Abstract
Ecological reasons for philopatry and cooperation are frequently invoked when kin selection is an insufficient explanation. The Ethiopian wolf (Canis simensis) is a specialised rodent hunter that forms family groups with cooperative breeding but also lives as monogamous pairs in suboptimal areas. Given the apparent absence of fitness gains to helpers from cooperative breeding, we set out to explore the benefits accrued by communal territorial defence measured as the acquisition and retention of habitats with more and most preferred rodent prey. Pairs defended relatively large territories to encompass critical amounts of key habitats within a matrix poor in rodents. Groups in optimal areas had relatively small territories and were expansionist, such that wolves in larger packs benefited per capita from increased good-quality foraging habitat. The fitness benefits of philopatry became evident after a rabies epizootic, when philopatry and expansionism prevailed in under-saturated conditions, until large groups split or provided dispersers that established locally. This study shows that high concentrations of prey can shift the balance of costs and benefits towards group living and cooperation in long-lived territorial carnivores, in so far as this dictates immediate rewards accrued from a given increment in territory size, namely greater foraging area per animal, leading to group enlargement and eventual inheritance of breeding space.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natal philopatry, the tendency for young to delay dispersal past their reproductive age, is thought to be at the root of sociality and cooperation in many species of birds and mammals. An overarching explanation for the evolution of philopatry is that the reproductive costs of group living are counterbalanced by fitness benefits of sociality (Alexander 1974), including indirect gains for helpers from the enhanced survival or reproduction of their kin (Hamilton 1964). This inclusive fitness theory have helped to understand philopatry and cooperation in many evolutionary scenarios (e.g. Clutton-Brock 2002), but not when inclusive fitness gains, or other more direct gains, are not apparent or restricted to interactions among kin. These cases can be better understood in the light of explanations such as the ecological constraints hypothesis (Emlen 1982), the habitat saturation hypothesis (Koenig and Pitelka 1981) and the benefits of philopatry hypothesis (Stacey and Ligon 1991), which stress ecological constraints on independent breeding, and the life time fitness gains of ‘staying at home’.
A diversity of evolutionary scenarios are now recognised as possible routes to philopatry and cooperation, involving direct and indirect fitness benefits, as well as immediate and long term (Koenig et al. 1992; Jennions and Macdonald 1994; Emlen 1995; Pen and Weissing 2000; Solomon 2003; Bach et al. 2006; Doerr and Doerr 2006; Lion and Gandon 2009; Sparkman et al. 2011). In the case of territorial species (e.g. Gaston 1978; Lindstroem 1986; Komdeur 1992), useful models include the territory inheritance hypothesis (Woolfenden and Fitzpatrick 1978) and the resource dispersion hypothesis (Macdonald 1983; Johnson et al. 2002). Long-lived territorial carnivores in particular offer opportunities to evaluate the costs and benefits of philopatry and cooperation as these are dictated by the abundance and distribution of food. The Ethiopian wolf Canis simiensis is particularly informative. This Afroalpine habitat specialist is a solitary rodent hunter that lives as monogamous pairs in less-productive areas, but forms familial groups in optimal habitat. All the members of the pack defend a common territory and care for the pups of the dominant pair (Sillero-Zubiri and Gottelli 1995a, b; Sillero-Zubiri et al. 1996b, 2004), but the benefits of cooperative breeding are not apparent. As neither survival nor reproduction increase with the number of helpers, maintaining and inheriting a high-quality range has been considered the major advantage of sociality in Ethiopian wolves. The wolves’ expansionist strategy (sensu Kruuk and Macdonald 1985), which results in larger groups defending larger territories, would be favoured by the abundance and predictability of Afroalpine rodents, which are also highly restricted in space (Sillero-Zubiri et al. 1995a, b, 1996b, 2004; Sillero-Zubiri and Macdonald 1998), but the precise relationship between group living and acquisition of food resources is yet to be explored.
To find out why Ethiopian wolves are social when they can also live in pairs, we set out to explore ecological benefits of philopatry and cooperation considering: (a) the pattern of distribution of prey, (b) the direct benefits derived from expansionism—the combination of group augmentation with territorial expansion, and (c) the longer-term benefits of philopatry and cooperation, namely:
-
(a)
The notion that the dispersion and abundance of resources, commonly food, can favour the evolution of group territoriality (Waser 1981; Macdonald 1983; Wrangham 1993) is supported by mathematical expressions of the resource dispersion hypothesis (Creel and Macdonald 1995; Carr and Macdonald 1986; Johnson et al. 2002) and by empirical evidence from species of social carnivores that are non-cooperative, or that do not evidently benefit from grouping (e.g. Eurasian badgers Meles meles (Kruuk 1978), brown hyaenas Hyaena brunnea (Mills 1982), Blanford's foxes Vulpes cana (Geffen and Macdonald 1992)), increasingly expanding to other mammals (e.g. striped mice Rhabdomys pumilio (Schradin and Pillay 2005), Gunnison's prairie dogs Cynomys gunnisoni (Verdolin 2009; Verdolin and Slobodchikoff 2009)). A corollary of this is that the dispersion of food imposes constraints upon group size and territory size independently, so that territory size can be affected by the distribution of food, whereas group size is determined by the quality of the food in a territory. If philopatry in Ethiopian wolves is resource based, we expect some fundamental difference in the availability of rodents between areas where they live in pairs and those were they form groups, which in turn should affect the cost–benefit ratio of group living.
-
(b)
Regarding the direct benefits of cooperation, theoretically cooperation can emerge from the benefits of group augmentation alone (Kokko et al. 2001) and the numerical advantages of sociality are often implicit in studies of territorial species (Stacey and Ligon 1991; Lazaro-Perea 2001; Campbell et al. 2005). These include access to higher-quality territories (Woolfenden and Fitzpatrick 1984; Kauffman et al. 2007), reduced costs due to shared territory defence (Clifton 1990) and winning inter-group contests (Carlson 1986; Adams 1990; Wilson and Wrangham 2003). Ethiopian wolves share the costs of territorial defence and larger groups fare better at territorial contests (Sillero-Zubiri and Macdonald 1998). If these benefits were dependent upon the acquisition of a portion of good-quality habitat, we would also expect that access to resources will increase with the addition of each new individual (Kokko et al. 2001).
