Abstract
We investigated patch quality assessment by the parasitoid Aphidius ervi Haliday (Hymenoptera: Braconidae) when exploiting colonies of the lettuce aphid Nasonovia ribisnigri Mosley (Hemiptera: Aphididae). Three host patches of different qualities were sequentially offered to a naïve female parasitoid. High quality patches (HQ) consisted of 20 N. ribisnigri reared on susceptible lettuce; low quality patches (LQ) consisted of 20 N. ribisnigri reared on partially resistant lettuce and mixed quality patches (MQ) consisted of equal numbers (10) of aphids reared on both lettuce types. Parasitized aphids were reared until parasitoid emergence; number of mummies and sex ratio were noted. On the first host patch encountered, the mean number of A. ervi mummies produced was significantly higher for HQ host patches (X ± SD; 7.1 ± 5.0) than for LQ host patches (3.8 ± 4.5). This suggests that female A. ervi do not need prior experience to assess patch quality; females probably using an innate estimate of patch quality when encountering a first host patch. This initial patch quality assessment can be modified with experience. When females encountered a MQ patch, they kept their exploitation level constant on the following patch, whatever its quality. Females increased their level of host acceptance on the third patch when three LQ patches were offered successively; accepting low-quality hosts could be preferable when better hosts are not currently available in the habitat. These results suggest that A. ervi females behave in a manner consistent with a Bayesian updating process when foraging for hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immature insect parasitoids complete their development using a single host organism. The value of this resource varies according to the species, age, size, parasitism status and availability (density, distribution, travel time between patches) of the host (Boivin et al. 2004). The quality of the host influences the fitness of both the ovipositing female and the emerging parasitoid. Ovipositing females must thus evaluate patch and host quality in order to optimize their foraging decisions (Godfray 1994). Female parasitoids can estimate patch quality in a given habitat by (i) relying on information gleaned from the exploitation of previous patches (the forager retains information from patch to patch), (ii) using information perceived upon arrival in the patch (the forager perceives sensory cues from potential resources) or (iii) sampling the patch (the forager inspects resource items to obtain an accurate estimate of patch quality) (Louâpre et al. 2011). Female parasitoids can estimate host suitability visually and by antennal contact, but final acceptance usually depends on host quality assessment following ovipositor insertion (Godfray 1994). A foraging female parasitoid will thus gather information from patch to patch about the distribution and quality of resources within the habitat.
Female parasitoids can optimize their use of the information obtained on the probability of encountering a given path type by using a Bayesian approach. Bayesian updating provides optimal learning rules in many foraging situations, the central feature being the dependance of a given behaviour on prior information and current experience (McNamara and Houston 1985; McNamara et al. 2006; Valone 2006). However, such information gathering is valuable only when it leads to behaviors that enhance the individual’s fitness (Eliassen et al. 2009). The efficiencie of the behavioural decisions depend on the estimate of the habitat quality and can change after each sample of the habitat, conceivably converging towards optimality. Information obtained from previous host patches may provide a proximate mechanism to optimize patch exploitation (Vos et al. 1998). For foraging parasitoids, the behaviors expected to be modified by an estimation of host suitability and host patch quality include the acceptance or rejection of hosts based on their suitability relative to other hosts in the habitat and the duration of the exploitation of a given patch (patch residence time) (Pierre et al. 2003). We would thus expect such change in behavior following previous patch experience to occur in species that could encounter several successing patches with differents values. Aphid parasitoids exploit aphid colonies that differ in size and composition and that are often clustered within an habitat.
Aphidius ervi Haliday (Hymenoptera: Braconidae) is a cosmopolitan hymenopteran that parasitizes a large range of aphid host species (Stilmant et al. 2008). This parasitoid demonstrates plasticity in its oviposition strategy in response to host species and immature stages both in its acceptance level and progeny allocation (He et al. 2011) and in its sex-ratio allocation (Wellings et al. 1986). A study exploring the learned component to host color morphs preference (Langley et al. 2006) showed that A. ervi exhibits an inherent preference for green aphid morphs of the pea aphid, Acyrthosiphon pisum (Harris), but this preference shows plasticity as initial encounters with red aphid morphs lead to a greater probability of subsequent orientation towards red aphids. Results of this study suggest that A. ervi can combine experience with further sampling information when taking foraging decisions.
