Abstract
When acoustically advertising animals call in dense clusters, problems in signal efficacy often arise. These problems are particularly acute in species where females ignore males who call immediately following a neighbor and males adjust call timing to avoid broadcasting following calls: Males may forego such adjustments and produce many ineffective calls, they may attend to all neighbors and call at a reduced rate, or they may selectively attend to certain neighbors, likely those who are nearby and/or more intense. We studied the problem of group calling in Ephippiger diurnus, a European bush cricket distributed in genetically isolated populations that vary considerably in male song and chorusing and in female preference for male song. Female E. diurnus ignore following male calls, and males adjust their call timing but only with respect to several loud neighbors. We found that males were more selective in attending to only their nearest neighbor in a population where chorusing yields sound during a high proportion of a collective singing bout, and more indiscriminate in attending to several neighbors where chorusing yields more intermittent sound. Such fine tuning can maintain a relatively high calling rate and may be generated by positive feedback loops operating between individual and group-level calling traits.
Significance statement
When animals sing in the company of conspecifics, individual singers often adjust their call timing such that they do not immediately follow neighbors. These adjustments become problematic in dense choruses, as adjusting for all neighbors could lead to a marked reduction in call rate. Consequently, some degree of selective attention, most likely to nearby and/or loud neighbors, is expected. We confirmed this expectation in the chorusing bush cricket E. diurnus, but we also found that the degree of attention varied among populations. In particular, singers were most selective, attending to only a single neighbor, where chorusing generated rather continuous sound and more indiscriminate attention would have led to sporadic calling. Thus, choruses appear to be finely tuned and controlled by feedback loops in which individual singers generate a collective display that, in turn, influences the singing behavior of those very same individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acoustically advertising animals often call within earshot of conspecific individuals, a situation that is particularly evident in species that broadcast loud songs and/or are found in relatively high density. Whether group calling arises either passively owing to narrow habitat requirements or actively because of the advantages of aggregation per se, members of the group may have to adjust their calling to accommodate or compete with neighbors (Greenfield 2015). In some cases, these adjustments involve changes in carrier frequency bands (Ulanovsky et al. 2004) or increases in intensity (Amichai et al. 2015), the so-called Lombard effect (Zollinger and Brumm 2011), but the typical adjustment is temporal (Greenfield 2005). Animals that broadcast discrete units of song may avoid overlapping a neighbor(s) or they may tend to do so, and individuals that sing with a given call repetition rate may adjust the call rhythm such that a particular phase angle or time delay is maintained with a neighbor’s rhythm (Greenfield 1994a). In some species, individuals adjust their free-running call rhythm (e.g., Sismondo 1990; Hartbauer et al. 2005; Nityananda and Balakrishnan 2007; Murphy et al. 2016), whereas in other species an individual delays or advances each call and thereby attains that particular phase angle (Greenfield 1994b).
The temporal adjustments that individuals make can generate an alternation or synchrony with neighbors, a collective timing that can sometimes improve the effectiveness of communication to conspecifics within or outside the group (Walker 1969; Greenfield and Schul 2008; cf. Moiseff and Copeland 2010). These adjustments can also serve as a means by which an individual avoids singing during a specific time interval relative to a neighbor’s call or, conversely, increases its singing during a relative time interval. For example, in diverse acoustic species, females ignore male calls that follow a neighbor’s calls by a brief delay (Dyson and Passmore 1988; Greenfield and Roizen 1993; Minckley and Greenfield 1995; Höbel 2010), a variant of the precedence effects known in psychoacoustics (Zurek 1987; Litovsky et al. 1999). Under such circumstances, temporal adjustments by which males reduce following calls may coevolve with the female perceptual trait (Greenfield et al. 2016), and males that produce a higher number of leading calls may attract more females (Party et al. 2014). A common way in which acoustic animals accomplish these adjustments is a type of phase-delay mechanism termed “inhibitory-resetting”: A focal individual’s central rhythm generator is reset to basal level upon perceiving a neighbor’s call, it remains inhibited at basal level until the end of that call, and it then rebounds and triggers the focal individual’s next call. Thus, the adjustment affects one and only one call cycle, which is normally longer than a cycle during free-running calling (Greenfield et al. 1997; cf. Hanson et al. 1971).
