Abstract
Models of prey choice in depleting patches predict an expanding specialist strategy: Animals should start as specialists on the most profitable prey and then at some point during patch exploitation switch to a generalist foraging strategy. When patch residence time is long, the switch to a generalist diet is predicted to occur earlier than when patch residence time is short. We tested these predictions under laboratory conditions using female parasitoids (Aphidius colemani) exploiting patches of mixed instars aphid hosts (Myzus persicae, L1 and L4). The duration of patch exploitation was manipulated by changing travel time between patches. As predicted, patch residence times increase with travel time between patches. Our results provide empirical support for the expanding specialist prediction: Parasitoid females specialized initially on the more profitable hosts (L4), and as the patch depleted, they switched to a generalist diet by accepting more frequently the less profitable hosts (L1). The point at which they switched from specialist to generalist occurred later when travel times and hence patch residence times were short. By affecting the patch exploitation strategy, travel time also determines the composition of hosts left behind, the “giving up composition.” The change in the relative density of remaining host types alters aphid populations’ age structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foraging theory deals mainly with two decisions: which prey to attack upon encounter and when to leave a depleting patch of resources to find another (MacArthur and Pianka 1966; Stephens et al. 2008). Although it is convenient to study each decision separately, in reality, animals are typically confronted with having to choose prey within depleting patches (Heller 1980). Surprisingly, few studies have considered both decisions concurrently (but see Heller 1980; Lucas and Schmid-Hempel 1988; Brown and Mitchell 1989; McNamara et al. 1993).
Classic prey models predict a solitary forager’s diet when searching within a patch for prey of different profitabilities (Emlen 1966; MacArthur and Pianka 1966; Schoener 1971; Werner and Hall 1974; Charnov 1976a). The main predictions are: (1) always accept prey of higher profitability; (2) never accept prey of lower profitability when the abundance of more profitable prey types is sufficiently high; (3) a prey type is either always or never included in the diet (no partial preference). Although a considerable body of experimental evidence supports these predictions (Stephens and Krebs 1986; Sih and Christensen 2001), the conditions under which they apply, for example constant prey encounter rates, are unlikely to be valid in natural conditions. Encounter rates are more likely to decline as patches deplete (Charnov 1976b), and prey choice is likely to be affected by declining encounter rates (Heller 1980; Mitchell 1990; Visser et al. 1990). To circumvent these intricacies, Heller (1980), for instance, used a computer simulation model to predict optimal diet choice strategies for depleting patches composed of two types of prey having different profitability. He considered three strategies: (1) specialist; accept only the most profitable prey; (2) generalist; accept all encountered prey; and (3) expanding specialist; accept only the most profitable prey at first and switch to a generalist diet once the most profitable prey is sufficiently depleted (Fig. 1).
Payoffs associated with generalist strategy (solid curve), specialist strategy (short-dashed curve), and expanding specialist strategy (long-dashed curve) in function of patch residence time (modified from Heller 1980)
Heller (1980) argued that the optimal strategy for a forager is to adopt the expanding specialist strategy, while adjusting the moment it switches from a specialist to a generalist diet based on the patch residence time (Fig. 1). Given that optimal patch residence time is predicted to increase with increasing travel time (i.e., time needed to go from one patch to another; Charnov 1976b; Stephens and Krebs 1986), travel time therefore also affects the optimal diet for prey in depleting patches. According to Heller (1980), the longer the patch residence time, the earlier the switch to a generalist diet should occur. Thus, as travel time increases, the forager should switch sooner to a generalist diet. No study has tested this optimal diet prediction for depleting patches.
Travel time is already known to influence patch residence time and hence patch density when the forager leaves the patch (giving up density, i.e., GUD; Brown 1988). Increased travel time leads to longer patch residence times and higher patch exploitation level (Brown 1988). However, if the forager changes its patch exploitation strategy as predicted by Heller’s (1980) model, travel time will not only affect the patch’s giving up density but also its giving up composition or GUC; i.e., the relative abundance of each prey type present within a patch after the forager has left). For instance, a poor quality prey should be less attacked when patches are closed together; therefore, its relative abundance in the habitat should increase after the predator leaves the patch. When the prey or hosts compete for resources, the strategy used by the predator, through its effect on giving up composition, is then likely to lead to apparent competition and may promote coexistence and habitat partitioning for the prey species (Holt and Kotler 1987; Brown and Kotler 2004).