-
(c)
The benefits of philopatry underlie cooperative behaviours in many birds and mammals with cooperative breeding and group territoriality, and whose offspring remain on the natal territory and become helpers (Gaston 1978; Emlen 1984; Koenig et al. 1992). When competition for space is high, a possible strategy for non-breeders is to remain at home and help, until opportunities emerge to inherit the space necessary for breeding (Woolfenden and Fitzpatrick 1984; Stacey and Ligon 1991; Stacey and Taper 1992). For example, we expect subordinate Ethiopian wolves to attempt independent reproduction locally, in response to the relaxation of ecological constraints brought about by the extinction of neighbouring packs.
Empirical tests of these hypotheses require measurements of critical resources at the relevant spatial and temporal scales, and knowledge of the structure of social groups. Considering that the abundance of giant mole rats (Tachyoryctes macrocephalus), the wolves’ preferred prey, and that of diurnal Murinae rats, both closely correlate with Ethiopian wolf abundance in the Bale Mountains (Sillero-Zubiri et al. 1995a, b), we propose that the distribution of habitats with different abundances of these prey types will reflect patterns of prey availability within territories. To measure access to key resources by monogamous pairs and expansionist groups, we mapped prey abundance in detail and associated this with the space use of packs. The data were collected from 17 wolf packs in the Bale Mountains of southern Ethiopia between 1987 and 2000. During this period, two local populations in optimal habitat crashed due to a rabies epizootic in 1990 and 1992, and subsequently recovered (Sillero-Zubiri and Gottelli 1995a, 1996a; Marino et al. 2006). By encompassing natural variations in density and pack structure, this study embraces a range of potential responses of wolves to prey availability and distribution, including decisions with fitness consequences in the longer term.
Methods
Study populations
In the Bale Mountains of southern Ethiopia (7° S, 39° 45′ E), optimal areas for Ethiopian wolves are found in the Web Valley at 3,500 m above sea level and the Sanetti Plateau at 3,800–4,050 m. Suboptimal areas are found in the rain shadow of Tullu Deemtu peak, on the southern declivity of the Sanetti plateau (Sillero-Zubiri et al. 1995a, b). The optimal habitat is dominated by short Afroalpine grasslands and meadows that sustain high rodent biomass and 1.2 adult wolves per square kilometre during high-density population phases. The less productive habitats are dominated by Helichrysum heaths with a fifth of the rodent prey biomass and an adult wolf density ca. 0.25/km2. The 17 packs studied over 16 years (totalling 64 pack/years) were distributed as follow: three neighbouring packs in ca. 40 km2 in Tullu Deemtu between 1989 and 1991; three to six neighbouring packs in ca. 35 km2 in Web Valley and two to three packs in ca. 20 km2 in Sanetti between 1988 and 2000 (Table 1). The monitoring period included a high density phase and a recovery phase after the rabies epizootics, each spanning 5 years in Web (1987–1991, 1996–2000) and three in Sanetti (1987–1989, 1998–2000) (Table 1)—the epizootic year was excluded from analyses (Marino et al. 2006). The monitoring gap in 1992–1995 was a period of political unrest and likely long enough for local populations to stabilise after the effects of massive mortality. During the high density phase, all habitat supporting a substantial rodent biomass was occupied by resident packs in the study areas, with competition for space placing a tight constraint on dispersal (Sillero-Zubiri and Gottelli 1995a). Males did not disperse whereas females tended to leave the natal pack, becoming floaters or facing long-distance dispersal and possibly death (Sillero-Zubiri et al. 1996b). At high density, packs in optimal areas had on average six wolves older than 1 year and a sex ratio close to two males per female. In suboptimal habitat (Tullu Deemtu), the predominant social unit was the breeding pair and the adult sex ratio close to one (Sillero-Zubiri and Gottelli 1995a). During the epizootics, 77 and 54 % of all the wolves studied in Web and Sanetti, respectively, died or disappeared over a short period of time; three of six packs went extinct in Web and one in Sanetti (Sillero-Zubiri et al. 1996a). The packs that survived the epizootics persisted until intensive monitoring restarted in 1997 (data from opportunistic visits in April 1995, March–August 1996, October–November 1996; C. Sillero-Zubiri and Edriss Ebu, unpublished data).
Measuring food resources
The relationships between rodent abundance and the structure and composition of the Afroalpine vegetation are well-known from empirical studies in the Bale Mountains, particularly for the diurnal species that are the most important prey for wolves, and which they also have access to all year round (Sillero-Zubiri and Gottelli 1995b; Sillero-Zubiri et al. 1995a). Our expectation is that the distribution of habitats with giant mole rats will be paramount for the configuration of territories and because wolf abundance also correlates with the abundance of diurnal Murinae rats (Sillero-Zubiri et al. 1995a, b), habitats in which these rats are abundant should also be important.
The Electronic supplementary material 1 (Mapping Ethiopian wolf habitats in the Bale Mountains) describes in detail the steps involved in the development of the habitat maps. These were: (a) identification of seven vegetation classes or vegetation–soil–rock complexes from cluster analyses of percentage cover data in 179 point samples of 5-m radius; (b) identification of three habitat categories based on their prey value, from comparisons of Murinae rat and Rhizomyinae mole rat burrow hole counts in habitat samples, using one-way ANOVA and post hoc Tukey tests (log-transformed data) and Kruskal–Wallis tests, respectively; (c) mapping the distribution of habitat quality types within the study, from a supervised classification of a Landsat image with habitat samples as training sites (IDRISI software, Clark Labs, Clark University, Worcester, MA, USA), validated by a satisfactory Kappa index; and (d) assessing accessibility to food by groups of different sizes by overlaying the territory of each pack in each year over the habitat map and calculating the area of each habitat quality type contained within them (RANGES software).