Inhibitory resetting mechanisms as described above are a prominent feature in various species of acoustic insects and anurans (Greenfield 1994b). The mechanisms function in a rather straightforward manner within small groups, pairs, and triads of singers, but as density and the number of neighbors increase, a dilemma arises: If a singer adjusts his calling in response to all audible neighbors, he risks calling at a much slower rate, but if he employs inhibitory resetting little or not at all, he will produce a great many ineffective following calls (Greenfield and Rand 2000). Short of moving outside of a high-density cluster (Nityananda et al. 2007), a potential solution to the dilemma is to be selective in attending to certain male neighbors while ignoring others, notably those who are more distant and/or weaker singers (Minckley et al. 1995; Snedden et al. 1998). By this method, the singer maintains a relatively high call rate while focusing attention on those males expected to be his strongest rivals for local females. This method is not only a cogent solution to the problem of group calling but is also consistent with general neuro-ethological principles: Animals often adapt to their sensory environment by raising or lowering their threshold level for physiological, and ultimately behavioral, response (e.g., Pollack 1988). In the context of the chorus produced by a local group of callers, a focal male is expected to respond to a high level of background noise coming from loud, nearby neighbors by raising his threshold and consequently ignoring the more distant neighbors (cf. Römer and Krusch 2000). More importantly, empirical studies on various acoustic insects and anurans report that males exhibit selective attention as predicted and that the attention may entail a sliding response threshold adjusted in accordance with the intensity of nearest neighbors (Greenfield and Rand 2000; Greenfield and Snedden 2003).
In most cases, the temporally structured chorus that a group of calling males generates is a “self-organized system” (cf. Camazine et al. 2001): There is no central control, and the structure emerges simply from the summation of multiple neighbor-neighbor interactions (Greenfield and Schul 2008). Moreover, females may exhibit no particular preference for the collective structure—synchrony, alternation, or some combination of the two formats—and the very males who created the chorus may not obtain any advantage from its overall timing structure (Greenfield et al. 2016). Nonetheless, the chorus represents the acoustic environment in which males sing and in which both males and females listen to conspecifics as well as to other environmental sounds (Greenfield 2015). Thus, the chorus that emerges from a group of calling males has the potential to influence the way in which individual males sing and interact with neighbors. Does chorus format impose selection pressure on how males adjust call rhythm and pay attention to their nearest neighbors? In turn, do such changes in male calling further influence the chorus format via feedback loops? And are changes in male calling due to the chorus environment that is experienced “real time” behavioral responses, evolutionary responses, or both?
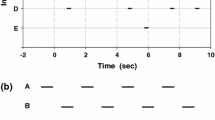
We addressed these above questions in the European bush cricket Ephippiger diurnus (Orthoptera: Tettigoniidae: Bradyporinae), a species in which males sing in small, local groups and females move toward singing males, ignoring those males whose songs follow their neighbors by a brief interval (Greenfield et al. 2004). Males adjust their call rhythms relative to their neighbors with an inhibitory resetting mechanism, and an elaborate chorus comprising both alternation and synchrony emerges from the inter-neighbor interactions (Fig. 1; Party et al. 2015). Recent experiments show that a male’s production of leading calls is a better predictor of his attractiveness to females than either his call length or rhythm (Party et al. 2014) and that the inhibitory resetting mechanism by which males can increase their incidence of leading calls has coevolved with female preference for such calls (Greenfield et al. 2016). However, females exhibit no preference for the overall chorusing format broadcast collectively by local males (Party et al. 2015). Recordings of a natural population indicate that males apply inhibitory resetting selectively to their nearest neighbor(s) (Greenfield and Snedden 2003). We note that female preference for song timing and male adjustments pertain to entire calls. There is no evidence of preferences and adjustments regarding within-call elements (syllables; Fig. 1a).
Songs of E. diurnus. a Oscillograms showing temporal features of male calls in the three populations studied, Vilamòs (two-syllable call), Col de Chioula (two-syllable call), and Peyriac de Mer (five-syllable call). b Chorusing by four males from the Peyriac de Mer population. Four-channel oscillogram shows a 60-s sample of singing by the males in a laboratory arena
Largely due to their flightlessness, E. diurnus are distributed in genetically distinct, geographically isolated populations throughout much of their range (Spooner and Ritchie 2006; Party et al. 2015). The various populations differ phenotypically in their developmental biology, morphology, male song, chorus format, and female preference for male song (Duijm 1990; Barbosa et al. 2016b). Because of this inter-population variation, E. diurnus was an ideal species with which to test the proposition that chorus format influences how individual males sing within a group. We measured the level of selective attention to neighbors during inhibitory resetting interactions in various populations and then compared this level with several parameters of chorus format and structure. We report a marked correspondence between the selective attention exhibited by individual males and the “collective duty cycle” of the choruses in which they normally sing.
