formation of mixed-species grouse leks: a role for predation in lek evolution? Proc R Soc Lond B 269:2503–2508
Greenfield MD (1983) Unsynchronized chorusing in the coneheaded katydid Neoconocephalus affinis (Beauvois). Anim Behav 31:102–112
Greenfield MD (1988) Interspecific acoustic interactions among katydids, Neoconocephalus: inhibition-induced shifts in diel periodicity. Anim Behav 36:684–695
Greenfield MD (1992) The evening chorus of the desert clicker, Ligurotettix coquilletti (Orthoptera: Acrididae): mating investment with delayed returns. Ethology 91:265–278
Greenfield MD (1994a) Cooperation and conflict in the evolution of signal interactions. Annu Rev Ecol Syst 25:97–126
Greenfield MD (1994b) Synchronous and alternating choruses in insects and anurans: common mechanisms and diverse functions. Am Zool 34:605–615
Greenfield MD (2002) Signalers and receivers: mechanisms and evolution of arthropod communication. Oxford University Press, Oxford
Greenfield MD (2005) Mechanisms and evolution of communal sexual displays in arthropods and anurans. Adv Study Behav 35:1–62
Greenfield MD, Minckley RL (1993) Acoustic dueling in tarbush grasshoppers: settlement of territorial contests via alternation of reliable signals. Ethology 95:309–326
Greenfield MD, Rand AS (2000) Frogs have rules: selective attention algorithms regulate chorusing in Physalaemus pustulosus (Leptodactylidae). Ethology 106:331–347
Greenfield MD, Roizen I (1993) Katydid synchronous chorusing is an evolutionarily stable outcome of female choice. Nature 364:618–620
Greenfield MD, Schul J (2008) Mechanisms and evolution of synchronous chorusing: emergent properties and adaptive functions in Neoconocephalus katydids (Orthoptera: Tettigoniidae). J Comp Psychol 122:289–297
Greenfield MD, Shaw KC (1983) Adaptive significance of chorusing with special reference to the Orthoptera. In: Gwynne DT, Morris GK (eds) Orthopteran mating systems: sexual competition in a diverse group of insects. Westview Press, Boulder, Colorado, pp 1–27
Greenfield MD, Snedden WA (2003) Selective attention and the spatio-temporal structure of orthopteran choruses. Behaviour 140:1–26
Greenfield MD, Tourtellot MK, Snedden WA (1997) Precedence effects and the evolution of chorusing. Proc R Soc Lond B 264:1355–1361
Hanson FE (1978) Comparative studies of firefly pacemakers. Fed Proc 37:2158–2164
Hartbauer M, Kratzer S, Steiner K, Römer H (2005) Mechanisms for synchrony and alternation in song interactions of the bushcricket Mecopoda elongata (Tettigoniidae: Orthoptera). J Comp Physiol A 191:175–188
Hartbauer M, Stabentheiner A, Römer H (2012) Signalling plasticity and energy saving in a tropical bushcricket. J Comp Physiol A 198:203–217
Hedwig B (1990) Modulation of auditory responsiveness in stridulating grasshoppers. J Comp Physiol A 167:847–856
Hill PSM (2008) Vibrational communication in animals. Harvard University Press, Cambridge, Massachusetts
Höbel G (2010) Interaction between signal timing and signal feature preferences: causes and implications for sexual selection. Anim Behav 79:1257–1266
Höglund J, Alatalo RV (1995) Leks. Princeton University Press, Princeton
Hunt RE, Morton TL (2001) Regulation of chorusing in the vibrational communication system of the leafhopper Graminella nigrifrons. Am Zool 41:1222–1228
Hutchinson JMC (2005) Is more choice always desirable? Evidence and arguments from leks, food selection, and environmental enrichment. Biol Rev 80:73–92
Jang Y, Greenfield MD (1998) Absolute versus relative measurements of sexual selection: assessing the contributions of ultrasonic signal characters to mate attraction in lesser wax moths, Achroia grisella (Lepidoptera: Pyralidae). Evolution 52:1383–1393
Jang Y, Greenfield MD (2000) Quantitative genetics of female choice in an ultrasonic pyralid moth, Achroia grisella: variation and evolvability of preference along multiple dimensions of the male advertisement signal. Heredity 84:73–80
Jia F-Y, Greenfield MD, Collins RD (2001) Ultrasonic signal competition among male wax moths. J Insect Behav 14:19–33