The habitat quality categories identified are:
-
Habitat quality 1 (HQ1) = vegetation classes with high rat and giant mole rat abundances: Alchemilla meadows, rocky grasslands and bogs; found in flat areas, valleys, and waterlogged depressions.
-
Habitat quality 2 (HQ2) = vegetation classes with high rat abundance and few giant mole rats: open Helichrysum heaths and short grasslands; found in ridges and hills with moderate slopes, and some flat areas with thin soils.
-
Habitat quality 3 (HQ3) = vegetation classes with few rats and no giant mole rats: Helichrysum heaths on the rain shadow of Tullu Deemtu and hill slopes, and Artemesia grasslands in hills and rocky ridges.
-
Others = a combination of sedge swamps and Alchemilla haumanii heaths, both markedly different from all other vegetation types and rare (represented by only a few sites in the sample, n = 4 and n = 8, respectively). The sedge swamps with Carex spp. occupy permanently flooded depressions with no value for wolves in terms of prey availability (Sillero-Zubiri et al. 1995a); A. haumanii heaths occupy a restricted area in the Sanetti Plateau.
The final habitat categories corresponded closely with well-known associations between vegetation, micro-topography and rodent abundance in Bale (Sillero-Zubiri and Gottelli 1995a, b; Sillero-Zubiri et al. 1995a, b; C. Sillero-Zubiri, unpublished field maps). Previous studies have validated the usefulness of rodent signs as a proxy for rodent abundance by contrasting them against rat live trapping and head counts of giant mole rats (Sillero-Zubiri et al. 1995a, b). Because the abundance and distribution of Murinae rats and giant mole rats correlate closely with wolf density in Bale, we assume that the distribution of these habitat types will reflect patterns of prey availability within territories.
Pack observations
Observational studies of Ethiopian wolves provide accurate information on the size and composition of social groups and on their reproductive success (Sillero-Zubiri and Gottelli 1995a; Sillero-Zubiri et al. 1996b). In Bale, Ethiopian wolves are diurnal and largely indifferent to people, thus family groups have been closely monitored from 20 to 500 m on foot or horseback, using binoculars. Social groups and their territories are typically stable and observations of large and complete groups are common, particularly during early morning and evening communal greetings, pack territorial patrols, and during the breeding season when the activity concentrates around the breeding den. Some individuals were recognised by unique coat patterns and with assistance of ear tags during the high density period (Sillero-Zubiri and Gottelli 1995a; Sillero-Zubiri et al. 1996b). Three age categories were visually identified: pups (<1 year old; since first observed emerging from the den at about 3 weeks of age), sub-adults (1 to <2 years old), and adults (≥2 years old, when they reach sexually maturity). The sex of adults and sub-adults and their dominance status was determined visually and from behavioural cues. In order to attain complete pack enumerations, data were recorded on the age and sex composition of each group sighted, until no new age/sex combinations were observed and the pack membership list was complete. The time window to define the size, composition, and territory of packs was October to March, which corresponds with the reproductive period in Bale—i.e. parturition through to pup independence (Sillero-Zubiri et al. 1998). Females dispersed just before the breeding season.
We defined group size as the number of adults and sub-adults in a pack in a given breeding season, and litter size the number of pups that emerged from the pack’s den in a given year. From consecutive data on pack size and composition (n = 47 pack/years), we derived adult survival rates (adults t + 1/(sub-adult + adults) t ); i.e. the combined survival of adults and sub-adults on the next breeding season when sub-adults become adults (age classes are difficult to differentiate after sexual maturity) and pup survival rates (sub-adults t + 1/pups t ); i.e. the survival of pups into the next breeding season when they become sub-adults (n = 39 pack/years, excluding the pack/years without litters emerging from the den on the previous years). Territory size was calculated as the minimum convex polygon with 5 % outlier removal of the pooled locations of independent sightings form each pack in each year (ArcView’s Animal Movement programme; Hooge and Eichenlaub 2000). This measure is known to be a good representation of the overlapping home ranges of all pack members (Sillero-Zubiri and Gottelli 1995a).
Statistical analyses
We used linear mixed models (MIXED procedure; SAS 2006) to explore the effects of group size upon survival, reproduction or territory size. We considered breeding events as units and used pack as a blocking factor to avoid this source of pseudoreplication (Carrete et al. 2008). To account for autocorrelation in the time series, the models were fitted with autoregressive covariance structure and year as subject effect (Bolker et al. 2009). Sequential generalised linear models (SAS 2006) were used to compare the average territory of packs in different population stages after accounting for pack effects (SAS 2006).
Results
Group living and the pattern of prey distribution
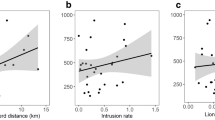
In the low-productivity Tullu Deemtu, wolves lived as mated pairs sometimes accompanied by a single offspring of the year (mean group size, 2.4 ± 0.5 SE), whereas in Web and Sanetti, the highly productive areas, wolves formed family groups of between 3 and 13 wolves (mean, 6.0 ± 1.9 and 6.8 ± 1.5 SE, respectively; Fig. 1). The territories of wolf pairs were approximately twice as large as those of groups (ANOVA, F 1,15 = 13.64, P = 0.002; Fig. 1) and this difference was largely due to different amounts of poor habitat contained within territories. The average area of HQ3 habitat within each territory was 6.4 km2 in Tullu Deemtu, compared to 0.53 km2 in Web and 0.34 km2 in Sanetti (ANOVA, F 2,14 = 17.957, P < 0.001), respectively, covering 60, 6 and 20 % of the territory. Tullu Deemtu territories were configured as to encompass good-quality habitat patches (HQ1 + HQ2) within a matrix of low-quality Helichrysum heaths (HQ3); these patches were mostly restricted to drainage lines with Alchemilla meadows and rocky grasslands with giant mole rat presence. Pack territories in Web and Sanetti were smaller (average, 4.4 km2) and more variable in size (CV = 0.5 compared with CV = 0.1 in Tullu Deemtu), as territory size increased with increasing group size (Spearman’s r = 0.72, P = 0.002; linear mixed model with pack as fixed effect, R = 0.43; F 1,21 = 8.95; P = 0.0070; Fig. 1). The smallest territories (1.8 and 2.0 km2) were held by three and four wolves, respectively, and contained almost identical amounts of good-quality habitat (HQ1 + Q2 = 1.3 and 1.4 km2), and area eight times smaller than the quality habitat contained within the largest territory (11.7 km2) defended by eight wolves.