We have recently shown that A. ervi performs poorly on lettuce aphids, Nasonovia ribisnigri Mosley (Hemiptera: Aphididae), reared on a partially aphid-resistant cultivar relative to an aphid-susceptible cultivar. When developing in aphids reared on partially resistant plants, fewer mummies were produced, adult parasitoids were smaller and females had a lower fecundity (Lanteigne et al. 2014). Aphids that developed on a partially aphid-resistant cultivar can thus be considered of lower quality than aphids that developed on a susceptible lettuce cultivar.
In this study, host plant resistance was used to generate aphids of different suitability. We investigated the host acceptance behaviour and sex allocation of A. ervi females when foraging on patches of high-quality hosts (HQ), reared on susceptible lettuce, on patches of low-quality hosts (LQ), reared on partially resistant plants and on patches of mixed-quality (MQ). Six treatments combining three-patches sequences were offered to a female parasitoid. We used the number of mummified aphids to estimate host acceptance by A. ervi. If no prior experience is needed by females A. ervi to estimate patch quality, we expect them to exploit the first patch in accordance to its intrinsic quality, i.e., high quality patches should be exploited more than low quality patches. When females base their patch evaluation on prior experience, we expect host acceptance and sex allocation to be similar in the first patch encountered, irrespective of its quality, but in subsequent patches oviposition decisions should vary according to the quality of the patches previously encountered, the females using their past experience to assess the quality of a given patch. Finally, a mixed model is also possible where the first patch encountered is exploited based on its quality and the subsequent patches are exploited differently depending on the quality of the previous patch.
Materials and Methods
Plant Genotypes
Complete and partial resistance to N. ribisnigri are conditioned in lettuce by two alleles: Nr (complete resistance) and nr (partial resistance) (McCreight and Liu 2012) known to cause antibiosis (van der Arend 2003) and antixenosis (Liu and McCreight 2006). The susceptible variety Lactuca sativa var. Estival (hereafter S lettuce), a heavy crisphead lettuce, does not possess the Nr gene (Jenni and Emery 2009) and was used to rear high-quality (HQ) aphids in our experiments. The wild lettuce L. serriola accession PI 491093 (hereafter PR lettuce) is partially resistant to N. ribisnigri and was used to produce low-quality (LQ) aphids. The partial resistance to lettuce aphid is due to a single dominant gene that appears to be identical to the gene for complete resistance in L. virosa accession IVT 273 and allelic to the gene for complete resistance in L. virosa accession IVT280, the source of resistance for all currently available aphid-resistant lettuce cultivars (McCreight and Liu 2012). Seeds were provided by the U.S. Department of Agriculture, Agricultural Research Service, Salinas, CA, USA.
Seedlings of S and PR lettuces were cultivated in climate-controlled rooms at 18 ± 2 °C, 50 ± 10 % r.h. with a 16L:8D photoperiod. Plants were watered daily, fertilized three times/week with 20N-8P-20K (0.75 g/l) and two times/week with 14N-0P-14K (1.0 g/l) while watering. Plant resistance to aphids tends to increase with age and the rate of this increase differs between varieties (van Emden and Bashford 1971). In order to minimize variation in plant resistance, all plants used were of a similar age (40–50 days old).
Aphids
Nasonovia ribisnigri colonies were established from individuals collected in Sainte-Clotilde (Québec, Canada) and maintained on susceptible lettuce plants at 17 ± 1 °C, 50 % ± 10 % r.h. and under a 14L:10D photoperiod.
To obtain cohorts of N. ribisnigri of specific age classes, about 60 apterous, parthenogenetic adult aphids were left in 2.5 cm diameter clip-on cages (five aphids per cage) on a lettuce plant of a given treatment for 12 h (Barrette et al. 2010). After 12 h, adults were removed and five offspring were left to develop until their third instar. Size (tibia length) and weight of aphids reared on PR lettuce are lower than for aphids reared on S lettuce, from the third and second instar onward, respectively (Lanteigne et al. 2014).
Parasitoids
Aphidius ervi is a cosmopolitan micro-hymenopteran that parasitizes a large range of aphid species (Stilmant et al. 2008). Parasitized aphids were purchased from Koppert Canada Ltd (Québec, Canada) and colonies were maintained on N. ribisnigri reared on S lettuce seedlings kept at 16 ± 1 °C, 50 % ± 10 % r.h. and a 18L:6D photoperiod.
