Abstract
The costs of dispersal are an important factor promoting natal philopatry, thereby encouraging the formation of social groups. The red fox, Vulpes vulpes, exhibits a highly flexible social system and one that is thought to represent a possible stage in the evolution of more complex patterns of group-living. Although the potential benefits accruing to philopatric offspring have previously been studied in this species, the potential costs of dispersal have received less attention. We contrasted survival rates, nutritional status, injuries and reproductive output of dispersing and non-dispersing male and female foxes in an urban population to assess the relative costs of dispersal versus natal philopatry. Mortality rates were not significantly higher for dispersing foxes, either in the short- or long-term. There was no evidence of increased nutritional stress in dispersing individuals. Dispersing individuals did, however, exhibit greater levels of wounding, although this did not appear to affect survival. Dispersing females were more likely to miss a breeding opportunity early in their reproductive lifespan. In contrast, both dispersing and non-dispersing males were unlikely to breed in their first year. We conclude that the major fitness component in females affected by dispersing is age at first reproduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The retention of grown offspring on the natal territory is probably the most common mechanism leading to the formation of social groups (Brown 1974; Gaston 1978; Emlen 1982). Two processes have generally been identified in the evolution of such groups. The first emphasises that ecological constraints, such as the lack of breeding opportunities, negatively influences a dispersing individual (Koenig and Pitelka 1981; Emlen 1982) and increases the fitness costs associated with dispersal. The second places a premium on the benefits non-dispersers may gain from staying, such as increased survival, the inheritance of the territory upon the death of the same-sex parent and/or indirect fitness benefits from helping to raise the young of related individuals (Zack 1990; Komdeur 1992). However, these processes are not mutually exclusive (Emlen 1994; Perrin and Lehmann 2001). In fact, the evolution of group-living is the result of the four-way trade-off between the costs and benefits associated with both dispersal and philopatry, which may affect males and females differently. Consequently, a clear understanding of the costs and benefits of both strategies is needed to evaluate their relative roles in promoting group formation.
Carnivora are a comparatively social order of mammals, with 10–15% of all species showing some form of non-reproductive aggregation (Gittleman 1989). Carnivore sociality is believed to have evolved by directional selection from the ancestral system of intra-sexual territoriality exhibited by most solitary species (Kruuk 1989; Sandell 1989; Creel and Macdonald 1995; but see Asa and Valdespino 1998). Some species, such as the red fox Vulpes vulpes and Eurasian badger Meles meles, have flexible social systems, being solitary, forming pairs or living in groups under different ecological conditions (Johnson et al. 2000). These species are, therefore, thought to represent one possible evolutionary stage in the development of more complex social systems, making them ideal models to examine the selection pressures that may drive group-living in this taxon (Macdonald 1979a).
One widely promulgated theorem for the evolution of group-living in these species, the resource dispersion hypothesis (RDH; Macdonald 1981, 1983; Macdonald and Carr 1989), proposes that social groups may form simply as the result of the rules by which a breeding pair configures a minimum economically defensible territory in relation to the spatial and temporal availability of resources during a key limiting period. Specifically, groups may arise because average resource availability is sufficient to allow subordinate individuals to remain on the natal territory at little or no cost to the dominant pair and with no explicit need for cooperation between group members. Once such spatial groups form, however, these may trigger the evolution of increased sociality (Macdonald 1983; Baker et al. 1998). Although this hypothesis suggests a mechanism for the passive development of social groups (Johnson et al. 2002), it fails, however, to address the evolutionary mechanism(s) whereby dominants and subordinates obtain some fitness benefit from group formation (Macdonald et al. 2004; Macdonald and Kays 2005). In other words, what is the net fitness advantage of natal philopatry or, conversely, what is the fitness disadvantage of dispersal?
The difference in mortality rates of dispersing and non-dispersing individuals is considered an important factor in the evolution of group-living (Bekoff 1987; Waser et al. 1994), and increased mortality has been suggested as a direct cost of dispersal that could promote group-living (Emlen 1982; Waser 1998). However, evidence supporting the assumption that dispersal movements are associated with increased mortality is mixed (Bélichon et al. 1996; Gillis and Krebs 2000). An increase in mortality is thought to occur as dispersing animals are likely to be moving through unfamiliar terrain, potentially making them more vulnerable to some mortality sources. However, whilst moving, dispersers may also suffer a reduction in physical condition due to short- and/or long-term nutritional stress arising from reduced foraging time, increased energetic expenditure and/or confinement to sub-optimal habitats (Palomares et al. 2000; Yoder et al. 2004). Dispersers are also likely to come into conflict with resident animals, leading to an increased risk of injury in aggressive conspecific interactions (Camenzind 1978; Hersteinsson and Macdonald 1982; Smale et al. 1993; Smith 1993). Nutritional stress and injuries are deferred costs of dispersal (sensu Stamps et al. 2005) that, although not necessarily fatal, can reduce physical condition, leading to e.g. increased susceptibility to parasites, a reduction in competitive ability and/or a decline in reproductive output (Clutton-Brock et al. 1982; Lochmiller et al. 1986; Cheeseman et al. 1988; Smith 1993; Drews 1996; Hughes and Kelly 2006).