Materials and methods
We studied males from three different E. diurnus populations in southern France and northeastern Spain chosen to include the two major clades of the species as well as geographic, song, and chorus variation (Table 1, Fig. 1a). In each population, we tested adults that were reared from eggs laid in the laboratory by a previous, field-collected population. Nymphs and adults were kept individually in transparent, plastic cages (20 cm height, 10 cm diameter) in an environmental room maintained at 25 ± 1 °C and under a 16:8 L/D photoperiod. We fed the insects cabbage, fish flakes, and pollen, ad libitum, and misted them daily. Adult males sang regularly in the cages, and screen mesh in the cage lids allowed each individual to hear the chorus produced collectively by all of the insects in the room. This feature was critical for behavioral development, as E. diurnus males sing rather little when in acoustic isolation. But at the same time, the plastic cages attenuated the songs of neighbors such that the males were not unduly influenced by the numerous insects within the environmental room. E. diurnus males begin singing approximately 10 days after the adult molt (Barbosa et al. 2016a). We recorded and tested males aged 15–57 days to ensure that they had fully matured. All recordings and playback tests were conducted in a room exposed to natural sunlight (temperature range at location of insects 25–32 °C). We restricted recordings and tests to 9:30–14:00 h, the natural activity period in all E. diurnus populations.
To design and analyze the playback tests with which we characterized selective attention in each population, we first recorded and evaluated patterns of chorusing in E. diurnus males. For the three populations, we chose four to seven groups of four singing males, using different individuals in each group, and held them in individual screen cages (10 cm height, 10 m diameter) placed 50 cm distant from one another within a 1-m diameter arena, all surrounded by acoustic insulation foam (cf. Party et al. 2015). A condenser microphone (model CM16/CMPA; Avisoft Bioacoustics, Berlin, Germany; frequency response ±3 dB, 10–150 kHz) suspended above each male recorded his calling; a tube of acoustic insulation placed around the microphone reduced crosstalk from neighboring males. Microphone outputs of a given chorus were sent to a multi-channel recorder (TASCAM DR-680, Teac Corporation), digitized, and saved as a four-channel sound file for later analysis with acoustic signal processing software (Audacity version 2.1.0, https://sourceforge.net/projects/audacity/files/audacity/2.1.0/; Seewave version 2.0.2, http://rug.mnhn.fr/seewave).
We recorded 3–10 min of chorusing from each four-male group and focused on a 60-s time interval when all four males called regularly. For each male, we sampled all consecutive calls during the interval and measured the delay between the onset of the last call by a neighbor and the onset of the focal male’s call. If several neighbors had overlapped calls prior to the focal male, we considered those several overlapping calls as one sound unit, as each neighbor’s call had approximately the same perceived amplitude. These delays were then organized in a “call delay histogram” for the male, and we interpreted the average of the three minimum call delays as the approximate interval during which he remained inhibited from initiating his next call within the chorus following a neighbor’s call (Fig. 2; cf. Greenfield et al. 1997). Within each population, we determined the average call delay (d) as the mean of the inhibition intervals calculated from the sampled males (Table 1). A comparative, phylogenetic study of E. diurnus populations showed that d is approximately equal to the maximum separation between two calls for which a female will ignore the second, following one (Greenfield et al. 2016).
Determination of motor delay (x) and call delay (d). a Call delay histogram for a representative male in the Peyriac de Mer population, showing the frequency of call onsets within delay bins measured from the onset of a song stimulus (0 ms). Several calls begin immediately after a stimulus is broadcast, and an interval with few calls then ensues, but the majority of calls do not begin until 950 ms after the stimulus onset. b Transformation of data in a to a call delay rank curve. The portion of the curve between points 1 and 2 corresponds with the interval of the call delay histogram during which few calls were initiated, and it is defined as that region of the curve where the slope is ≥3 times that found for lower or higher ranks. The motor delay is defined as the mean of the three longest delays to the left of point 1 (x = 159 ms); the call delay is defined as the mean of the three shortest delays to the right of point 2 (d = 984 ms)
To evaluate the acoustic environment within the choruses further, we measured the “duty cycle” that a focal male would typically hear during 60-s intervals of regular calling by all four males (cf. Fig. 1b). For each focal male, we summed all of the time intervals during which song was broadcast by at least one of his three neighbors and divided this total by 60 s. Thus, the chorus duty cycle reflected the call length and rhythm of the individual males as well as how they timed their calls relative to one another. As in determining d above, within each population we calculated the mean chorus duty cycle by averaging the values measured among the sampled males (Table 1).