Aphid parasitoids are ideal model organisms to test how travel time affects diet choice in depleting patches. They are naturally confronted with patches of hosts of different profitabilities (Dixon 1977), as it is common to observe aphid colonies containing a mixture of individuals of all immature and adult stages (Dixon 1977, 1985). Aphid colonies are not uniformly distributed in the habitat, and the distance and time needed to travel between colonies vary accordingly.
In this study, we explored under laboratory conditions the impact of travel time on the strategies used by females of a solitary parasitoid (Aphidius colemani) (Viereck) (Hymenoptera, Braconidae, Aphidiinae) exploiting patches of its aphid host (Myzus persicae) (Sulzer) (Homoptera, Aphididae). Patches were composed of two host types [first (L1) and fourth (L4) instar aphids] in equal abundance. Travel time was manipulated by isolating the parasitoid female and imposing either a short or a long delay before being introduced into another host patch. Females’ patch residence times and patch exploitation strategies are compared to Heller’s predictions.
Methods
Study organisms
A. colemani is a solitary parasitoid of several Aphididae species, including the green peach aphid, M. persicae (Starý 1975). Parasitoid females oviposit in the hemocoel of the host, and during subsequent larval development, the immature parasitoid feeds on aphid tissues ultimately killing the host. At the end of its development, the parasitoid larva spins a cocoon, pupates inside the empty aphid skin (called the “mummy”), and emerges as a free-living adult.
Aphids are small, sedentary, plant-sucking insects that form large colonies of mainly apterous individuals. Aphids have a complex life cycle involving parthenogenetic and sexual reproduction, as well as seasonal heteroecy. They are exploited by a wide array of natural enemies such as predators, parasitoids, and entomopathogens (Völkl et al. 2007).
A colony of M. persicae, established from individuals collected in greenhouses from the Horticultural Research and Development Center, was maintained on sweet pepper plants (Capsicum annuum L.). A. colemani was purchased from Koppert Canada (Scarborough, ON, Canada) and reared on M. persicae. Insect cultures were maintained at 20 ± 0.5°C, 60–65% relative humidity and under a 16L:8D photoperiod, standard conditions for all experiments.
Cohorts of M. persicae of specific age class were obtained by isolating about 150 apterous, parthenogenetic adult aphids on a sweet pepper leaf for 8 h. After this period, the adult aphids were removed, and the offspring were reared on excised leaves placed in a Petri dish. Based on larval developmental time of M. persicae under our rearing conditions, first and fourth instars larvae (here forth, L1 and L4) were respectively aged 1 and 7 days.
Parasitoid females used in this study were standardized as follows. Third instar aphids were exposed to female parasitoids for 4 h, at a parasitoid/host ratio of 1:10. Parasitized aphids were then reared in Petri dishes on excised leaves of sweet pepper. Following emergence, males and females remained caged together to allow mating and had access to a dilute honey solution (20%). Prior to each test, naive females (no previous encounter with hosts), aged of 36 ± 12 h, were randomly selected.
Experimental setup
The foraging behavior of parasitoid females exploiting patches of immobilized aphids was observed and scored. An experimental patch consisted of a square (2.5 × 2.5 cm) piece of sweet pepper leaf, in which 16 holes (1.5 mm in diameter) had been pierced. A transparent tape (Scotch® sealing tape) was applied to the underside of the patch such that surface of the 16 holes in the leaf was sticky. The aphids were individually placed on the sticky holes which prevented them from moving during the test. L1 and L4 instars were alternatively assigned over holes. A previous study had shown that, when aphids are unable to escape or defend themselves, L4 provide a better fitness gain (around 2.5 times more) than L1 (Barrette et al. 2009).