Under some ecological conditions, red foxes form social groups consisting of a dominant breeding pair and (primarily) non-dispersing offspring (Macdonald 1979b; von Schantz 1981; Voigt and Macdonald 1984; Baker et al. 1998). Typically it was thought that philopatric offspring were females (Macdonald 1979b, 1981), but the numbers of male and female subordinates are equal in some populations (Baker and Harris 2004). Behavioural studies on both captive and wild foxes originally suggested that the major cost associated with natal philopatry was the complete suppression of subordinate reproduction (Macdonald 1979b; von Schantz 1981), but genetic evidence has demonstrated more widespread breeding than previously supposed (Baker et al. 2004). Conversely, subordinates were thought to obtain indirect fitness benefits through alloparental care (but see von Schantz 1981), either by increasing cub survival directly (Zabel and Taggart 1989) or by reducing the provisioning load on dominant animals (Zabel and Taggart 1989; Baker et al. 1998). Philopatric animals may also inherit dominant status upon the death of their same-sex parent (Lindström 1986).
In comparison, the relative costs and benefits associated with dispersing from the natal group have been largely overlooked. In this paper, we compare a range of potential costs and benefits between dispersing and non-dispersing individuals in an urban fox population in Bristol, UK. In particular, we examine whether there are significant differences between dispersers and non-dispersers in: (a) patterns and rates of mortality; (b) short- and long-term measures of nutritional stress; (c) patterns of bite-wounding; (d) the attainment of dominant status within social groups; and (e) reproductive success.
Materials and methods
The Bristol fox study began in 1977. Prior to 1990, animals were captured throughout the city as part of an extensive capture–mark–recapture (CMR) programme. Since 1990, the study has focussed on a number of social groups in the north-west of the city. In this paper, foxes recovered dead during 1977–1993 were used to estimate mortality rates and patterns of nutritional stress in terms of dietary changes and utilisation of fat reserves; density during this period was 7.8–25.8 adult foxes/km2 (Soulsbury et al. 2007a). Animals captured during 2002–2005 were used to estimate patterns of bite-wounding, the attainment of dominant status, reproductive output and short-term nutritional stress; density during this period was 4.0–5.5 foxes/km2 (Soulsbury et al. 2007a). Sub-sets of data have been utilised to address specific questions due to inherent differences in the short-term objectives within the overall study.
Foxes were captured using baited box traps (Baker et al. 2001) or netted from den locations. Foxes were ear-tagged (Rototags, Dalton Supplies Ltd, Nettlebed, Henley-on-Thames, Oxfordshire, UK), weighed, sexed and aged by incisor wear (Harris 1978). From 1992, skin ejected during tagging was kept for genetic analysis. Originally, white ear tags were used. However, from spring 1998 these were progressively replaced with brightly coloured tags: a tag was placed in each ear and there were 100 possible colour combinations, thereby allowing individuals to be identified at a distance. For ageing, all individuals were assumed to have been born on April 1st each year (Harris and Trewhella 1988); animals <6 months, 6–12 months and >12 months are termed cubs, subadults and adults, respectively (Harris and Trewhella 1988).
Foxes were categorised as dispersers or non-dispersers based upon the straight-line distance between their point of capture as a cub (i.e. the location of their natal territory) and their recovery dead relative to average home range size as determined by radio-tracking (Harris and Trewhella 1988); dispersers were classified as all individuals recovered dead >1 territory diameter from their point of first capture (Harris and Trewhella 1988). During the course of the study, large changes in population density and home range size occurred; therefore, average home range diameter was taken as 700 m and 480 m in the periods 1977–1989 and 1992–1993, respectively.
Patterns of mortality
Patterns of mortality of dispersing and non-dispersing foxes were calculated using only those individuals tagged as cubs and recovered dead as a subadult or adult during 1977–1993. Dead foxes were reported principally by the general public and Bristol City Council cleansing department. Prior to an outbreak of sarcoptic mange in 1994 (Baker et al. 2000), <5% of radio-collared foxes died in a location where they would not have been discovered by a member of the general public (authors’ unpublished data). Therefore, we consider that there are no inherent biases between dispersing and non-dispersing animals in the likelihood of being recovered dead per se, nor in the likelihood of being recovered dead from a specific source of mortality.
Dispersal occurs principally in juveniles aged 6–12 months old (Harris and Trewhella 1988). Chi-squared tests were used to compare: (a) the relative number of dispersing and non-dispersing males and females recovered dead as a subadult versus those recovered dead as an adult as an estimate of sex-strategy-specific mortality rates; and (b) the relative number of dispersing and non-dispersing males and females recovered dead as subadults from specific causes (collision with vehicle; culled, i.e. killed with dogs, shot, snared, trapped, dug out of den; and disease, fights, misadventure, e.g. electrocution and unknown causes combined).