Cohorts of A. ervi females were obtained by exposing second-instar aphids to female parasitoids (parasitoid:host ratio of 1:10) in Petri dishes (2.5 cm diameter) with an excised leaf disc of S lettuce. After a period of 4 h, the parasitoid was removed and aphids were placed in Petri dishes (12 cm diameter) with an excised leaf disc of S lettuce and moist filter paper and reared until parasitoid emergence. Newly-emerged parasitoids had access to dilute honey solution (20 %) and males and females were kept together in Petri dishes (12 cm diameter) to allow mating. Female parasitoids used in experiments were 36 ± 12 h-old, naive (no previous host experience) and randomly selected (Barrette et al. 2010).
Experimental Setup
To measure the level of patch exploitation, three patches of 20 third-instar aphids, confined within a Petri dish (2.5 cm diameter) with an excised leaf disc of S lettuce, were successively offered to a female parasitoid. Three types of patch were tested: patch of high-quality hosts (HQ), reared on susceptible lettuce, patch of low-quality hosts (LQ), reared on partially resistant plants and patch of mixed-quality hosts (MQ), containing ten HQ aphids and ten LQ aphids. Parafilm was used to seal the top of the Petri dishes. A density of 20 aphids per patch is commonly encountered on infested lettuce by N. ribisnigri (M.E. Lanteigne, personal observation). The parasitoid was constrained to remain in each patch for 2 h, allowing enough time for the female to exploit the patch. The parasitoid was gently transferred with an aspirator to the next patch within 30 s.
Six treatments were tested:
-
(i)
Three patches of 20 LQ aphids;
-
(ii)
Three patches of 20 HQ aphids;
-
(iii)
20 LQ aphids, 20 LQ aphids, 20 HQ aphids;
-
(iv)
20 HQ aphids, 20 HQ aphids, 20 LQ aphids;
-
(v)
20 HQ aphids, 20 MQ aphids, 20 HQ aphids;
-
(vi)
20 LQ aphids, 20 MQ aphids, 20 LQ aphids.
Sixteen replicates were performed per treatment. At the end of the experiment, the parasitoid was removed and aphids of a given patch were placed in Petri dishes (12 cm diameter) with an excised leaf disc of S lettuce on moist filter paper and reared under the above-mentioned conditions until parasitoid emergence. The number of mummified aphids and the offspring secondary sex ratio (number of males/total number of parasitoids) were determined. All experiments were performed at 20 ± 1 °C, 50 ± 10 % r.h., and with a 16L:8D photoperiod.
Statistical Analyses
To determine the ability of A. ervi females to estimate patch quality without prior experience, we compared the number of mummies produced in the first patch encountered when it was a HQ patch (treatments 2, 4 and 5) to those produced when it was a LQ patch (treatments 1, 3 and 6) using a Wilcoxon test (a non-parametric test was used as the data were not normally distributed). In order to pool the data from the treatments where the first patch encountered was a high quality patch (treatments 2, 4 and 5) or a low quality patch (treatments 1, 3 and 6), we verified, using a Kruskal-Wallis test, if there was a significant difference in the number of mummies between treatments. As no significant difference was found (HQ: KW = 5.85, P = 0.054; LQ: KW = 1.32, P = 0.517), the data were pooled. To determine if previous patch experience influenced the number of mummies produced in the subsequent patches, we compared for each treatment the total number of mummies produced in the first, second and third patches. We used a chi-square test with expected values set as 1/3-1/3-1/3 (the null hypothesis being that all patches were exploited equally) for treatment 1 and 2 where the three patches encountered had the same composition. For the other treatments, we used as expected values for HQ and LQ patches the average number of mummies produced when the first patch encountered was a HQ patch (treatments 2, 4 and 5) or a LQ patch (treatments 1, 3 and 6). For the medium quality patch (treatment 5 and 6), we used the mid-point between a high and a low quality patch. Sex ratios for each patch within a treatment were compared using a chi-square test. All statistical analyses were carried out using R software version 2.15.2 (R Development Core Team 2012).