Jones DL, Jones RL, Ratnam R (2014) Calling dynamics and call synchronization in a local group of unison bout callers. J Comp Physiol A 200:93–107
Ketola T, Kortet R, Kotiaho JS (2009) Endurance in exercise is associated with courtship call rate in decorated crickets, Gryllodes sigillatus. Evol Ecol Res 11:1131–1139
Kokko H (1997) The lekking game: can female choice explain aggregated male displays? J Theor Biol 187:57–64
Lafaille M, Bimbard G, Greenfield MD (2010) Risk trading in mating behavior: forgoing anti-predator responses reduces the likelihood of missing terminal mating opportunities. Behav Ecol Sociobiol 64:1485–1494
Lim H-K, Greenfield MD (2007) Female pheromonal chorusing in an arctiid moth (Utetheisa ornatrix). Behav Ecol 18:165–173
Litovsky RY, Colburn HS, Yost WA, Guzman SJ (1999) The precedence effect. J Acoust Soc Am 106:1633–1654
Liu YB, Haynes KF (1992) Filamentous nature of pheromone plumes protects integrity of signal from background chemical noise in cabbage looper moth, Trichoplusia ni. J Chem Ecol 18:299–307
Marshall VT, Gerhardt HC (2010) A precedence effect underlies preferences for calls with leading pulses in the grey treefrog, Hyla versicolor. Anim Behav 80:139–145
Milner RNC, Jennions MD, Backwell PRY (2012) Keeping up appearances: male fiddler crabs wave faster in a crowd. Biol Lett 8:176–178
Minckley RL, Greenfield MD (1995) Psychoacoustics of female phonotaxis and the evolution of male signal interactions in Orthoptera. Ethol Ecol Evol 7:235–243
Minckley RL, Greenfield MD, Tourtellot MK (1995) Chorus structure in tarbush grasshoppers: inhibition, selective phonoresponse, and signal competition. Anim Behav 50:579–594
Moiseff A, Copeland J (2010) Firefly synchrony: a behavioral strategy to minimize visual clutter. Science 329:181
Muller KL (1998) The role of conspecifics in habitat settlement in a territorial grasshopper. Anim Behav 56:479–485
Narins PM (1992) Reduction of tympanic membrane displacement during vocalization of the arboreal frog, Eleutherodactylus coqui. J Acoust Soc Am 91:3551–3557
Nityananda V, Balakrishnan R (2007) Synchrony during acoustic interactions in the bushcricket Mecopoda ‘chirper’ (Tettigoniidae: Orthoptera) is generated by a combination of chirp-by-chirp resetting and change in intrinsic chirp rate. J Comp Physiol A 193:51–65
Nityananda V, Balakrishnan R (2008) Leaders and followers in katydid choruses in the field: call intensity, spacing and consistency. Anim Behav 76:723–735
Nityananda V, Balakrishnan R (2009) Modeling the role of competition and cooperation in the evolution of katydid acoustic synchrony. Behav Ecol 20:484–489
Nityananda V, Stradner J, Balakrishnan R, Römer H (2007) Selective attention in a synchronising bushcricket: physiology, behaviour and ecology. J Comp Physiol A 193:983–991
Party V, Brunel-Pons O, Greenfield MD (2014) Priority of precedence: receiver psychology, female preference for leading calls and sexual selection in insect choruses. Anim Behav 87:175–185
Pollack GS (1988) Selective attention in an insect auditory neuron. J Neurosci 8:2635–2639
Poulet JFA, Hedwig B (2002) A corollary discharge maintains auditory sensitivity during sound production. Nature 418:872–876
Reinhold K, Greenfield MD, Jang Y, Broce A (1998) Energetic cost of sexual attractiveness: ultrasonic advertisement in waxmoths. Anim Behav 55:905–913
Richards DG, Wiley RH (1980) Reverberations and amplitude fluctuations in the propagation of sound in a forest: implications for animal communication. Am Nat 115:381–399
Ritchie MG (1996) The shape of female mating preferences. Proc Nat Acad Sci USA 93:14628–14631
Römer H, Krusch M (2000) A gain-control mechanism for processing of chorus sounds in the afferent auditory pathway of the bushcricket Tettigonia viridissima (Orthoptera: Tettigoniidae). J Comp Physiol A 186:181–191
Römer H, Lewald J (1992) High-frequency sound transmission in natural habitats—implications for the evolution of insect acoustic communication. Behav Ecol Sociobiol 29:437–444
Römer H, Bailey WJ, Dadour IR (1989) Insect hearing in the field. 3. Masking by noise. J Comp Physiol A 164:609–620