The experiment was conducted in three steps. First, naive females were allowed to exploit a patch of 16 immobile aphids (eight L1 and eight L4) for 5 min providing them with experience of the available hosts. Second, the female was removed from the patch and placed in an Ependorf (1.5 ml), with free access to water, for either 0.25 or 24 h simulating short or long travel times, respectively. These durations of isolation were chosen in order to obtain qualitative differences in the perception the female had of patch availability in the habitat (Boivin et al. 2004). Although these differences in travel time were relatively large, we consider them to be representative of the range of foraging time for female parasitoids. Female parasitoids foraging in a natural habitat and leaving a plant to search for another host patch may have to fly several minutes before encountering a new host plant. Much longer travel time, in the order of several hours, may occur if for example weather conditions prevent a parasitoid female from flying in search of a new host patch.
After this delay, the female was placed in a fresh patch with the same host composition and distribution as previously described, and her foraging (host selection) was scored directly using Observer XT version 7.0 (Noldus Information Technology, Leesburg, VA, USA) software.
The following behaviors were recorded only from the second patch: entering the patch, leaving the patch for more than 60 s, antennal contact of a host, bending of the ovipositor, and insertion of the ovipositor. Leaving a host without ovipositing in a host is defined as a rejection while inserting the ovipositor into a host is defined as an acceptance. A trial starts when the female enters the patch and ends when she leaves it for more than five consecutive minutes. The dietary switching point is defined as the moment where the first L1 host is accepted. Because the switch from L4 to L1 always occurred before the females began to accept parasitized L4, only host instar was considered as an indication of host profitability, and no distinction was made between unparasitized and parasitized hosts. However, the female often resampled the same hosts during a trial. Each of the two travel time treatments was replicated 30 times.
Statistical analyses
Mean patch residence times, mean percentage of L4 hosts accepted before switching, mean number of encounters, mean number of rejections before switching, and mean percentage of healthy hosts left were compared between both conditions (short vs long travel time) using one-way analyses of variance with SPSS 10.0 for Windows (Chicago, IL, USA).
Results
Results were obtained from 60 parasitoid females observed for a total of 887.3 min. The majority of these females (93%, i.e., 56/60) parasitized both types of hosts (L1 and L4) during trials. The other four females were thus discarded from the analyses. Travel time did not affect the rate of host encounters (F 1,55 = 1.9; P = 0.16; Table 1).
Patch residence time
Travel time influenced the patch residence time of parasitoid females; they stayed longer in patches when travel time was long rather than short (F 1,55 = 4.70; P = 0.03; Table 1).
Patch exploitation strategies
Travel time between patches shaped the patch exploitation strategies of parasitoid females. At the beginning of patch exploitation, parasitoid females from both treatments specialized and accepted the majority of L4 hosts encountered (F 1,55 = 0.31; P = 0.58; Table 1).
However, the switching point from specialist to generalist strategy occurred at a later moment during patch exploitation when travel time was short rather than long (F 1,55 = 32.00; P < 0.001). Furthermore, parasitoid females accepted their first L1 host after fewer encounters with either L1 or L4 when travel time between patches was long (F 1,55 = 43.21; P < 0.001). The number of L1 rejected before the first one was accepted was lower when travel time was long (F 1,55 = 40.20; P < 0.001; Table 1).
Giving up composition
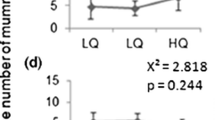
More healthy hosts remained in the patch after the female left when travel time was short (F 1,55 = 13.74; P < 0.001). In the short travel time condition, the proportion of healthy L1 hosts remaining after female departure was higher than the proportion of healthy L4 hosts (F 1,55 = 12.86; P < 0.001). There was no significant difference between the proportion of healthy L1 and healthy L4 hosts remaining in the patch after female departure in the long travel time condition (F 1,55 = 0.69; P = 0.41; Fig. 2).
Discussion
Our results support the prediction that patch residence time increases with travel time, but they go beyond by demonstrating that, through its effect on patch residence time, travel time also affects the optimal diet choice of a forager. As predicted by a model of optimal prey choice for depleting patches (Heller 1980), the parasitoids first specialized on the most profitable hosts, and, as the patch depleted, they switched to a generalist diet by accepting less profitable hosts. Furthermore, as predicted, the dietary switch occurred later when travel time between patches and hence patch residence times were short. Our results also show that travel time affects the composition of hosts left behind; smaller hosts are less parasitized when patches are aggregated. We therefore argue that foraging costs such as travel time can affect both the GUD and the GUC of patches. The change in the relative density of remaining hosts will likely affect population age structure.