We determined the level of selective attention in the three E. diurnus populations with a series of playback experiments. A focal male was held in a screen cage (30 cm height) surrounded by four speakers 50 cm distant, all of which were enclosed within a barrier of acoustic insulation foam. Each speaker repeatedly broadcast a standard male call from the respective population (see Party et al. 2014, 2015), and we adjusted the timing of the four speakers such that successive calls were broadcast to the focal male after an interval slightly shorter than d milliseconds (d-x ms; see experiment 1 below and Fig. 2 for definition and determination of x). Thus, the male never experienced an interval of silence as long as or longer than his minimum call delay, forcing him to either not sing or ignore one or more of the speakers and sing following its broadcast (cf. Greenfield and Rand 2000). For example, in Fig. 3, the test male (lowest trace) twice sang following the 78-dB speaker (uppermost trace); i.e., his call began from x to d ms after the onset of the speaker. Otherwise, he refrained from singing. A centrally located condenser microphone recorded the focal male’s calls, and a second condenser microphone situated above the arena recorded the broadcasts of the four speakers as well as the focal male’s calling.
Timing of song stimuli in four-speaker playback trials in experiment 1. Thick horizontal bars indicate stimuli broadcast by the 78-, 82-, 86-, and 90-dB speakers, which are organized in blocks of four broadcasts that are re-randomized without replacement within each successive block. The interval between successive broadcasts is d-x, where d and x are the call delay and motor delay, respectively, in the population (see text). Double-headed arrows indicate the interval, d-x in length, for which a male’s call onset is considered to “follow” a given speaker broadcast. Thus, both call 1 and call 2 by the test male follow the 78-dB speaker, represented by yellow bars
Experiment 1
We calibrated the amplitudes of the four speakers to 90, 86, 82, and 78 dB sound pressure level (SPL) (cf. Party et al. 2014 for calibration protocol) as perceived by the focal male in the center. The 90-dB broadcast represented a typical male neighbor 1 m distant; the lower amplitudes represented neighbors correspondingly farther away. We timed these song stimuli according to a pseudorandom sequence in which the four speakers (amplitudes) each broadcast in a block 4∙(d-x) milliseconds in length that was repeated for 3 min, with the order of broadcasts within each successive block being re-randomized without replacement (Fig. 3). This sequence precluded the possibility that a focal male could anticipate which speaker would broadcast next and then adjust his call rhythm appropriately. We tested 18–20 males in each population with trials approximately 9 min in length, and we analyzed the timing of the male’s initial 30 calls. For each of these 30 calls, we noted which speaker he ignored (= “followed”), following defined as a call beginning during the interval starting x ms after the onset of the speaker broadcast and continuing until d ms have elapsed (Table 1; Fig. 3, which shows that the test male twice followed the 78-dB speaker). Here, x is a brief “motor delay” that accounts for the insect’s inability to inhibit a call that had already been triggered by the central rhythm generator (cf. Buck et al. 1981a, 1981b), and its length is determined from the call delay histograms (Fig. 2) in the population. Thus, calls that began less than x ms after a speaker onset did not indicate that the male ignored the broadcast but rather that he followed (ignored) the previous broadcast by the speaker. For example, the second call by the test male in Fig. 3 followed the 78-dB speaker, not the 86-dB one.
We had an a priori expectation that a male would attend to the loudest, 90-dB speaker and produce relatively few calls following its broadcasts. Assuming a binomial distribution with n = 30 (number of calls sampled), p = 0.25 (random probability that a given call follows the 90-dB speaker), and k = the number of successes (calls following the 90-dB speaker), we determined the one-tailed cumulative distribution function (CDF) for k = 0. . . 30. These calculations indicated that 3 was the greatest integer k for which the CDF was <0.05, and we therefore designated males who followed the 90-dB speaker with three or fewer calls as paying attention to it. Using the same approach, we determined whether a male attended to the two loudest speakers (86 and 90 dB): Assuming n = 30 and p = 0.50, we calculated that 10 was the greatest integer k for which the CDF was <0.05. To be conservative, we only designated males who followed both the 86- and 90-dB speakers with five (= 10/2) and three or fewer calls, respectively, as paying attention to them. Similarly we determined whether a male attended to the three loudest speakers (82, 86, and 90 dB): Assuming n = 30 and p = 0.75, we calculated that 17 was the greatest k for which the CDF was <0.05. Conservatively, we only designated males who followed the three speakers with five (≈ 17/3), five, and three or fewer calls, respectively, as paying attention to them. Conceivably, a male might attend to a low-amplitude speaker (e.g., 82 dB) without attending to one or more higher ones (e.g., 86 and/or 90 dB). However, this complication was very rare, occurring only once, and we ignored it in our designations of selective attention.