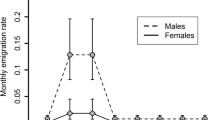
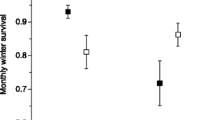
Sex-strategy-specific mortality rates for cohorts of cubs each year during 1979–1989 and 1992–1993 inclusive were calculated as: M = (S / (S + A)) × 100, where S and A are the number of animals recovered dead as subadults and adults, respectively. Minimum sample size in any given year was 16 recoveries. Rates were compared between dispersing and non-dispersing males and females using repeated-measures ANOVA. To examine temporal patterns of survival in the short- (6–12 months) and long-term (>12 months), we compared survival patterns of the four sex-strategy categories using the Kaplan–Meier estimation technique, calculated in MINITAB version 13.0. For animals recovered dead as adults, we used a Kruskal–Wallis test to determine whether there was any difference in the median age at death between the four sex-strategy categories.
To determine whether individuals that dispersed further were more likely to perish, we investigated the relationship between mortality rate and distance dispersed for successive territory intervals, i.e. the proportion of dispersing animals that died as subadults and which were recovered dead 1, 2, 3, etc. territory diameters from their original point of capture as a cub versus the number of adults recovered dead 1, 2, 3, etc. territory diameters from their point of capture, up to a maximum of eight territory diameters. Males and females were analysed separately using Pearson’s correlation coefficient.
Nutritional stress
Dispersing individuals have to cross unfamiliar terrain. Consequently, such movements may be a period of nutritional stress. In the short term, this may be manifested as a reduction in the amount of food consumed per se and/or differences in the relative importance of different food items in the diet. In the longer term, nutritional stress may result in the depletion of fat reserves.
During 1977–1989, individual food items (earthworm fragments; scavenged meat; scavenged bread; fruit and vegetables; insects) in the stomachs of subadult foxes recovered dead were scored on a scale of 0 (item absent) to 5 (filled stomach; Harris 1981); vertebrate food items were recorded too infrequently to include in the analyses. Stomachs that had been damaged at death, e.g. from vehicle collisions, were not examined. The numbers of stomachs of dispersing and non-dispersing subadults containing no food items were compared using a chi-squared test; data from males and females were combined due to small samples sizes. Excluding individuals with empty stomachs, the total volume scores of food in each stomach and the volume scores of each food category were compared using Mann–Whitney tests.
Subadult individuals necropsied during the three-year period 1988–1991 were examined for the extent of kidney fat reserves, scored on a scale of 0 (no fat) to 5 (kidney completely covered in fat). Kidneys that had been damaged at death were not examined. Differences in kidney fat scores between dispersers and non-dispersers were compared using a Mann–Whitney test; data from males and females were combined due to small sample sizes. To assess whether there was a temporal effect of the timing of dispersal movements on kidney fat scores, a Spearman’s rank correlation was used to compare the kidney fat scores of dispersing subadult foxes against month of recovery.
Urine was collected from subadult foxes captured alive during August 2004–April 2005. Due to restrictions on the length of time and how animals could be kept prior to release, samples were collected opportunistically. Samples were stored in 1.5-ml Eppendorf tubes at −20°C for a maximum of 3 days before being sent to Langford Veterinary Diagnostics (School of Clinical Veterinary Science, Langford House, Langford, Bristol, UK) for analysis of urea and creatinine content. Urinary urea nitrogen (UN) concentration varies in relation to protein intake: it is elevated in animals that have recently fed, and drops the longer an animal has not fed as the body recycles rather than excretes nitrogen, although it will also increase in advanced stages of starvation as muscle is catabolised to obtain protein. UN values must therefore be standardised by comparison with urinary creatinine concentration (DelGiudice et al. 1987). Urea/creatinine ratios were compared between dispersing and non-dispersing foxes using a two-sample t-test. Status (i.e. dispersing or non-dispersing) was assigned by comparing the straight-line distance between the point of capture when urine samples were collected and the point of first capture as a cub.
Injuries
All subadult foxes captured between October and March 2002–2005 were scored for the number and severity of bite wounds on each of eight regions of the body: muzzle; face and ears; neck; thorax (area over the ribcage); abdomen; forelegs; hind legs; and tail. Wounds were classified as minor (<3 cm in length and restricted to cutaneous layer) or major (>3 cm in length and/or subcutaneous penetration) wounds. The total numbers of (a) minor and (b) major wounds were compared between dispersing and non-dispersing individuals using Mann–Whitney tests: data for males and females were combined due to the small number of dispersing foxes recaptured. The frequency of minor and major wounds combined on different parts of the body were compared within dispersers and non-dispersers using Friedman tests, with post-hoc analyses where appropriate (Siegel and Castellan 1988). Status (i.e. dispersing or non-dispersing) was assigned by comparing the straight-line distance between the point of capture when wounding data were recorded and the point of first capture as a cub.
Dominance attainment and reproductive success
One potential benefit of dispersing is the more rapid attainment of dominant status. Conversely, a reduction in physical condition following a dispersal movement could lead to missed breeding opportunities. However, the attainment of dominant status does not guarantee that an individual will breed successfully, and philopatric individuals may also be able to reproduce even if they are not the dominant animal in their group. Using dispersing and non-dispersing individuals studied in the period 2002–2005, we compared (a) the relative success of dispersal and natal philopatry as routes to attaining dominance and (b) the proportion of animals successfully producing young in their first and second years using Fisher’s exact tests. Social hierarchy was determined using the methods described by Baker et al. (1998, 2000). Dominant individuals were those that elicited submissive behaviours from same-sex conspecifics within their social group; dominance was assumed where only one individual of a given sex was present on the territory. Interactions were observed during nocturnal radio-tracking sessions of focal individuals and at known feeding sites; dispersing and non-dispersing individuals were radio-tracked for between 6 and 20 nights as subadults and between 4 and 6 nights per 3-month season as adults.