Direct benefits of group augmentation
Territory size was positively correlated with both group size and the amount of best habitat (HQ1 + HQ2), within a territory (respectively: Spearman’s r = 0.92, P < 0.001 and r = 0.88, P < 0.001 in Web; and r = 0.96, P < 0.001 and r = 0.55, P > 0.05 in Sanetti. The linear relationships, combining Web and Sanetti packs, were territory size = 1.25 + 1.64 HQ1, R 2 = 0.88, F 1,11 = 80.49, P < 0.001; territory size = 1.26 + 2.28 HQ2, R 2 = 0.77, F 1,11 = P < 0.001; n = 12 packs). Group size also increased with increasing area of good-quality habitat within the territory (HQ1 + HQ2), with an apparent asymptote above around pack size seven to eight (Fig. 2), as confirmed statistically by the linear regression of the log-transformed values of group size on HQ1 or HQ2 with a slope significantly lower than zero (HQ1, slope 0.42 (CI = 0.21–0.63); HQ2, slope 0.42 (CI = 0.23–0.61); n = 12 packs). Indeed, the function that best explained the variation in group size as a function of territory size was a linear regression of the log-transformed values of territory size on log group size (log group size = −1.51 + 0.55 log home range size; R 2 = 0.77, F 1,11 = 36.66, P < 0.001). The slope was significantly <1 (t 11 = −4.9982, P < 0.001) indicating that larger territories contained larger areas per wolf. This tendency was apparent across all packs and was not explained by the territories of larger packs containing a lesser area of high-quality habitat (Fig. 3).
No other apparent advantage of larger-pack membership was detected by correlations between group size and reproduction (i.e. litter size) or recruitment (i.e. pup survival in the first year). A negative relationship between group size and adult survival (linear mixed model; group size effect, −0.020; F 1,32 = 8.53; P = 0.0093) reflected the tendency of non-breeding females’ to disperse when groups become large (e.g. in 86 % of all pack/years, there were just one or two adult females in the social group), as well as the acceptance of immigrants by small- and medium-size packs, an event recorded only twice and during the high-density phase only (a floating female that joined a pack and a subordinate female that rejoined her pack after a failed pack-splitting attempt).
Longer-term benefits of philopatry and cooperation
During the high-density phase, pack territories were stable forming a tessellated mosaic across all suitable habitat (Fig. 4). Territories drifted only when neighbouring packs colonised the area vacated by a small pack that collapsed after the death of its dominant female (Sillero-Zubiri and Gottelli 1995a). During the recovery phase, the packs that survived the rabies epizootics expanded spatially to cover again all suitable habitat (Fig. 4). Progressively, these packs enlarged their membership via the delayed dispersal of young (Fig. 5); new packs first appeared 5 years after the population crash. One such pack originated from a split of a large group and two others by coalescence of dispersers. At least some of the dispersers that coalesced into new groups originated locally, including two known individuals from established packs; this was corroborated by the reduction of established packs in that year (Fig. 5).
Pack territories in the Web Valley: a just before epizootic, b surviving packs at the beginning of the recovery period (4 years after the epizootic) and (c) packs established in the area 8 years after epizootic, including new packs (asterisks). Background: habitat quality types sensu Gottelli and Sillero-Zubiri (1992); light grey optimal wolf habitat, medium grey good wolf habitat, dark grey marginal wolf habitat
New packs carved out a territory locally, including part of their natal range and the fringes and interstices between neighbouring territories (Fig. 4). As a result of fission, the territory of the original group contracted from 11.7 to 9.4 km². This was the first successful pack-splitting attempt recorded for the species. It started when a subordinate female from a pack of 13 became pregnant and built a den on the fringes of her natal territory, where she gave birth and established a new pack accompanied by three other natal pack members. Two unsuccessful events (in 1991 in Web and 1999 in Sanetti) ended with the break-away group rejoining the natal pack after the subordinate’s litter had died. Splitting attempts always occurred within unusually large groups (11–13 members) and the successful fission coincided with depressed population density and highest per capita area for wolves (Fig. 3). After accounting for the effect of pack size, the packs that persisted throughout the epizootics had disproportionately larger territories (7.3 km2 ± 0.72 SE) compared with those that existed at high density before the epizootics (5.0 km2 ± 0.64 SE) or those that formed during the recovery phase (6.8 km2 ± 0.45 SE; sequential GLM predicting pack size, F 2,11 = 4.27, P = 0.0495, R 2 = 0.82; fixed effects: pack size, F 1,11 = 28.3, P = 0.001, partial eta 2 = 0.78 and population stage F 2,11 = 4.23, P = 0.056, partial eta 2 = 0.51).
Discussion
Group living and the pattern of prey distribution
In suboptimal areas, Ethiopian wolves need to defend large territories in order to include and maintain critical amounts of key habitats, with prey aggregated in depressions and drainage lines. This critical amount is bound to reflect the minimum requirements to maintain a pair of wolves with room for one philopatric youngster in a good year (Kruuk and Macdonald 1985). Including an extra high-quality patch would have involved expanding an already large territory to an extent no longer economically defensible and/or exceeding the potential benefits of group enlargement. In low-productive areas, territorial expansion would clearly not ensure the rate of acquisition of key resources that expansionist wolves enjoy in prime areas.