Patch residence time and travel time
The patch residence time of female parasitoids increased with travel time. This result is in agreement with patch model predictions and has been demonstrated several times in different taxa (Schaefer and Messier 1995; Giraldeau et al. 1994; Bonser et al. 1998; Houston 2009), including parasitoids (Boivin et al. 2004; Thiel and Hoffmeister 2004; Tentelier et al. 2006). However, increased travel time and aging are difficult to isolate experimentally. In our experiments, we simulated increased travel time by isolating A. colemani females before they start foraging. The increased patch residence time that we observed could therefore also be due to differences in the age of the females in the two experimental conditions. Patch residence time of parasitoid females has been predicted to increase after 60% of their expected lifetime (Wajnberg et al. 2006). The females tested in the short and long travel time conditions were aged 24 and 72 h, respectively, representing between 10% and 30% of their lifetime (Hofsvang and Hågvar 1975). Because these females were still young, we are confident that the increased patch residence time we observed can be ascribed to increased travel time rather than to female age.
Expanding specialist—a switching strategy
Classical optimal diet theory does not predict partial preferences, where a prey type is sometimes accepted and sometimes rejected (McNamara and Houston 1987). The expanding specialist strategy, however, predicts such partial preferences because a less profitable prey that is initially rejected is then later accepted. A number of earlier studies on prey choice have reported the occurrence of partial preferences (e.g., Pulliam 1974; Werner and Hall 1974; Davies 1977; Krebs et al. 1977; Mittelbach 1981; Rechten et al. 1983; Jones 1990), and such partial preferences have been attributed to different constraints such as recognition mistakes (Hughes 1979), imperfect information concerning prey densities (Krebs et al. 1978), or physiological state of the forager (Richards 1983). However, if the evidence of partial preference was based on the level of patch exploitation, it is possible that some of these results can be attributed to the use of a switching strategy such as the expanding specialist. For instance, Davidson (1978) exposed ants (Pogonornyrmex rugosus and Pogonornyrmex barbatus) to seed patches containing a combination of seeds of different sizes; she then concluded, from the number of foraged seeds of different sizes, that ants exhibit partial preferences. In fact, some recent studies did explain cases of partial preferences by the possible use of an expanding specialist strategy (Brown and Morgan 1995; Garb et al. 2000; Hayslette and Mirarchi 2002).
In our study, initial rejection of less profitable hosts followed by their consistent acceptance can hardly be explained by recognition mistakes and imperfect information. Our parasitoid females had had access to both types of hosts prior to the experiment, and A. colemani females are known to be able to assess accurately the profitability of different M. persicae instars (Barrette et al. 2009). Physiological state such as egg load is also known to affect patch exploitation (Fletcher et al. 1994). Parasitoids with large egg loads are expected to be least selective in their oviposition behavior while those with small egg loads should preferentially choose high-quality hosts (Fletcher et al. 1994). One could argue that egg load covaries with age such that the older females in the long travel time condition had the highest egg loads and were thus the least selective of their hosts. Egg load, however, is unlikely to have an important effect in A. colemani because the females emerge with a large egg load of more than 140 mature eggs (Barrette et al. 2009). During the first step of our experiment, females had access to only 16 hosts. Even if young females had oviposited in several hosts, they would still have plenty of eggs left over to oviposit after the short travel time. Therefore, the differences observed in patch exploitation strategies are unlikely to be attributed to differences in egg load.
Implications for population age structure
The distribution of resources in the habitat is known to influence the intensity of patch exploitation (Charnov 1976b; Brown 1988). The closer are the resource patches, the shorter will be the average travel time between patches and thus the shorter will be the patch residence time (Brown 1988). In turn, the patch will be less depleted when the forager quits the patch because residence time is shorter. Our results bring an additional perspective to the effect of travel time on patch exploitation. Travel time also contributes to the composition of prey left behind, the giving up composition (i.e., the relative abundance of each prey type present within a patch after the forager has left).