Patterns of breeding by males and females were determined using individual genotypes from 10 microsatellites using the basic methodology described in Soulsbury et al. (2007b). As two loci showed significant levels of allelic dropout (Soulsbury et al. 2007b), parentage was assigned using a decision matrix (Soulsbury 2005). Only individuals that were radio-collared throughout the period 2002–2005 were included in this analysis. Radio-collared vixens were tracked to diurnal resting sites during the pre- and post parturition periods, allowing us to identify the exact location of the den. Following their first emergence from the den at ca. 3–4 weeks of age, we attempted to capture all cubs. Depending on the type of den site (under a garden shed or underground in a complex of holes), cubs were either netted (sheds) or cage traps were placed in the immediate vicinity of the den (holes). Additionally, a semi-permanent network of traps was spread across the entire study site, totalling ca. 10,500 trap nights per annum.
We used two measures of fitness for female foxes; lifetime reproductive success (LRS) and individual fitness (λ individual; McGraw and Caswell 1996; Brommer et al. 2002). Data were collected from females that had been followed throughout their lifetime but which may still have been alive at the end of the study; there were insufficient data to derive comparable estimates for males. A rate-sensitive estimate of individual fitness was utilised as it incorporates quantitative information on reproductive timing, a major component of fitness (Stearns 1992), and is therefore considered a more appropriate surrogate than LRS (McGraw and Caswell 1996). LRS was calculated as the total number of cubs produced across the individual’s lifetime. Individual fitness for each animal was calculated as the dominant eigenvalue from a matrix incorporating reproductive output in each year and annual female survival throughout her lifetime (McGraw and Caswell 1996).
For both measures, collection of quantified data on actual litter size was problematic, as breeding dens were frequently located in badger tunnel systems (Newman et al. 2003). Both badgers and their refugia are legally protected in Britain, such that catching entire litters was very difficult. Therefore, in deriving estimates of LRS and λ, we have assumed a constant litter size at each reproductive attempt where the female was known to have bred. At a population level, mean litter size does not vary markedly with age (Harris and Smith 1987), and so we assumed a litter size of four cubs, as this was mean emergent litter size during the course of the study (Soulsbury et al. 2007a). However, it must be noted that both LRS and λ are affected by variation in litter size between reproductive attempts. Consequently, the estimates presented here must be treated as provisional.
Results
Patterns of mortality
During the period 1977–1993, there was no significant difference in the relative number of male dispersers (32.2%, N = 214), male non-dispersers (36.1%, N = 122), female dispersers (29.0%, N = 100) and female non-dispersers (28.9%, N = 149) recovered dead as a subadult versus those recovered dead as adults (chi-squared test: \(\chi _{\text{3}}^{\text{2}} = 2.00\), p = 0.57). Annual mortality rates for dispersing and non-dispersing male and female foxes were not significantly different (repeated-measures ANOVA: Pillai’s trace F 3,10 = 0.45, p = 0.10; Fig. 1). Furthermore, there was no significant difference in the relative importance of different mortality factors between the four sex-strategy categories for those animals that died as subadults (\(\chi _6^2 = 2.33\), p = 0.89; Table 1).
Patterns of survival for the four categories of individuals were not significantly different in the short- (Kaplan–Meier survival estimation: \(\chi _3^2 = 3.08\), p = 0.38) or long-term (\(\chi _3^2 = 2.93\), p = 0.40; Fig. 2). There was no significant difference in the median age at death for dispersing males (median ± IQR: 29.0 ± 24.0 months, N = 145), philopatric males (28.0 ± 20.5, N = 78), dispersing females (34.0 ± 25.0, N = 71) or philopatric females (33.0 ± 24.0, N = 106) that had survived to adulthood (Kruskal–Wallis test: H 3 = 2.34, p = 0.5). There was no correlation between dispersal distance and mortality rates for dispersing males (Pearson’s correlation coefficient: r = −0.25, p = 0.54) or dispersing females (r = 0.20, p = 0.64).
Nutritional stress
The number of stomachs that were empty at necropsy did not differ between dispersing (42.4%, N = 33) and non-dispersing subadults (41.5%, N = 41, males and females combined; chi-squared test: \(\chi _1^2 = 0.007\), p = 0.93). Similarly, there was no significant difference on the total volume score of all stomach contents combined (dispersers: mean ± SE = 6.8 ± 0.6, N = 19; non-dispersers: 8.0 ± 1.0, N = 24; Mann–Whitney test: W = 450.5, p = 0.43) nor the volume scores of any individual food item: scavenged meat (2.2 ± 0.3 versus 2.3 ± 0.4; W = 509.0, p = 0.65), scavenged bread (1.2 ± 0.4 versus 0.8 ± 0.3; W = 469.0, p = 0.15), fruit/vegetables (2.0 ± 0.2 versus 2.2 ± 0.2; W = 496.5, p = 0.45), insects (0.3 ± 0.2 versus 0.4 ± 0.1; W = 512.0, p = 0.71), earthworms (0.1 ± 0.1 versus 0.2 ± 0.1; W = 512.0, p = 0.71). Kidney fat scores of dispersing (1.7 ± 0.2, N = 17) and non-dispersing (1.9 ± 0.3, N = 8) subadult foxes recovered dead were not significantly different (Mann–Whitney test: W = 115.0, p = 0.54). Kidney fat scores of dispersing subadult foxes did not decline significantly through the dispersal period (r s = −0.10, P = 0.707).