Similar relationships between territory size, shape and the dispersion of key resources have been reported in other carnivore species (e.g. Eurasian badgers (Kruuk and Parish 1982), Blanford’s foxes (Geffen et al. 1992) and Arctic foxes Alopex lagopus (Hersteinsson and Macdonald 1996)), and interpreted as congruent with the resource dispersion hypothesis. This predicts that when resources are more variable in space or time (i.e. more heterogeneous), territory size will increase because of the need to defend larger areas that contain enough food patches to satisfy even a single animal or the basic social unit (Carr and Macdonald 1986). At odds with expectations, the area of good habitat contained within an average territory in Tullu Deemtu was similar to that of the average territory in Web and Sanetti, albeit dispersed over a much larger expanse of poor-quality terrain. There is a possibility that good-quality habitats on the rain shadow of Tullu Deemtu have lower prey abundance than the equivalent categories in Web and Sanetti, and that this difference was not detected because these habitats were underrepresented in the Tullu Deemtu sample. In any case, in order to understand fully the limitations imposed by resource dispersion on the potential for group enlargement in Ethiopian wolves, a more profound understanding of their foraging ecology in prey-poor habitat is needed. Prey renewal rates, travelling costs, daily foraging time and overall energy balance are bound to differ between the areas of contrasting productivity.
There is a simple mechanistic explanation for the smaller Ethiopian wolf territories in the more productive habitats; an increase in overall prey abundance will lead to an increase in habitat quality per unit area, and thus higher animal densities and smaller ranges (Gittleman and Harvey 1982; Reiss 1988). Interestingly, Ethiopian wolves have the highest densities and smallest home ranges of any medium-sized canids, and their home ranges are substantially smaller to those predicted by their metabolic needs (Sillero-Zubiri and Gottelli 1995a; Johnson et al. 2002; Macdonald and Sillero-Zubiri 2004). As many small- and medium-sized canids, they rely upon small prey that are locally abundant and easy to catch (Carbone et al. 1999) and/or patchy and rapidly renewed (Johnson et al. 2002; Macdonald and Sillero-Zubiri 2004). In prime wolf habitats, the biomass of rodent prey reaches up to 24–26 kg/ha (Sillero-Zubiri et al. 1995a), considerably higher than the densities of rodents in any other African habitat (Yalden 1988). Not surprisingly, the wolves' daily hunting ranges in these areas overlap considerably within packs, because of low feeding competition—animals foraging as close as <10 m do not seem to suffer from interference (Sillero-Zubiri and Gottelli 1994). These conditions can lead to spatial groups even if individuals do not gain any additional benefit from forming groups per se (Alexander 1974; Macdonald 1983; Wrangham 1993). One example is the Arctic fox in coastal areas where prey is concentrated in predictable patches (Eide et al. 2004).
While the richness of rodent prey makes conditions favourable for group living in Ethiopian wolves, some additional benefit from group living is necessary to explain why wolves in optimal habitats do not subdivide the space among more pairs or small groups, and in doing so avoiding the costs of helping and delayed breeding.
The benefits of group augmentation
Ethiopian wolves actively patrol and mark the boundaries of their territory, and group size determines the outcome of any territorial boundary clashes (Sillero-Zubiri and Macdonald 1998). Thus, larger packs can hold on to and defend larger territories, gaining control of limited space. This study revealed a net benefit of expansionism, in the form of a disproportionate increase in the per capita foraging area in larger packs, while the defence costs incurred by territorial expansion were shared with a greater number of wolves (scent-marking rates per pack increase less rapidly than predicted by group size (Sillero-Zubiri and Macdonald 1998)). The data showed consistent ecological gains up to an apparent inflexion at six to eight wolves per group, close to the average pack size in Web and Sanetti. This may indicate an economically optimal group size in optimal habitats, and a critical point in complex social relationships triggering pack fissions in larger groups. Our findings reinforce the notion that for Ethiopian wolves, philopatry offers food security (sensu Carr and Macdonald 1986) while waiting for a chance to attain reproductive status locally (Sillero-Zubiri et al. 1996a), coinciding with the ecological constraints and habitat saturation hypotheses.
There are other potential benefits of group living in carnivores that can be confidently discarded as important for these wolves, such as increased efficiency through group hunting and/or the defence of kills, and alloparental behaviour. This is because Ethiopian wolves are solitary hunters of small prey and top predators of the Afroalpine ecosystem, without direct competitors except perhaps for birds of prey to a limited extent (Sillero-Zubiri and Gottelli 1995a, b). Alloparental behaviour in Ethiopian wolves involves guarding the den and regurgitating or carrying rodents to feed the pups of the dominant female, but nonbreeding helpers do not seem to enhance the reproductive output of the group (i.e. no correlation between food-provisioning rates, pup survival and numbers of helpers; Sillero-Zubiri et al. 2004). This study confirmed the lack of correspondence between group size and survival or reproduction over a broader range of group sizes and habitat saturation levels.
The high level of relatedness within an Ethiopian wolf pack (Randall et al. 2010) entails that kin selection may be contributing to stabilise sociality and cooperation, but the inclusive fitness gains for subordinates are insignificant or very small, and easily overshadowed by limited opportunities to disperse and breed. Under the hypotheses of ecological constraints and habitat saturation, we would expect subordinate female Ethiopian wolves to disperse opportunistically when breeding space is made available, for example, after epizootics. Their opportunities to breed within the natal territory are minimal (average, 0.12 ± 0.09 SD/year/pack; Sillero-Zubiri et al. 1996a), because of the longevity and enhanced lifetime breeding success of dominant females (whose reproductive costs are reduced by cooperative breeding (Sillero-Zubiri et al. 1996a)). Males have access to more mating opportunities due to a higher turnover of the dominant position and sneaky matings with dominant females of neighbouring packs (Sillero-Zubiri et al. 1996a).
The longer-term benefits of philopatry and cooperation
Incorporating the dynamics of social groups contributes a longer-term perspective of the advantages of philopatry and cooperation. Against our expectations, subordinate wolves remained philopatric when new breeding space was vacated after pack extinctions. The surviving packs expanded into the vacated areas and enlarged steadily in pack membership. We have shown how animals in larger groups enjoy incremental gains in terms of access to food. How does expansionism affect the prospects for territory inheritance?