Results
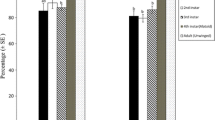
Different patterns of mummies produced were observed among treatments and the following main patterns can be identified. The number of mummies produced in the first patch encountered by A. ervi was significantly higher for HQ patches (X ± SD; 7.1 ± 5.0) than for LQ patches (3.8 ± 4.5) (W = 2270, P < 0.001). The average numbers of mummies produced in the second and third patch were not statistically different from the first patch in the second (HQ, HQ, HQ), third (LQ, LQ, HQ) and fourth (HQ, HQ, LQ) treatment (Fig. 1b, c, d). In the first treatment (LQ, LQ, LQ), the number of mummies was similar for the first two patches but significantly increased in the third patch (Fig. 1a). In treatments 5 and 6, where a MQ patch was offered in the middle of the sequence, the number of mummies produced decreased and increased when the MQ patch followed a HQ and LQ patch, respectively, but stayed constant in the third patch (Fig. 1e, f).
The secondary sex-ratios (Fig. 2) remained statistically constant for the three patches of the first (LQ, LQ, LQ), fourth (HQ, HQ, LQ), fifth (HQ, MQ, HQ) and sixth (LQ, MQ, LQ) treatment but decreased significantly in the second (HQ, HQ, HQ) and third (LQ, LQ, HQ) treatments (Fig. 2).
Discussion
Aphidius ervi females have the capacity to assess host suitability and patch quality without prior experience; the average number of mummified N. ribisnigri in the first host patch encountered was significantly higher for HQ patches (16.5 % more mummies) than for LQ patches. Naive females thus used their innate knowledge to discriminate between high and low quality aphids when foraging and adjusted their host exploitation accordingly. Aphids in HQ patches were larger than those from LQ patches (Lanteigne et al. 2014) and since larger aphids defend themselves more vigorously and increase handling time of parasitoids (Barrette et al. 2009), better defense from aphids in LQ patches is unlikely.
Our results suggest that foraging A. ervi females follow a mixed model and behave as if they use a Bayesian updating process as shown in Asobara tabida by Louâpre et al. (2011). The exploitation level of the second and third patches varied according to the experience gained in the previous patches. When females encountered three HQ patches (Treatment 2), the number of hosts attacked remained similar, suggesting that females had adjusted their patch exploitation based on their estimation of the quality of the first patch and, because patch quality remained constant from one patch to the next, they did not change their patch exploitation strategy. In contrast, when three patches of LQ were offered successively to the females, patch exploitation level increased in the third patch (Treatment 1) suggesting that females, that first allowed a reduced number of progeny due to the low quality of patches 1 and 2, increased their investment when encountering a third consecutive low quality patch. They may then have perceived the habitat as being homogeneous and of low quality. This pattern contrasts with another aphid parasitoid, Aphidius rhopalosiphi, whose females only take into account the last visited patch in their patch exploitation decision (Outreman et al. 2005). In a poor environment, the female reproductive success is likely to become time-limited as females could be unable to exploit enough suitable hosts in their life time (van Baaren et al. 2005). Accordingly, aphid parasitoids have been shown to adapt their patch exploitation strategy to the local host availability (Outreman et al. 2005). Aphidius ervi females thus changed their level of host exploitation in the third patch because ovipositing in low-quality hosts became the best strategy as their experience in previous patches showed that better-quality hosts were not available.
When A. ervi females experienced two patches of a given quality before being offered a different one (Treatments 3 and 4), no significant differences in the exploitation level was found (Fig. 1c and d). This suggests that females based their evaluation of habitat quality on several patches. When exploiting a highly variable habitat, it may be adaptive to respond with a delay to a perceived change in patch quality (Boivin et al. 2004).
This strategy is also supported by results from treatments 5 and 6. When female A. ervi were offered patches of MQ hosts, their level of exploitation increased when it followed a LQ patch and decreased when it followed a HQ patch. This was expected as MQ patches represent a higher quality than LQ patches and a lower quality than HQ patches. As the change in patch quality occurred on the second patch visited, the females modified their behavior immediately. However, the level of exploitation remained constant for the third patch, regardless whether it was a HQ or LQ patch. Aphidius ervi females thus seem to adjust their patch exploitation based on their experience on several patches, as would do a Bayesian forager. For a Bayesian forager, each sampling experience provides additional information, so that the estimate of patch quality gets closer to its real value as the number of samples increases (McNamara et al. 2006). As the patch exploitation did not increase for HQ or decrease for LQ in the third patch (Treatments 5 and 6), it suggests that when females experienced heterogeneity in patch quality, they adapted their exploitation behaviour accordingly, exploiting both HQ and LQ patches to a level corresponding to the perceived average quality of patches available in this habitat.