Urea/creatinine ratios did not differ between dispersing (mean ± SE = 22.90 ± 3.17, N = 5) and non-dispersing subadult foxes (23.99 ± 3.31, N = 7; two-sample t-test: t 10 = 0.23, p = 0.82). Although sample sizes were small, projected differences between well-fed and fasted individuals (see DelGiudice et al. 1987) would have been detectable.
Injuries
Dispersers (N = 7) had significantly more minor (2.6 ± 0.6 versus 0.5 ± 0.2; W = 133.0, p < 0.01) and major wounds (1.1 ± 0.3 versus 0.4 ± 0.2; W = 121.0, p = 0.04) than non-dispersers (N = 17; Fig. 3). Samples sizes were not sufficient to examine sex differences in the total number of wounds for dispersing individuals. However, there were no significant differences in the number of minor (Mann–Whitney test: W = 88.5, p = 0.104) or major wounds (Mann–Whitney test: W = 75.5, p = 0.630) between non-dispersing males (N = 9) and non-dispersing females (N = 8).
Minor and major wounds combined were not equally spread across the body for either dispersing (Friedman test: S 7 = 14.91, p = 0.037, adjusted for ties) or non-dispersing (Friedman test: S 7 = 27.58, p < 0.001, adjusted for ties) subadult foxes; post-hoc analyses indicated that the muzzle had significantly more wounds than all other regions of the body. In the case of dispersing individuals, the number of wounds on the muzzle was not, however, significantly different from the number of wounds on the face.
Dominance attainment and reproductive success
The numbers of individual males attaining dominant status as a consequence of dispersing (4/4) versus those that did not disperse (4/5; Fisher’s exact test, p = 1.00) were not significantly different. In contrast, significantly more female dispersers attained dominant status (5/6) than those that remained on their natal territory (3/11; Fisher’s exact test; p = 0.05); in the latter, all three non-dispersing females attained dominance following the death of the previous dominant female.
The number of male dispersers (1/9) and non-dispersers (1/5) that bred in their first year was not significantly different (Fisher’s exact test; p = 1.00). Although samples sizes were small, it appeared that there was no difference in the number of male dispersers (2/3) and non-dispersers (4/5) that bred aged ≥2 years (Fisher’s exact test; p = 0.89). In contrast, significantly fewer females that dispersed (1/7) bred in their first year compared to non-dispersing females (9/11; Fisher’s exact test; p = 0.013). However, by their second year, there was no significant difference in the number of female dispersers and non-dispersers breeding (7/7 versus 6/6, respectively, p = 1.00). This pattern did not cause significant differences in the LRS of dispersing and non-dispersing females (Mann–Whitney U test: W = 39.0, p = 1.00), but did cause a significant difference in fitness (W = 125.0, p = 0.05; Fig. 4).
Discussion
Contrary to expectations, dispersing foxes were not nutritionally stressed relative to non-dispersing individuals, either using short-term (urea/creatinine ratios) or long-term (kidney fat scores) measures. This is perhaps not surprising, however, as dispersing foxes in this population do not appear to use poorer quality habitats or to have a significantly higher rate of energy expenditure (Soulsbury 2005). However, as had been reported in other species (Woodroffe et al. 1993; Woodroffe and Macdonald 1995; Kays et al. 2000; Cant et al. 2001), levels of wounding were significantly higher in dispersing individuals. The majority of bite wounds were located in the facial region, particularly on the muzzle, and were minor, suggesting they were the result of ritualised fights; red foxes fight by standing face-to-face on their hind feet with forepaws on each other’s chest and attempt to push their opponent backwards, thereby forcing it to flee (Vincent 1958; Fox 1969; Macdonald 1987). Biting, when it occurs, is directed at the muzzle, lower jaws and cheeks of the opponent (Fox 1969). However, severe wounding can occur, and this was also higher in dispersing individuals. Furthermore, these data were collected at a time when density was relatively low for this population (4.0–5.5 adult foxes/km2). As the number of encounters with residents and their associated risks are likely to increase with density, the role of injuries as a cost to dispersal may be increasingly important as density increases (Harris and Smith 1987; White et al. 1995).
Given the similarities in nutritional indices of dispersing and non-dispersing individuals and that wounding did not appear to be overtly serious, it is perhaps not surprising that the mortality rates of dispersing and non-dispersing subadult foxes were not significantly different. Baker et al. (2007) found that subadult mortality was higher during autumn/winter (the dispersal period) due to increased rates of crossing roads. However, since non-dispersers also show heightened activity during the autumn and winter, dispersal did not increase the risk of any mortality source.