Experiment 2
To determine whether an absence of calls following one or more loud speakers in experiment 1 reflected attention to an absolute amplitude level or attention that was set relative to the loudest stimulus, we repeated the protocol but used loudspeakers broadcasting 82, 78, 74, and 70 dB SPL. A similar pattern of attention to one or more loud speakers in experiment 2 would be interpreted as adaptation relative to the loudest stimulus present in the environment. In each of the three populations, we used five of the males tested in experiment 1 for these trials, allowing a minimum of 24 h between the experiments.
Experiment 3
To determine whether attention to certain speakers in experiments 1 and 2 reflected, in part, a rule by which a male selected a fixed number of stimuli or neighbors to attend, we conducted a third experiment in which all four speakers broadcast the same intermediate amplitude, 82 dB. Here, attention to certain of the speakers might also indicate that arbitrary factors, e.g., a stimulus to the left, play a role. As above, in each population we used five of the males tested in experiments 1 and 2, allowing a minimum of 24 h between the experiments. The same protocol as in experiments 1 and 2 was used, but in analysis the expected probability that a male attended to any given speaker was 4x the CDF. Thus, 2 was the greatest integer k for which the probability that a male attended to a speaker was <0.05.
Analysis of the focal male’s behavior in all experiments was blinded in that we first used information transcribed from the two recording microphones to specify only his call timing relative to the overall sequence of speaker broadcasts presented during the entire trial without noting the identity, i.e., amplitude level, of the individual speakers. We then consulted the pseudorandom sequence of speaker broadcasts and ascertained the speaker that each call had followed.
We predicted that males would attend to relatively few stimuli, possibly only the loudest one, in populations with a higher chorus duty cycle. In this acoustic environment, a male would potentially be inhibited by neighbors during a significant percentage of time, and attention directed toward several neighbors could seriously reduce his singing. On the other hand, where chorus duty cycle is lower, a male might attend to his loudest neighbor plus several others and still maintain a high singing rate.
Results
Experiment 1
We found selective attention to the louder speakers in all three populations tested. Between 70 and 94 % of the males attended to one or more speakers: Males from Peyriac de Mer attended to a mean of 1.00 speakers (mode = 1), those from Col de Chioula attended to a mean of 1.63 speakers (mode = 2), and those from Vilamòs attended to a mean of 2.06 speakers (mode = 2) (Fig. 4). These levels of attention were correlated with both the mean number of syllables per call broadcast by males in the respective population (r 2 adj = 0.23; t reg. Coeff. = −4.28 ; p < 0.01, n = 57) and the chorus duty cycle (Fig. 4; r 2 adj = 0.25; t reg. Coeff. = −4.45; p < 0.01) that we measured in our laboratory recordings. We then recalculated these two regressions after omitting those males who did not pay attention to any of the four speakers, and we found similar results (r 2 adj = 0.24, t reg. Coeff. = −3.97, p < 0.01, n = 48; r 2 adj = 0.27, t reg. Coeff. = −4.36, p < 0.01).
Number of speakers attended to by test males vs. chorus duty cycle in experiment 1. For the three populations, each distinguished by a different chorus duty cycle, data show the number of speakers that a given test male avoided “following” (see Fig. 3 for illustration of following and text for statistical treatment). 0: attention to no speaker and following (ignoring) of all; 1: attention to the 90-dB speaker and following of the 78-, 82-, and 86-dB speakers; 2: attention to the 90- and 86-dB speakers; 3: attention to the 90-, 86-, and 82-dB speakers. Symbols are slightly dispersed vertically to reveal all of the males that attended to a specific number of speakers. Least-squares linear regression line for attention vs. chorus duty cycle is shown (y = 4.76–6.87x; r 2 adj = 0.25 ; t reg. Coeff. = −4.45 ; p < 0.01 )
Experiment 2
Between 40 % (Vilamòs) and 80 % (Peyriac de Mer) of the males attended to one or more of the louder speakers in the three populations. The failure of every one of the males who attended to the loudest speaker in this experiment, 82 dB, to have also attended to 82 dB in experiment 1 is best interpreted as sensory adaptation to the louder, 86 and 90 dB, speakers in experiment 1. That is, the 82-dB speaker was below threshold level for most males in experiment 1, as their “sliding” range of sensitivity extended only s dB below the loudest, 90-dB speaker. Based on the numbers of speakers attended to in experiment 1 (Fig. 4), s was 3.5, 5.1, and 6.6 dB in the Peyriac de Mer, Col de Chioula, and Vilamòs population, respectively (s = amplitude difference between the loudest stimulus and the weakest one that elicited attention). In calculating s, we omitted males that did not pay attention to any speaker, as we could not distinguish a very high threshold from an absence of selectivity in their inhibitory resetting mechanism. Additionally, we assumed that males paying attention to only the 90-dB speaker had a threshold of 88 dB SPL, those paying attention to the 86- and 90-dB speakers had a threshold of 84 dB, and those paying attention to the 82-, 86-, and 90-dB speakers had a threshold of 80 dB. Unlike experiment 1, we did not find differences in levels of attention among the three populations in experiment 2, a result that probably reflects the small samples of males tested.