Thus, when patches are close together in a habitat, not only will they be less depleted by foragers, but the proportion of low quality resources will be higher in the resources left behind by the forager. This effect is due not only to different quitting harvest rates but also to the fact that foragers delay switching to a generalist diet when travel time is short. As a result, when parasitoid females specialize on profitable hosts for long periods, the less profitable hosts have a higher probability of escaping parasitism.
Our study suggests that when patches are closely aggregated, the survival rates of the different aphid types vary, smaller aphids surviving better than larger ones, which is likely to change the age structure of the populations. Future studies could determine in the field if closely aggregated aphid colonies are, indeed, not only less parasitized (lower proportion of parasitized aphids per colony) than distant aphid colonies but also composed of a larger proportion of younger aphids.
References
Barrette M, Wu G-M, Brodeur J, Giraldeau LA, Boivin G (2009) Testing competing measures of profitability for mobile resources. Oecologia 158:757–764
Boivin G, Fauvergue X, Wajnberg E (2004) Optimal patch residence time in egg parasitoids: innate versus learned estimate of patch quality. Oecologia 138:640–647
Bonser R, Wright PJ, Bament S, Chukwu UO (1998) Optimal patch use by foraging workers of Lasius fuliginosus. L. niger and Myrmica ruginodis. Ecol Entomol 23:15–21
Brown JS (1988) Patch use as an indicator of habitat preference, predation risk, and competition. Behav Ecol Sociobiol 22:37–47
Brown JH, Mitchell WA (1989) Diet selection on depletable resources. Oikos 54:33–43
Brown JS, Morgan RA (1995) Effects of foraging behavior and spatial scale on diet selectivity: a test with fox squirrels. Oikos 74:122–136
Brown JH, Kotler BP (2004) Hazardous duty pay and the foraging cost of predation. Ecol Lett 7:999–1014
Charnov EL (1976a) Optimal foraging: attack strategy of a mantid. Am Nat 110:141–151
Charnov EL (1976b) Optimal foraging, the marginal value theorem. Theor Popul Biol 9:129–136
Davidson DW (1978) Experimental tests of the optimal diet in two social insects. Behav Ecol Sociobiol 4:35–41
Davies N (1977) Prey selection and the search strategy of the spotted flycatcher (Muscicapa striata): a field study of optimal foraging. Anim Behav 25:1016–1033
Dixon AFG (1977) Aphid ecology: life cycles, polymorphism, and population regulation. Annu Rev Ecol Syst 8:329–353
Dixon AFG (1985) Structure of aphid populations. Annu Rev Entomol 30:155–174
Emlen JM (1966) The role of time and energy in food preferences. Am Nat 100:611–617
Fletcher JP, Hughes JP, Harvey IF (1994) Life expectancy and egg load affect oviposition decision of a solitary parasitoid. Proc R Soc Lond B Biol Sci 258:163–167
Garb J, Kotler BP, Brown JS (2000) Foraging and community consequences of seed size for coexisting Negev desert granivores. Oikos 88:291–300
Giraldeau L-A, Kramer DL, Deslandes I, Lair H (1994) The effect of competitors and distance on central place foraging in eastern chipmunks, Tamias striatus. Anim Behav 47:621–632
Hayslette SE, Mirarchi RE (2002) Foraging-patch use and within-patch diet selectivity in mourning doves, Zenaida macroura. Ecology 83:2637–2641
Heller R (1980) On optimal diet in a patchy environment. Theor Popul Biol 17:201–214
Hofsvang T, Hågvar EB (1975) Duration of development and longevity in Aphidius ervi and Aphidius platensis (Hym.: Aphidiidae), two parasites of Myzus persicae (Hom.: Aphididae). Entomophaga 20:11–22
Holt RD, Kotler BP (1987) Short-term apparent competition. Am Nat 130:412–430
Houston AI (2009) Flying in the face of nature. Behav Processes 80:295–305
Hughes RN (1979) Optimal diets under the energy maximization premise: the effects of recognition time and learning. Am Nat 113:209–221