Instead, the principal cost associated with dispersal movements for females appeared to be the increased likelihood of missed breeding opportunities at the onset of the individual’s reproductive lifespan; no such pattern, however, was evident for males. Similar reproductive costs have been observed in some other species, e.g. kit foxes Vulpes macrotis mutica (Koopman et al. 2000), lions Panthera leo (Pusey and Packer 1987) and black bears Ursus americanus (Rogers 1977). It is unclear, however, why this delay in reproduction occurred, as females in their first year are physiologically capable of breeding, as indicated by the high number of philopatric females reproducing, and whilst nutritional stress during dispersal may cause reproductive failure in some species (Johnson 1986), this did not appear to be the case in this study. Rather, the stress of dispersing may be an important factor, as high cortisol concentrations are associated with reproductive failure in female foxes (Hartley et al. 1994).
Unequal reproductive costs have been highlighted as one factor that may cause sex-biased dispersal rates (Johnson 1986; Pusey 1987), with the sex that breeds at an earlier age being at greater risk of missing the first reproductive period following a dispersal movement (Johst and Brandl 1997): in this study, juvenile females appeared to be at greater risk of missing breeding opportunities following dispersal movements. Consequently, such asymmetric reproductive costs appear to favour the retention of female over male offspring in red foxes, leading to a female bias in social groups (Baker and Harris 2004). However, increased access to early reproduction for subordinate males, as found at higher densities (Baker et al. 2004), may increase the reproductive benefits of male philopatry. Coupled with increased risk of injuries, this may explain why group sex ratios decline from a female bias to parity at higher densities (Baker et al. 2004; Baker and Harris 2004).
In summary, dispersal costs, in particular elevated mortality, have been highlighted as important in promoting natal philopatry and group-living. However, we found that mortality was not increased by dispersing and that deferred costs, principally in the form of lost breeding opportunities, especially for females, may be a more important determinant of dispersal strategies in this population. Opportunities for direct reproduction by philopatric subordinates would therefore appear to be a crucial component affecting the formation of social groups (Vehrencamp 1983a, b; Keller and Reeve 1994) and, therefore, warrant further investigation.
References
Asa CS, Valdespino C (1998) Canid reproductive biology: an integration of proximate mechanisms and ultimate causes. Am Zool 38:251–259
Baker PJ, Harris S (2004) Red foxes: the behavioural ecology of red foxes in urban Bristol. In: Macdonald DW, Sillero-Zubiri C (eds) Biology and conservation of wild canids. Oxford University Press, Oxford, pp 207–216
Baker PJ, Robertson CPJ, Funk SM, Harris S (1998) Potential fitness benefits of group living in the red fox, Vulpes vulpes. Anim Behav 56:1411–1424
Baker PJ, Funk SM, Harris S, White PCL (2000) Flexible spatial organization of urban foxes, Vulpes vulpes, before and during an outbreak of sarcoptic mange. Anim Behav 59:127–146
Baker PJ, Harris S, Robertson CPJ, Saunders G, White PCL (2001) Differences in the capture rate of cage-trapped red foxes Vulpes vulpes and an evaluation of rabies control measures in Britain. J Appl Ecol 38:823–835
Baker PJ, Funk SM, Bruford MW, Harris S (2004) Polygynandry in a red fox population: implications for the evolution of group living in canids? Behav Ecol 15:766–778
Baker PJ, Dowding CV, Molony SE, White PCL, Harris S (2007) Activity patterns of urban red foxes (Vulpes vulpes) reduce the risk of traffic-induced mortality. Behav Ecol 18:716–724
Bekoff M (1987) Group living, natal philopatry, and Lindström’s lottery: it's all in the family. Trends Ecol Evol 2:115–116
Bélichon S, Clobert J, Massot M (1996) Are there differences in fitness components between philopatric and dispersing individuals? Acta Oecol 17:503–517
Brommer JE, Merilä J, Kokko H (2002) Reproductive timing and individual fitness. Ecol Lett 5:802–810
Brown LH (1974) Alternate routes to sociality in jays—with a theory for the evolution of altruism and communal breeding. Am Zool 14:63–80
Camenzind FJ (1978) Behavioral ecology of coyotes on the National Elk Refuge, Jackson, Wyoming. In: Bekoff M (ed) Coyotes: biology, behavior and management. Academic, New York, pp 267–294
Cant MA, Otali E, Mwanguhya F (2001) Eviction and dispersal in co-operatively breeding banded mongooses (Mungos mungo). J Zool 254:155–162
Cheeseman CL, Wilesmith JW, Stuart FA, Mallinson PJ (1988) Dynamics of tuberculosis in a naturally infected badger population. Mamm Rev 18:61–72