The tendency shown by Ethiopian wolves to remain cohesive below the carrying capacity of the environment has also been observed in red foxes (Baker et al. 2000; Soulsbury et al. 2010), Eurasian badgers (Revilla and Palomares 2002) and Arctic foxes (Eide et al. 2004; Goltsman et al. 2005). In these cases, the stay-and-wait strategy might give local animals a competitive edge over immigrants, by rendering vacated areas unavailable and exacerbating settlement costs for immigrant pairs or small groups. In other well-known examples, the relaxation of ecological constraints has prompted dispersal and limited cooperation, as predicted by the ecological constraints hypothesis (e.g. red-cockaded woodpecker Picoides borealis (Walters et al. 1992), Seychelles warbler Acrocephalus sechellensis (Komdeur 1992), prairie voles Microtus ochrogaster (Lucia et al. 2008)). In Ethiopian wolves, the stay-and-wait strategy paid off because the costs of delayed reproduction were compensated in the longer-term by the benefits of territory inheritance and the enhanced lifetime reproduction of the new breeding pairs (Woolfenden and Fitzpatrick 1978). Once groups became large, subordinate wolves dispersed locally, established new territories and bred. Dispersers left the natal group as a group and retained control of part of their natal range (pack fission), or coalesced into a pool of mostly local dispersers and carved out territories in-between establish packs.
A dilemma we cannot overlook, because of the high relatedness within Ethiopian wolf packs (Randall et al. 2010), is that expansionism can enhance kin competition within groups (Lehmann et al. 2006). The process of pack fissions, also described as budding behaviour or dispersal by propagules, offers a solution, because group dispersal relaxes local competition without reducing kinship (Gardner and West 2006). The same applies to local dispersers that coalesce into new packs because of the high relatedness between neighbouring packs in the study area.
To better understand the benefits of philopatry in Ethiopian wolves and other long-live organisms, future efforts should quantify breeding opportunities and life-long reproductive success (e.g. Soulsbury et al. 2008; Sparkman et al. 2010). Empirical studies in that direction are revealing new and interesting links between philopatry/cooperation and demography (Lehmann et al. 2006), habitat availability (e.g. Lucia et al. 2008; Iossa et al. 2009), mating systems (Wolff 1992), life history traits (Hatchwell and Komdeur 2000; Hatchwell 2009) and predation (Kamler et al. 2004; Beckerman et al. 2011).
Conclusion
Ecological constraints favour philopatry in specialised organisms such as the Ethiopian wolf when the numerical advantages of territorial defence ensure access to and inheritance of a portion of high-quality habitat. Intense competition for breeding space impose group size requirements for successful establishment of new territories, and to deter colonisation from outsiders, which explain why pack fission lags behind availability of high-quality space. This study provides a compelling example of how the relative contributions of direct and indirect benefits of sociality can be determined by the distribution of prey.
References
Adams ES (1990) Boundary disputes in the territorial ant Azteca trigona—effects of asymmetries in colony size. Anim Behav 39:321–328
Alexander RD (1974) The evolution of social behaviour. Annu Rev Ecol Syst 5:325–383
Bach LA, Thomsen R, Pertoldi C, Loeschcke V (2006) Kin competition and the evolution of dispersal in an individual-based model. Ecol Modell 192:658–666
Baker P, Funk S, Harris S, White P (2000) Flexible spatial organization of urban foxes, Vulpes vulpes, before and during an outbreak of sarcoptic mange. Anim Behav 59:127–146
Beckerman AP, Sharp SP, Hatchwell BJ (2011) Predation and kin-structured populations: an empirical perspective on the evolution of cooperation. Behav Ecol. doi:10.1093/beheco/arr131
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Campbell RD, Rosell F, Nolet BA, Dijkstra VAA (2005) Territory and group sizes in Eurasian beavers (Castor fiber): echoes of settlement and reproduction? Behav Ecol Sociobiol 58:597–607
Carbone C, Mace GM, Roberts SC, Macdonald DW (1999) Energetic constraints on the diet of terrestrial carnivores. Nature 402(286):288
Carlson A (1986) Group territoriality in the rattling cisticola, Cisticola chiniana. Oikos 47:181–189
Carr GM, Macdonald DW (1986) The sociality of solitary foragers: a model based on resource dispersion. Anim Behav 34:1540–1549
Carrete M, Tella JL, Sanchez-Zapata JA, Moleon M, Gil-Sanchez JM (2008) Current caveats and further directions in the analysis of density-dependent population regulation. Oikos 117:1115–1119
Clifton KE (1990) The costs and benefits of territory sharing for the Caribbean coral reef fish, Scarus iserti. Behav Ecol Sociobiol 26:139–147
Clutton-Brock T (2002) Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296:69–72
Creel SR, Macdonald DW (1995) Sociality, group size, and reproductive suppression among carnivores. Adv Stud Behav 24:203–257
Doerr ED, Doerr VAJ (2006) Comparative demography of treecreepers: evaluating hypotheses for the evolution and maintenance of cooperative breeding. Anim Behav 72:147–159
Eide NE, Jepsen JU, Prestrud P (2004) Spatial organization of reproductive Arctic foxes Alopex lagopus: responses to changes in spatial and temporal availability of prey. J Anim Ecol 73:1056–1068
Emlen ST (1982) The evolution of helping.1. An ecological constraints model. Am Nat 119:29–39