Experiment 3
Only one male, an individual in the Vilamòs population, attended to one of the speakers, all four of which broadcast at 82 dB SPL. No other male showed significant attention to any of the broadcasting speakers.
Discussion
As predicted, and as seen in a preliminary, earlier study (Greenfield and Snedden 2003), E. diurnus males cope with the dilemma of group singing by selectively attending to neighbors. The current study confirms that attention is regulated by a sliding threshold with which a focal male adjusts his singing in relation to his nearest, loudest neighbor and other nearby neighbors whose song amplitude, as perceived by the focal male, is within s dB of that loudest neighbor. Experiment 3 showed that attention is probably not regulated further by a fixed number rule in which a focal male adjusts his song with respect to a certain number of neighbors: When all speakers broadcast the same amplitude, a value that generally elicited attention in experiment 2, the test males apportioned their attention more or less evenly among the stimuli. Similarly, there was no evidence that arbitrary rules, e.g., adjustments with respect to a neighbor on the left, or on the right, governed attention.
The three populations that we tested differed considerably in their mean level of attention, as measured by the depth of the sliding threshold s. We found that attention was strongly correlated with both the mean number of syllables per call in a population (cf. Table 1) as well as the chorus duty cycle. While call syllable number reflects call length and may also reflect the duty cycle of an individual male’s song, we note that E. diurnus are reluctant to call when alone. Rather, call duty cycle is a more realistic index of the acoustic environment that a male would normally be exposed to, and it reflects the opportunity that a male using inhibitory resetting to control his rhythm would have to sing. Thus, finding that attention was most restricted in the population (Peyriac de Mer) where call duty cycle was highest conforms to our prediction: Males need to be more selective in their responses to neighbors where indiscriminate attention would seriously interfere with singing.
The strong correlation observed between call duty cycle and the selectivity of attention to neighbors may represent an evolved response genetically fixed in a population, a plastic response to the immediate acoustic environment, or both. Some evidence from our experiments suggests that at least some component of the response is genetically fixed. All males in our experiments had been kept in an environmental room where they heard a mixed chorus of several E. diurnus populations prior to testing. Moreover, in the playback trials they were all tested with a chorus of speaker broadcasts that required them to ignore some broadcasts and attend to others if they were to sing. Despite these similar experiences and test conditions—a “common garden” approach—the various populations exhibited marked differences in attention. Nonetheless, plastic responses occurring in real time are possible as well (Rebar et al. 2016). Natural populations in the field would be habitually exposed to a certain chorus format, characterized by a chorus duty cycle, itself generated by call duration, call rhythm, and inter-male temporal interactions, and males may be expected to modify attention accordingly: Be restrictive to the nearest neighbor only where duty cycle is high, but more indiscriminate where it is lower. Our experimental design, however, does not allow us to discern this second, potential component.
We have characterized chorus structure in E. diurnus as largely representing an emergent property that simply arises from the summation of temporal interactions between male neighbors. But our findings reported here indicate that these temporal interactions are influenced by the collective chorus that they themselves create (Fig. 5; cf. Greenfield and Schul 2008; Greenfield 2015). We now ask whether these modified temporal interactions—highly selective vs. more indiscriminate attention to nearest neighbors—may further influence the chorus generated by individual males. For example, highly selective attention, as seen in populations with a higher chorus duty cycle, is expected to yield a more pronounced chorus structure in which a male alternates with his nearest neighbor but, by default, synchronizes with his neighbor’s neighbor(s) (Fig. 1b; Party et al. 2015). This temporal structure may yet increase the chorus duty cycle that local males perceive. Analogously, attention to several neighbors, as seen where chorus duty cycle is low, may reduce that duty cycle even further. These potential scenarios demonstrate how positive feedback loops can arise and drive both individual and group behavior toward more exaggerated levels via “runaway processes.” Overall, they demonstrate the intricate nature of interactions possible within a collective behavioral event and how such elaborations might originate in rather simple behavior exhibited by individuals.