Römer H, Hedwig B, Ott SR (2002) Contralateral inhibition as a sensory bias: the neural basis for a female preference in a synchronously calling bushcricket, Mecopoda elongate. Eur J Neurosci 15:1655–1662
Schöneich S, Hedwig B (2011) Neural basis of singing in crickets: central pattern generation in abdominal ganglia. Naturwissenschaften 98:1069–1073
Schwartz JJ (1991) Why stop calling? A study of unison bout singing in a neotropical treefrog. Anim Behav 42:565–578
Schwartz JJ, Ressel SJ, Bevier CR (1995) Carbohydrate and calling. Depletion of muscle glycogen and the chorusing dynamics of the neotropical treefrog Hyla microcephala. Behav Ecol Sociobiol 37:125–135
Shaw KC (1968) An analysis of the phonoresponse of males of the true katydid, Pterophylla camellifolia (Fabricius) (Orthoptera: Tettigoniidae). Behaviour 31:203–260
Siegert ME, Römer H, Hashim R, Hartbauer M (2011) Neuronal correlates of a preference for leading signals in the synchronizing bushcricket Mecopoda elongata (Orthoptera, Tettigoniidae). J Exp Biol 214:3924–3934
Sismondo E (1990) Synchronous, alternating, and phase-locked stridulation by a tropical katydid. Science 249:55–58
Snedden WA, Greenfield MD (1998) Females prefer leading males: relative call timing and sexual selection in katydid choruses. Anim Behav 56:1091–1098
Snedden WA, Greenfield MD, Jang Y (1998) Mechanisms of selective attention in grasshopper choruses: who listens to whom? Behav Ecol Sociobiol 43:59–66
Strogatz SH, Mirollo RE (1990) Synchronization of pulse-coupled biological oscillators. SIAM J Appl Math 50:1645–1662
Thiele D, Bailey WJ (1980) The function of sound in male spacing behaviour in bush-crickets (Tettigoniidae, Orthoptera). Austral J Ecol 5:275–286
Trobe D, Schuster R, Römer H (2011) Fast and reliable decisions for a dynamic song parameter in field crickets. J Comp Physiol A 197:131–135
Turchin P, Kareiva P (1989) Aggregation in Aphis varians: an effective strategy for reducing predation risk. Ecology 70:1008–1016
Tuttle MD, Ryan MJ (1982) The role of synchronized calling, ambient light, and ambient noise in the anti-bat-predator behavior of a treefrog. Behav Ecol Sociobiol 11:125–131
Vencl FV, Carlson AD (1998) Proximate mechanisms of sexual selection in the firefly Photinus pyralis (Coleoptera: Lampyridae). J Insect Behav 11:191–207
Virant-Doberlet M, King RA, Polajnar J, Symondson WOC (2011) Molecular diagnostics reveal spiders that exploit prey vibrational signals used in sexual communication. Mol Ecol 20:2204–2216
von Helversen O, Elsner N (1977) Stridulatory movements of acridid grasshoppers recorded with an opto-electronic device. J Comp Physiol A 122:53–64
Walker TJ (1969) Acoustic synchrony: two mechanisms in the snowy tree cricket. Science 166:891–894
Walker TJ (1983) Diel patterns of calling in nocturnal Orthoptera. In: Gwynne DT, Morris GK (eds) Orthopteran mating systems: sexual competition in a diverse group of insects. Westview Press, Boulder, Colorado, pp 45–72
Walker TJ, Brandt JF, Dew D (1970) Sound-synchronized, ultra-high speed photography: a method for studying stridulation in crickets and katydids (Orthoptera). Ann Entomol Soc Am 63:910–912
Wiley RH (1991) Lekking in birds and mammals: behavioral and evolutionary issues. Adv Study Behav 20:201–291
Wyttenbach RA, Hoy RR (1993) Demonstration of the precedence effect in an insect. J Acoust Soc Am 94:777–784
Acknowledgments
I thank the National Science Foundation (U.S.A.) and the Agence National de la Recherche (France; contrat EVOLCHOR; ANR-11-BSV7-025-01) for funding my studies of insect acoustic communication which led to many of the syntheses expressed in this review. Critiques by Carl Gerhardt and several anonymous referees improved accuracy of the manuscript’s final version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greenfield, M.D. Signal interactions and interference in insect choruses: singing and listening in the social environment. J Comp Physiol A 201, 143–154 (2015). https://doi.org/10.1007/s00359-014-0938-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-014-0938-7