In treatment 2 (HQ, HQ, HQ) and 3 (LQ, LQ, HQ) offspring sex ratio decreased with the exploitation of the three patches, regardless of patch quality. Typically, in aphid parasitoids, relatively more males are allocated to small hosts than to large hosts (Wellings et al. 1986). Aphids reared on PR lettuce (LQ patch) are smaller than aphids reared on S lettuce (HQ patch) (Lanteigne et al. 2014). When a HQ patch was offered after two LQ patches (Treatment 3), a decrease in the sex-ratio of the progeny was expected, due to an increase in host quality. The observed tendency to a lower sex-ratio regardless of patch quality remains difficult to explain. It might be that inexperienced females underestimated patch quality therefore allocating a male biased sex-ratio in the first patch. Alternatively, females may use a precise sex allocation strategy and allocate more males when they start to exploit a new habitat (Hardy 1992). Precise sex allocation has been found in aphid parasitoids exploiting a patch (Mackauer and Volkl 2002). Our results may suggest that such a strategy can also occur between patches. Finally, male biased sex ratio at the beginning of the oviposition period could also be due to constrained sex allocation by the female, for example when oviposition starts too soon after mating (King 1987; Boivin 2013). However, this is unlikely as A. ervi males and females were kept together for 36 h before the experiment, allowing ample time for mating and for sperm to move to the female spermatheca.
In these experiments, we noted the number of mummies produced, not directly host acceptance. As immature mortality may occur, the number of mummies is a conservative estimate of the number of aphids accepted by the female parasitoid. However, the presence of immature mortality is not likely to affect the results when two similar patches that differ only by the previous experience of the female that exploited them are compared. Our results were obtained using females of standard condition (all of same age, mated, etc.) under laboratory condition (artificial patches, confinement in a petri dish) and the level of exploitation observed may not represent what occurs in nature. However the differences observed between treatments remain valid as all females experienced similar conditions in all treatments.
This parasitoid species thus uses its innate estimate of patch quality when exploiting a first patch, followed by relative estimate modulated through previous experience. Patch quality can vary both within a generation of parasitoids, due to changes in host quality (species, size, parasitization status) in a given habitat, and among generations, due to seasonal variations (Sequeira and MacKauer 1993). In such conditions, natural selection should select an innate estimate that is close to the average value of patch quality in the habitat. This strategy could be advantageous when there is a low variability between generations and a high variability within a generation (Boivin et al. 2004) and, as A. ervi can discriminate between HQ and LQ patches without previous experience, females could adequately exploit their first patch. This innate estimate could then be improved by the female through habitat sampling, thereby allowing the female to adjust patch exploitation. As the average size of aphids can change during the season (Sequeira and MacKauer 1993), females would change their strategy to the available host quality. Heterogeneity in patch quality could also impact the dispersal pattern of parasitoids (Outreman et al. 2005). Females that experienced several low quality patches could engage in more long-range dispersal than females that experienced high quality patches.
These results have implications in agro-ecosystems where both susceptible and resistant cultivars are present and where female parasitoids are likely to move from one field to the next. The presence of susceptible cultivars near resistant cultures could increase parasitism rate in the resistant field, as A. ervi increased their patch exploitation level when MQ patches were offered before LQ patches. These results can provide an avenue of interest for the utilisation of susceptible lettuce in fields of resistant plants.