Evolution of chorusing and feedback loops that amplify expression of individual and group-level traits within choruses. 1 Female preference for calls that happen to lead those of a neighbor selects for inhibitory resetting mechanism in males, which generally includes some level of selective attention to nearby, loud neighbors. 2 A temporally structured chorus that includes alternation and/or synchrony of calling by neighboring males emerges. Call length, call rhythm, and the amount of synchrony vs. alternation between neighbors influence the percentage of time during which sound is broadcast by the group, a parameter termed “chorus duty cycle.” 3a When chorus duty cycle is high, attention to only the nearest neighbor is favored, which in turn sustains a higher duty cycle in the chorus. 3b When chorus duty cycle is low, attention to several nearby neighbors is favored, which in turn sustains a lower duty cycle in the chorus
References
Amichai E, Blumrosen G, Yovel Y (2015) Calling louder and longer: how bats use biosonar under severe acoustic interference from other bats. Proc R Soc Lond B 282:20152064. doi:10.1098/rspb.2015.2064
Barbosa F, Rebar D, Greenfield MD (2016a) Reproduction and immunity trade-offs constrain mating signals and nuptial gift size in a bushcricket. Behav Ecol 27:109–117
Barbosa F, Rebar D, Greenfield MD (2016b) Female preference functions drive inter-population divergence in male signaling: call diversity in the bushcricket Ephippiger diurnus. J Evol Biol (in press)
Buck J, Buck E, Case JF, Hanson FE (1981a) Control of flashing in fireflies. V. Pacemaker synchronization in Pteroptyx cribellata. J Comp Physiol A 144:287–298
Buck J, Buck E, Hanson FE, Case JF, Mets L, Atta GJ (1981b) Control of flashing in fireflies. IV. Free run pacemaking in synchronic Pteroptyx. J Comp Physiol A 144:277–286
Camazine S, Deneubourg J-L, Franks N, Sneyd J, Theraulaz G, Bonabeau E (2001) Self-organization in biological systems. Princeton University Press, Princeton, p. 560
Duijm M (1990) On some song characteristics in Ephippiger (Orthoptera, Tettigonioidea) and their geographic variation. Neth J Zool 40:428–453
Dyson ML, Passmore NI (1988) Two-choice phonotaxis in Hyperolius marmoratus (Anura: Hyperoliidae): the effect of temporal variation in presented stimuli. Anim Behav 36:648–652
Greenfield MD (1994a) Cooperation and conflict in the evolution of signal interactions. Annu Rev Ecol Syst 25:97–126
Greenfield MD (1994b) Synchronous and alternating choruses in insects and anurans: common mechanisms and diverse functions. Am Zool 34:605–615
Greenfield MD (2005) Mechanisms and evolution of communal sexual displays in arthropods and anurans. Adv Study Behav 35:1–61
Greenfield MD (2015) Signal interactions and interference in insect choruses: singing and listening in the social environment. J Comp Physiol A 201:143–154 Special issue: Insect hearing: From physics to ecology
Greenfield MD, Rand AS (2000) Frogs have rules: selective attention algorithms regulate chorusing in Physalaemus pustulosus (Leptodactylidae). Ethology 106:331–347
Greenfield MD, Roizen I (1993) Katydid synchronous chorusing is an evolutionarily stable outcome of female choice. Nature 364:618–620
Greenfield MD, Schul J (2008) Mechanisms and evolution of synchronous chorusing: emergent properties and adaptive functions in Neoconocephalus katydids (Orthoptera: Tettigoniidae). J Comp Psychol 122:289–297
Greenfield MD, Snedden WA (2003) Selective attention and the spatio-temporal structure of orthopteran choruses. Behaviour 140:1–26
Greenfield MD, Esquer-Garrigos Y, Streiff R, Party V (2016) Animal choruses emerge from receiver psychology. Sci Rep, in review.