Emlen ST (1984) Cooperative breeding in birds and mammals. Behavioural ecology: an evolutionary approach, 2nd edn. Blackwell, London
Emlen ST (1995) An evolutionary theory of the family. P Roy Soc Lond B 92:8092–8099
Gardner A, West SA (2006) Demography, altruism, and the benefits of budding. J Evol Biol 19:1707–1716
Gaston AJ (1978) Evolution of group territorial behavior and cooperative breeding. Am Nat 112:1091–1100
Geffen E, Macdonald DW (1992) Small size and monogamy: spatial organization of Blanford's foxes, Vulpes cana. Anim Behav 44:1123–1130
Geffen E, Hefner R, Macdonald DW, Ucko M (1992) Habitat selection and home range in the Blanford fox, Vulpes cana—compatibility with the resource dispersion hypothesis. Oecologia 91:75–81
Gittleman JL, Harvey PH (1982) Carnivore home-range size, metabolic needs and ecology. Behav Ecol Sociobiol 10:57–63
Goltsman M, Kruchenkova EP, Sergeev S, Volodin I, Macdonald DW (2005) ‘Island syndrome’ in a population of Arctic foxes (Alopex lagopus) from Mednyi Island. J Zool (Lond) 267:405–418
Gottelli D, Sillero-Zubiri C (1992) The Ethiopian wolf - an endangered endemic canid. Oryx 26:205–214
Hamilton WD (1964) The genetical evolution of social behaviour. J Theor Biol 7:1–52
Hatchwell BJ (2009) The evolution of cooperative breeding in birds: kinship, dispersal and life history. Philos T Roy Soc B 364:3217–3227
Hatchwell BJ, Komdeur J (2000) Ecological constraints, life history traits and the evolution of cooperative breeding. Anim Behav 59:1079–1086
Hersteinsson P, Macdonald DW (1996) Diet of arctic foxes (Alopex lagopus) in Iceland. J Zool (Lond) 240:457–474
Hooge PN, Eichenlaub B (2000) Animal movement extension to Arcview. Version 2.0. Alaska Science Center—Biological Science Office, U.S. Geological Survey, Anchorage, AK, USA
Iossa G, Soulsbury CD, Baker PJ, Edwards KJ, Harris S (2009) Behavioral changes associated with a population density decline in the facultatively social red fox. Behav Ecol 20:385–395
Jennions MD, Macdonald DW (1994) Cooperative breeding in mammals. Trends Ecol Evol 9:89–93
Johnson DDP, Kays R, Blackwell PG, Macdonald DW (2002) Does the resource dispersion hypothesis explain group living? Trends Ecol Evol 17:563–570
Kamler JF, Ballard WB, Lemons PR, Mote K (2004) Variation in mating system and group structure in two populations of swift foxes, Vulpes velox. Anim Behav 68:83–88
Kauffman MJ, Varley N, Smith DW, Stahler DR, MacNulty DR, Boyce MS (2007) Landscape heterogeneity shapes predation in a newly restored predator–prey system. Ecol Lett 10:690–700
Koenig W, Pitelka F (1981) Natural selection and social behavior. In: Alexander R, Tinkle D (eds) Ecological factors and kin selection in the evolution of cooperative breeding in birds. Chiron Press Inc, New York, pp 261–280
Koenig WD, Pitelka FA, Carmen WJ, Mumme RL, Stanback MT (1992) The evolution of delayed dispersal in cooperative breeders. Q Rev Biol 67:111–150
Kokko H, Johnstone RA, Clutton-Brock TH (2001) The evolution of cooperative breeding through group augmentation. P Roy Soc Lond B 268:187–196
Komdeur J (1992) Importance of habitat saturation and territory quality for evolution of cooperative breeding in the Seychelles warbler. Nature 358:493–495
Kruuk H (1978) Spatial-organization and territorial behavior of European badger Meles meles. J Zool (Lond) 184:1–19
Kruuk H, Macdonald DW (1985) Group territories of carnivores: empires and enclaves. In: Sibly RM, Smith RH (eds) Behavioral ecology. Ecological consequences of adaptive behaviour. Blackwell, Oxford, UK, pp 521–536
Kruuk H, Parish T (1982) Factors affecting population density, group size and territory size of the European badger, Meles meles. J Zool (Lond) 196:31–39
Lazaro-Perea C (2001) Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defence and assessment of neighbours. Anim Behav 62:11–21
Lehmann L, Perrin N, Rousset F (2006) Population demography and the evolution of helping behaviors. Evolution 60:1137–1151
Lindstroem E (1986) Territory inheritance and the evolution of group-living in carnivores. Anim Behav Forum 34:1825–1835
Lion S, Gandon S (2009) Habitat saturation and the spatial evolutionary ecology of altruism. J Evol Biol 22:1487–1502
Lucia KE, Keane B, Hayes LD, Lin YK, Schaefer RL, Solomon NG (2008) Philopatry in prairie voles: an evaluation of the habitat saturation hypothesis. Behav Ecol 19:774–783
Macdonald DW (1983) The ecology of carnivore social behaviour. Nature 301(379):384
Macdonald DW, Sillero-Zubiri C (eds) (2004) Biology and conservation of wild canids. Oxford University Press, Oxford
Marino J, Sillero-Zubiri C, Macdonald DW (2006) Trends, dynamics and resilience of an Ethiopian wolf population. Anim Conserv 9:49–58
Mills MGL (1982) Factors affecting group size and territory size of the Brown hyaena, Hyaena brunnea in the southern Kalahari. J Zool (Lond) 198:39–51
Pen I, Weissing FJ (2000) Towards a unified theory of cooperative breeding: the role of ecology and life history re-examined. Proc Biol Sci 267:2411–2418
Randall D, Pollinger J, Argaw K, Macdonald D, Wayne R (2010) Fine-scale genetic structure in Ethiopian wolves imposed by sociality, migration, and population bottlenecks. Conserv Genet 11:89–101
Reiss M (1988) Scaling of home range size: body size, metabolic needs and ecology. Trends Ecol Evol 3:85–86
Revilla E, Palomares F (2002) Spatial organization, group living and ecological correlates in low-density populations of Eurasian badgers, Meles meles. J Anim Ecol 71:497–512
SAS (2006) SAS/STAT 9.1 user's guide. SAS Institute Inc, Cary, NC, USA