Jones G (1990) Prey selection by the greater horseshoe bat (Rhinolophus ferrumequinum): optimal foraging by echolocation? J Anim Ecol 59:587–602
Krebs JR, Erichsen JT, Webber MI, Charnov EL (1977) Optimal prey selection in the great tit (Parus major). Anim Behav 25:30–38
Krebs JR, Kacelnik A, Taylor P (1978) Test of optimal sampling by foraging great tits. Nature 275:27–31
Lucas JR, Schmid-Hempel P (1988) Diet choice in patches: time-constraint and state-space solutions. J Theor Biol 131:307–332
MacArthur R, Pianka E (1966) On optimal use of a patchy environment. Am Nat 100:603–609
McNamara JM, Houston AI (1987) Partial preferences and foraging. Anim Behav 35:1084–1099
McNamara JM, Houston AI, Weisser WW (1993) Combining prey choice and patch use: what does rate-maximizing predict? J Theor Biol 164:219–238
Mitchell WA (1990) An optimal control theory of diet selection: the effects of resource depletion and exploitative competition. Oikos 58:16–24
Mittelbach GG (1981) Foraging efficiency and body size: a study of optimal diet and habitat use by bluegills. Ecology 62:1370–1386
Pulliam HR (1974) On the theory of optimal diets. Am Nat 108:59–74
Rechten C, Avery M, Stevens A (1983) Optimal prey selection: why do great tits show partial preferences? Anim Behav 31:576–584
Richards LJ (1983) Hunger and the optimal diet. Am Nat 122:326–334
Schaefer JA, Messier F (1995) Winter foraging by muskoxen: a hierarchical approach to patch residence time and cratering behaviour. Oecologia 104:39–44
Schoener TW (1971) Theory of feeding strategies. Annu Rev Ecol Syst 2:369–404
Sih A, Christensen B (2001) Optimal diet theory: when does it work, and when and why does it fail? Anim Behav 61:379–390
Starý P (1975) Aphidius colemani Viereck: its taxonomy, distribution and host range (Hymenoptera, Aphidiidae). Acta Entomol Bohemoslov 72:156–163
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, Princeton
Stephens DW, Brown JS, Ydenberg RC (2008) Foraging: behavior and ecology. University of Chicago Press, Chicago
Tentelier C, Desouhant E, Fauvergue X (2006) Habitat assessment by parasitoids: mechanisms for patch use behavior. Behav Ecol 17:515–521
Thiel A, Hoffmeister TS (2004) Knowing your habitat: linking patch-encounter rate and patch exploitation in parasitoids. Behav Ecol 15:419–425
Visser ME, van Alphen JJM, Nell HW (1990) Adaptive superparasitism and patch time allocation in solitary parasitoids: the influence of the number of parasitoids depleting a patch. Behaviour 114:21–36
Völkl W, Mackauer M, Pell J, Brodeur J (2007) Predators, parasitoids and pathogens. In: van Emden H, Harrington R (eds) Aphids as crop pests. CAB International, Oxford, pp 187–233
Wajnberg E, Bernhard P, Hamelin F, Boivin G (2006) Optimal patch time allocation for time-limited foragers. Behav Ecol Sociobiol 60:1–10
Werner EE, Hall DJ (1974) Optimal foraging and the size selection of prey by the bluegill sunfish (Lepomis macrochirus). Ecology 55:1042–1052
Acknowledgments
This study and MB were financially supported by grants from the National Sciences and Engineering Research Council of Canada (NSERC) to GB, JB, and LAG. MB received additional financial support from a Graduate Scholarship from the Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT) and a Major Fellowship from McGill University. We thank Jenny Vosburg and Josiane Vaillancourt for technical assistance with insects’ rearing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Lindström
Rights and permissions
About this article
Cite this article
Barrette, M., Boivin, G., Brodeur, J. et al. Travel time affects optimal diets in depleting patches. Behav Ecol Sociobiol 64, 593–598 (2010). https://doi.org/10.1007/s00265-009-0876-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0876-5