Clutton-Brock TH, Guiness FE, Albon SD (1982) Red deer—behavior and ecology of two sexes. University Press, Edinburgh
Creel SR, Macdonald DW (1995) Sociality, group size, and reproductive suppression among carnivores. Adv Study Behav 24:203–257
DelGiudice GD, Seal US, Mech LD (1987) Effects of feeding and fasting on wolf blood and urine characteristics. J Wildl Manage 51:1–10
Drews C (1996) Contexts and patterns of injuries in free-ranging male baboons (Papio cynocephalus). Behaviour 133:443–474
Emlen ST (1982) The evolution of helping. I. An ecological constraints model. Am Nat 119:29–39
Emlen ST (1994) Benefits, constraints and the evolution of the family. Trends Ecol Evol 9:282–285
Fox MW (1969) The anatomy of aggression and its ritualization in Canidae: a developmental and comparative study. Behaviour 35:242–258
Gaston AJ (1978) The evolution of group territorial behavior and cooperative breeding. Am Nat 112:1091–1100
Gillis EA, Krebs CJ (2000) Survival of dispersing versus philopatric juvenile snowshoe hares: do dispersers die? Oikos 90:343–346
Gittleman JL (1989) Carnivore group living: comparative trends. In: Gittleman JL (ed) Carnivore behavior, ecology and evolution. Cornell University Press, New York, pp 183–207
Harris S (1978) Age determination in the red fox (Vulpes vulpes)—an evaluation of technique efficiency as applied to a sample of suburban foxes. J Zool 184:91–117
Harris S (1981) The food of suburban foxes (Vulpes vulpes), with special reference to London. Mamm Rev 11:151–168
Harris S, Smith GC (1987) Demography of two urban fox (Vulpes vulpes) populations. J Appl Ecol 24:75–86
Harris S, Trewhella WJ (1988) An analysis of some of the factors affecting dispersal in an urban fox (Vulpes vulpes) population. J Appl Ecol 25:409–422
Hartley FGL, Follet BK, Harris S, Hirst D, McNeilly AS (1994) The endocrinology of gestation failure in foxes (Vulpes vulpes). J Reprod Fert 100:341–346
Hersteinsson P, Macdonald DW (1982) Some comparisons between red and arctic foxes, Vulpes vulpes and Alopex lagopus, as revealed by radio tracking. Symp Zool Soc Lond 49:259–289
Hughes S, Kelly P (2006) Interactions of malnutrition and immune impairment, with specific reference to immunity against parasites. Paras Immuno 28:577–588
Johnson CN (1986) Sex-biased philopatry and dispersal in mammals. Oecologia 69:626–627
Johnson DDP, Macdonald DW, Dickman AJ (2000) An analysis and review of models of the sociobiology of the Mustelidae. Mamm Rev 30:171–196
Johnson DDP, Kays R, Blackwell PG, Macdonald DW (2002) Does the resource dispersion hypothesis explain group living? Trends Ecol Evol 17:563–570
Johst K, Brandl R (1997) Evolution of dispersal: the importance of the temporal order of reproduction and dispersal. Proc Roy Soc B 264:23–30
Kays RW, Gittleman JL, Wayne RK (2000) Microsatellite analysis of kinkajou social organization. Molec Ecol 9:743–751
Keller L, Reeve HK (1994) Partitioning of reproduction in animal societies. Trends Ecol Evol 9:98–102
Koenig WD, Pitelka FA (1981) Ecological factors and kin selection in the evolution of cooperative breeding in birds. In: Alexander RD, Tinkle DW (eds) Natural selection and social behavior: recent research and new theory. Chiron, New York, pp 261–280
Komdeur J (1992) Importance of habitat saturation and territory quality for evolution of cooperative breeding in the Seychelles warbler. Nature 358:493–495
Koopman ME, Cypher BL, Scrivner JH (2000) Dispersal patterns of San Joaquin kit foxes (Vulpes macrotis mutica). J Mammal 81:213–222
Kruuk H (1989) The social badger–ecology and behaviour of a group–living carnivore (Meles meles). Oxford University Press, Oxford
Lindström E (1986) Territory inheritance and the evolution of group–living in carnivores. Anim Behav 34:1825–1835
Lochmiller RL, Hellgren EC, Grant WE (1986) Reproductive responses to nutritional stress in adult female collared peccaries. J Wildl Manage 50:295–300
Macdonald DW (1979a) The flexible social system of the golden jackal, Canis aureus. Behav Ecol Sociobiol 5:17–38
Macdonald DW (1979b) ‘Helpers’ in fox society. Nature 282:69–71
Macdonald DW (1981) Resource dispersion and the social organization of the red fox (Vulpes vulpes). In: Chapman J, Pursley D (eds) Proceedings of the Worldwide Furbearer Conference. University of Maryland Press, Maryland, pp 918–949
Macdonald DW (1983) The ecology of carnivore social behaviour. Nature 301:379–384
Macdonald DW (1987) Running with the fox. Unwin Hyman, London
Macdonald DW, Carr GM (1989) Food security and the rewards of tolerance. In: Standen V, Foley RA (eds) Comparative socioecology: the behavioural ecology of humans and other mammals. Blackwell, Oxford, pp 75–99
Macdonald DW, Creel S, Mills MGL (2004) Society. In: Macdonald DW, Sillero-Zubiri C (eds) Biology and conservation of wild canids. Oxford University Press, Oxford, pp 85–106
Macdonald DW, Kays RW (2005) Carnivores of the world: an introduction. In: Nowak RM (ed) Walker’s carnivores of the world. John Hopkins University Press, Baltimore, pp 1–67