References
Barrette M, Wu GM, Brodeur J, Giraldeau L-A, Boivin G (2009) Testing competing measures of profitability for mobile resources. Oecologia 158:757–764
Barrette M, Boivin G, Brodeur J, Giraldeau L-A (2010) Travel time affects optimal diets in depleting patches. Behav Ecol Sociobiol 64:593–598
Boivin G (2013) Sperm as a limiting factor in mating success in Hymenopteran parasitoids. Entomol Exp Appl 146:149–155
Boivin G, Fauvergue X, Wajnberg E (2004) Optimal patch residence time in egg parasitoids: innate versus learned estimate of patch quality. Oecologia 138:640–647
Eliassen E, Jorgensen C, Manger M, Giske J (2009) Quantifying the adaptive value of learning in foraging behaviour. Am Nat 174:478–489
Godfray HCJ (1994) Parasitoids: behavioral and evolutionary ecology. Princeton University Press, Princeton
Hardy ICW (1992) Non-binomial sex allocation and brood sex ratio variances in the parasitoid Hymenoptera. Oikos 65:143–158
He X, Wang Q, Teulon D (2011) Host age preference behavior in Aphidius ervi Haliday (Hymenoptera: Aphidiidae). J Insect Behav 24:447–455
Jenni S, Emery GC (2009) Estival lettuce. Can J Plant Sci 89:99–101
King BH (1987) Offspring sex ratios in parasitoid wasps. Q Rev Biol 62:367–396
Langley S, Tilmon K, Cardinale B, Ives A (2006) Learning by the parasitoid wasp, Aphidius ervi (Hymenoptera: Braconidae), alters individual fixed preferences for pea aphid color morphs. Oecologia 150:172–179
Lanteigne M, Brodeur J, Jenni S, Boivin G (2014) Partial aphid resistance in lettuce negatively affects parasitoids. Environ Entomol 43:1240–1246
Liu YB, McCreight JD (2006) Responses of Nasonovia ribisnigri (Homoptera: Aphididae) to susceptible and resistant lettuce. J Econ Entomol 99:972–978
Louâpre P, van Baaren J, Pierre JS, van Alphen JJM (2011) Information gleaned and former patch quality determine foraging behavior of parasitic wasps. Behav Ecol 22:1064–1069
Mackauer M, Volkl W (2002) Brood-size and sex-ratio variation in field populations of three species of solitary aphid parasitoids (Hymenoptera: Braconidae, Aphidiinae). Oecologia 131:296–305
McCreight JD, Liu YB (2012) Resistance to lettuce aphid (Nasonovia ribisnigri) biotype 0 in wild lettuce accessions PI 491093 and PI 274378. HortSci 47:179–184
McNamara JM, Houston AI (1985) Optimal foraging and learning. J Theor Biol 117:231–249
McNamara JM, Green RF, Olsson O (2006) Bayes’ theorem and its applications in animal behaviour. Oikos 112:243–251
Outreman Y, Le Ralec A, Wajnberg E, Pierre JS (2005) Effects of within- and among-patch experiences on the patch-leaving decision rules in an insect parasitoid. Behav Ecol Sociobiol 58:208–217
Pierre JS, van Baaren J, Boivin G (2003) Patch leaving decision rules in parasitoids: do they use sequential decisional sampling? Behav Ecol Sociobiol 54:147–155
R Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0, URL http://www.R-project.org/.
Sequeira R, MacKauer M (1993) Seasonal variation in body size and offspring sex ratio in field populations of the parasitoid wasp, Aphidius ervi (Hymenoptera: Aphidiidae). Oikos 68:340–346
Stilmant D, Van Bellinghen C, Hance T, Boivin G (2008) Host specialization in habitat specialists and generalists. Oecologia 156:905–912
Valone TJ (2006) Are animals capable of Bayesian updating? An empirical review. Oikos 112:252–259
van Baaren J, Boivin G, Outreman Y (2005) Patch exploitation strategy by an egg parasitoid in constant or variable environment. Ecol Entomol 30:502–509
van der Arend JM (2003) The possibility of Nasonovia ribisnigri resistance breaking biotype development due to plant host resistance: a literature study. In: van Hintum TJL, Lebeda A, Pink D, Schut JW (eds) Eucarpia leafy vegetables. CGN, Gravenzande, pp 75–81
van Emden HF, Bashford MA (1971) The performance of Brevicoryne brassicae and Myzus persicae in relation to plant age and leaf amino acids. Entomol Exp Appl 14:349–360
Vos M, Hemerik L, Vet LEM (1998) Patch exploitation by the parasitoids Cotesia rubecula and Cotesia glomerata in multi-patch environments with different host distributions. J Anim Ecol 67:774–783
Wellings PW, Morton R, Hart PJ (1986) Primary sex-ratio and differential progeny survivorship in solitary haplo-diploid parasitoids. Ecol Entomol 11:341–348
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lanteigne, ME., Brodeur, J., Jenni, S. et al. Patch Experience Changes Host Acceptance of the Aphid Parasitoid Aphidius ervi . J Insect Behav 28, 436–446 (2015). https://doi.org/10.1007/s10905-015-9515-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-015-9515-3