Greenfield MD, Siegfreid E, Snedden WA (2004) Variation and repeatability of female choice in a chorusing katydid, Ephippiger ephippiger: an experimental exploration of the precedence effect. Ethology 110:287–299
Greenfield MD, Tourtellot MK, Snedden WA (1997) Precedence effects and the evolution of chorusing. Proc R Soc Lond B 264:1355–1361
Hanson FE, Case JF, Buck E, Buck J (1971) Synchrony and flash entrainment in a New Guinea firefly. Science 174:161–164
Hartbauer M, Kratzer S, Steiner K, Römer H (2005) Mechanisms for synchrony and alternation in song interactions of the bushcricket Mecopoda elongata (Tettigoniidae: Orthoptera). J Comp Physiol A 191:175–188
Höbel G (2010) Interaction between signal timing and signal feature preferences: causes and implications for sexual selection. Anim Behav 79:1257–1266
Litovsky RY, Colburn HS, Yost WA, Guzman SJ (1999) The precedence effect. J Acoust Soc Am 106:1633–1654
Minckley RL, Greenfield MD (1995) Psychoacoustics of female phonotaxis and the evolution of male signal interactions in Orthoptera. Ethol Ecol Evol 7:235–243
Minckley RL, Greenfield MD, Tourtellot MK (1995) Chorus structure in tarbush grasshoppers: inhibition, selective phonoresponse, and signal competition. Anim Behav 50:579–594
Moiseff A, Copeland J (2010) Firefly synchrony: a behavioral strategy to minimize visual clutter. Science 329:181
Murphy MA, Thompson NL, Schul J (2016) Keeping up with the neighbor: a novel mechanism of call synchrony in Neoconocephalus ensiger katydids. J Comp Physiol A 202:225–234
Nityananda V, Balakrishnan R (2007) Synchrony during acoustic interactions in the bushcricket Mecopoda ’chirper’ (Tettigoniidae: Orthoptera) is generated by a combination of chirp-by-chirp resetting and change in intrinsic chirp rate. J Comp Physiol A 193:51–65
Nityananda V, Stradner J, Balakrishnan R, Römer H (2007) Selective attention in a synchronising bushcricket: physiology, behaviour and ecology. J Comp Physiol A 193:983–991
Party V, Brunel-Pons O, Greenfield MD (2014) Priority of precedence: receiver psychology, female preference for leading calls and sexual selection in insect choruses. Anim Behav 87:175–185
Party V, Streiff R, Marin-Cudraz T, Greenfield MD (2015) Group synchrony and alternation as an emergent property: elaborate chorus structure in a bushcricket is an incidental by-product of female preference for leading calls. Behav Ecol Sociobiol 69:1957–1973
Pollack GS (1988) Selective attention in an insect auditory neuron. J Neurosci 8:2635–2639
Rebar D, Barbosa F, Greenfield MD (2016) Acoustic experience influences male and female pre- and postcopulatory behaviors in a bushcricket. Behav Ecol 27:434–443
Römer H, Krusch M (2000) A gain-control mechanism for processing of chorus sounds in the afferent auditory pathway of the bushcricket Tettigonia viridissima (Orthoptera; Tettigoniidae). J Comp Physiol A 186:181–191
Sismondo E (1990) Synchronous, alternating, and phase-locked stridulation by a tropical katydid. Science 249:55–58
Snedden WA, Greenfield MD, Jang Y (1998) Mechanisms of selective attention in grasshopper choruses: who listens to whom? Behav Ecol Sociobiol 43:59–66
Spooner LJ, Ritchie MG (2006) An unusual phylogeography in the bushcricket Ephippiger ephippiger from southern France. Heredity 97:398–408
Ulanovsky N, Fenton MB, Tsoar A, Korine C (2004) Dynamics of jamming avoidance in echolocating bats. Proc R Soc Lond B 271:1467–1475
Walker TJ (1969) Acoustic synchrony: two mechanisms in the snowy tree cricket. Science 166:891–894
Zollinger SA, Brumm H (2011) The Lombard effect. Curr Biol 21:R614–R615
Zurek PM (1987) The precedence effect. In: Yost WA, Gourevitch G (eds) Directional hearing. Springer-Verlag, New York, pp. 85–105
Acknowledgments
We thank Guy Bourdais, Séverine Devers, Yann Emmelin, Alicia Jarrige, Virginie Party, and Justine Penin for technical assistance in the laboratory, and the Agence Nationale de la Recherche de France (contrat ANR-11-BSV7-025-01; EVOLCHOR), the Centre National de la Recherche Scientifique (CNRS), and the Université François Rabelais de Tours for their financial support. Virginie Party, Darren Rebar, and several referees provided valuable criticisms of earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was funded by grant ANR-11-BSV7-025-01 from the Agence Nationale de la Recherche de France. The authors declare that they have no conflicts of interest pertaining to the research reported in this manuscript. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Communicated by S. Sakaluk
Rights and permissions
About this article
Cite this article
Marin-Cudraz, T., Greenfield, M.D. Finely tuned choruses: bush crickets adjust attention to neighboring singers in relation to the acoustic environment they create. Behav Ecol Sociobiol 70, 1581–1589 (2016). https://doi.org/10.1007/s00265-016-2166-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2166-3