McGraw JB, Caswell H (1996) Estimation of individual fitness from life-history data. Am Nat 147:47–64
Newman TJ, Baker PJ, Simcock E, Saunders G, White PCL, Harris S (2003) Changes in red fox habitat preference and rest site fidelity following a disease-induced population decline. Acta Theriol 48:79–91
Palomares F, Delibes M, Ferreras P, Fedriani JM, Calzada J, Revilla E (2000) Iberian lynx in a fragmented landscape: predispersal, dispersal, and postdispersal habitats. Cons Biol 14:809–818
Perrin N, Lehmann L (2001) Is sociality driven by the costs of dispersal or the benefits of philopatry? A role for kin-discrimination mechanisms. Am Nat 158:471–483
Pusey AE (1987) Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends Ecol Evol 2:295–299
Pusey AE, Packer C (1987) The evolution of sex-biased dispersal in lions. Behaviour 101:275–310
Rogers LC (1977) Social relationships, movements, and population dynamics of black bears in northern Minnesota. PhD Dissertation, University of Minnesota, Minneapolis
Sandell M (1989) The mating tactics and spacing patterns of solitary carnivores. In: Gittleman JL (ed) Carnivore behavior, ecology and evolution, Vol 1. Cornell University Press, New York, pp 164–182
Siegel S, Castellan NJ (1988) Nonparametric statistics for the behavioral sciences, 2nd edition. McGraw-Hill, New York
Smale L, Frank LG, Holekamp KE (1993) Ontogeny of dominance in free-living spotted hyaenas: juvenile rank relations with adult females and immigrant males. Anim Behav 46:467–477
Smith JLD (1993) The role of dispersal in structuring the Chitwan tiger population. Behaviour 124:165–195
Soulsbury CD (2005) The costs and benefits of red fox Vulpes vulpes dispersal. PhD Dissertation, University of Bristol, Bristol
Soulsbury CD, Iossa G, Baker PJ, Cole NC, Funk SM, Harris S (2007a) The impact of sarcoptic mange Sarcoptes scabiei on the British fox Vulpes vulpes population. Mamm Rev 37:278–296
Soulsbury CD, Iossa G, Edwards KJ, Baker PJ, Harris S (2007b) Allelic dropout from a high-quality DNA source. Cons Genetics 8:733–738
Stamps JA, Krishnan VV, Red ML (2005) Search costs and habitat selection by dispersers. Ecology 86:510–518
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Vehrencamp SL (1983a) A model for the evolution of despotic versus egalitarian societies. Anim Behav 31:667–682
Vehrencamp SL (1983b) Optimal degree of skew in cooperative societies. Am Zool 23:327–335
Vincent RE (1958) Observations of red fox behavior. Ecology 39:755–757
Voigt DR, Macdonald DW (1984) Variation in the spatial and social behaviour of the red fox, Vulpes vulpes. Acta Zool Fennica 171:261–265
von Schantz T (1981) Female cooperation, male competition, and dispersal in the red fox Vulpes vulpes. Oikos 37:63–68
Waser PM (1998) Patterns and consequences of dispersal in gregarious carnivores. In: Gittleman JL (ed) Carnivore behavior, ecology and evolution, Volume 2. Cornell University Press, New York, pp 267–295
Waser PM, Creel SR, Lucas JR (1994) Death and disappearance: estimating mortality risks associated with philopatry and dispersal. Behav Ecol 5:135–141
White PCL, Harris S, Smith GC (1995) Fox contact behaviour and rabies spread: a model for the estimation of contact probabilities between urban foxes at different population densities and its implications for rabies control in Britain. J Appl Ecol 32:693–706
Woodroffe R, Macdonald DW (1995) Female/female competition in European badgers Meles meles: effects on breeding success. J Anim Ecol 64:12–20
Woodroffe R, Macdonald DW, da Silva J (1993) Dispersal and philopatry in the European badger, Meles meles. J Zool 237:227–239
Yoder JM, Marschall EA, Swanson DA (2004) The cost of dispersal: predation as a function of movement and site familiarity in ruffed grouse. Behav Ecol 15:469–476
Zabel CJ, Taggart SJ (1989) Shift in red fox, Vulpes vulpes, mating system associated with El Niño in the Bering Sea. Anim Behav 38:830–838
Zack S (1990) Coupling delayed breeding with short-distance dispersal in cooperatively breeding birds. Ethology 86:265–286
Acknowledgements
We thank the Natural Environment Research Council (CDS), the International Fund for Animal Welfare (PJB), the Rotary Foundation of Rotary International, Newby Trust Ltd and the Sir Richard Stapley Educational Trust (GI), and The Dulverton Trust (SH) for financial support and Keith Edwards and Jane Coghill of the University of Bristol Transcriptomics Unit for help in analysing the genetic data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P.B. Banks
Rights and permissions
About this article
Cite this article
Soulsbury, C.D., Baker, P.J., Iossa, G. et al. Fitness costs of dispersal in red foxes (Vulpes vulpes). Behav Ecol Sociobiol 62, 1289–1298 (2008). https://doi.org/10.1007/s00265-008-0557-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-008-0557-9