Schradin C, Pillay N (2005) Intraspecific variation in the spatial and social organization of the African striped mouse. J Mammal 86:99–107
Sillero-Zubiri C, Gottelli D (1994) Canis simensis. Mamm Species 485:1–6
Sillero-Zubiri C, Gottelli D (1995a) Diet and feeding behavior of Ethiopian wolves (Canis simensis). J Mammal 76:531–541
Sillero-Zubiri C, Gottelli D (1995b) Spatial organization in the Ethiopian wolf Canis simensis: large packs and small stable home ranges. J Zool (Lond) 237:65–81
Sillero-Zubiri C, Macdonald DW (1998) Scent-marking and territorial behaviour of Ethiopian wolves Canis simensis. J Zool (Lond) 245:351–361
Sillero-Zubiri C, Tattersall FH, Macdonald DW (1995a) Bale mountains rodent communities and their relevance to the Ethiopian wolf (Canis simensis). Afr J Ecol 33:301–320
Sillero-Zubiri C, Tattersall FH, Macdonald DW (1995b) Habitat selection and daily activity of giant molerats Tachyoryctes macrocephalus: significance to the Ethiopian wolf Canis simensis in the Afroalpine ecosystem. Biol Conserv 72:77–84
Sillero-Zubiri C, Gottelli D, Macdonald DW (1996a) Male philopatry, extra-pack copulations and inbreeding avoidance in Ethiopian wolves (Canis simensis). Behav Ecol Sociobiol 38:331–340
Sillero-Zubiri C, King AA, Macdonald DW (1996b) Rabies and mortality in Ethiopian wolves (Canis simensis). J Wildl Dis 32:80–86
Sillero-Zubiri C, Johnson PJ, Macdonald DW (1998) A hypothesis for breeding synchrony in Ethiopian wolves (Canis simensis). J Mammal 79:853–858
Sillero-Zubiri C, Marino J, Gottelli D, Macdonald DW (2004) Afroalpine ecology, solitary foraging and intense sociality amongst Ethiopian wolves. In: Macdonald DW, Sillero-Zubiri C (eds) Biology and conservation of wild canids. Oxford University Press, Oxford, UK, pp 311–323
Solomon NG (2003) A reexamination of factors influencing philopatry in rodents. J Mammal 84:1182–1197
Soulsbury C, Baker P, Iossa G, Harris S (2008) Fitness costs of dispersal in red foxes (Vulpes vulpes). Behav Ecol Sociobiol 62:1289–1298
Soulsbury CD, Baker PJ, Iossa G, Harris S (2010) Red foxes (Vulpes vulpes). In: Gehrt SD, Riley SPD, Cypher BL (eds) Urban carnivores: ecology, conflict, and conservation. Johns Hopkins University Press Baltimore, Maryland, pp 63–75
Sparkman AM, Adams J, Beyer A, Steury TD, Waits L, Murray DL (2010) Helper effects on pup lifetime fitness in the cooperatively breeding red wolf (Canis rufus). Proc Roy Soc B 278:1381–1389
Sparkman AM, Adams JR, Steury TD, Waits LP, Murray DL (2011) Direct fitness benefits of delayed dispersal in the cooperatively breeding red wolf (Canis rufus). Behav Ecol 22:199–205
Stacey PB, Ligon JD (1991) The benefits-of-philopatry hypothesis for the evolution of cooperative breeding—variation in territory quality and group-size effects. Am Nat 137:831–846
Stacey PB, Taper M (1992) Environmental variation and persistence of small populations. Ecol Appl 2:18–29
Verdolin JL (2009) Gunnison's prairie dog (Cynomys gunnisoni): testing the resource dispersion hypothesis. Behav Ecol Sociobiol 63:789–799
Verdolin JL, Slobodchikoff CN (2009) Resources, not kinship, determine social patterning in the territorial gunnison’s prairie dog (Cynomys gunnisoni). Ethology 115:59–69
Walters J, Copeyon C, Carter J (1992) Test of the ecological basis of cooperative breeding in red-cockaded woodpeckers. Auk 109:90–97
Waser PM (1981) Sociality or territorial defence? The influence of resource renewal. Behav Ecol Sociobiol 8:231–237
Wilson ML, Wrangham RW (2003) Intergroup relations in chimpanzees. Ann Rev Anthropol 32:363–392
Wolff JO (1992) Parents supress reproduction and stimulate dispersal in opposite-sex juvenile white-footed mice. Nature 359:409–410
Woolfenden GE, Fitzpatrick JW (1978) The inheritance of territory in group-breeding birds. Bioscience 28:104–108
Woolfenden GE, Fitzpatrick JW (1984) The Florida scrub jay. Princeton University Press, Princeton
Wrangham RW (1993) Constraints on group size in primates and carnivores: population density and day-range as assays of exploitation competition. Behav Ecol Sociobiol 32:199–209
Yalden DW (1988) Small mammals of the Bale Mountains, Ethiopia. Afr J Ecol 26:281–294
Acknowledgments
We thank the Ethiopian Wildlife Conservation Authority and the Bale Mountains National Park for their support and permission to work in Bale. We are grateful to the Ethiopian Wolf Conservation Programme team, particularly those involved with data collection: Edriss Ebu, Alo Hussein, Phillip Stephens, Candy d’Sa, Scott Newey, David Switzer, Aleks Malkovich and many more. Mike Packer read an earlier version of the manuscript and provided valuable comments. This work was funded by the Wildlife Conservation Society, the Born Free Foundation, People's Trust for Endangered Species and the Frankfurt Zoological Society among others.
Ethical standards
Observations of Ethiopian wolves in the Bale Mountains National Park were conducted under agreements with the Ethiopian Wildlife Conservation Organization and under the auspices of the IUCN Canid Specialist Groups, complying with the laws of the country and with best-practice international guidelines.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Banks
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
DOCX 157 kb
Rights and permissions
About this article
Cite this article
Marino, J., Sillero-Zubiri, C., Johnson, P.J. et al. Ecological bases of philopatry and cooperation in Ethiopian wolves. Behav Ecol Sociobiol 66, 1005–1015 (2012). https://doi.org/10.1007/s00265-012-1348-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-012-1348-x