Abstract
Although many avian eggs appear to be cryptically colored, many species also lay vibrant blue green eggs. This seemingly conspicuous coloration has puzzled biologists since Wallace, as natural selection should favor reduced egg visibility to minimize predation pressure. The sexual signaling hypothesis posits that blue green egg coloration serves as a signal of female quality and that males exert post-mating sexual selection on this trait by investing more in the nests of females laying more intensely blue green eggs. This hypothesis has received mixed support to date, and most previous studies have been conducted in cavity-nesting species where male evaluation of his partner’s egg coloration, relative to that of other females, may be somewhat limited. In this study, we test the sexual signaling hypothesis in colonially nesting ring-billed gulls (Larus delawarensis) where males have ample opportunity to assess their mate’s egg coloration relative to that of other females. We used correlational data and an experimental manipulation to test four assumptions and predictions of the sexual signaling hypothesis: (1) blue green pigmentation should be limiting to females; (2) extent of blue green egg coloration should relate to female quality; (3) extent of blue green egg coloration should relate to offspring quality; and (4) males should provide more care to clutches with higher blue green chroma. Our data provide little support for these predictions of the sexual signaling hypothesis in ring-billed gulls. In light of this and other empirical data, we encourage future studies to consider additional hypotheses for the evolution of blue green egg coloration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The evolution of conspicuous traits, such as elaborate displays and vibrant colors, has long interested biologists and naturalists (Darwin 1871; Wallace 1889). While theoretical models and empirical studies have provided a satisfying explanation for the evolution of sexually selected ornaments (Andersson 1994), other exaggerated traits remain perplexing. One particularly bewildering example is that of conspicuous egg coloration. In several avian species, females lay eggs that are strikingly blue green in color (Underwood and Sealy 2002; Moreno and Osorno 2003; Kilner 2006). This blue green coloration is acquired through deposition of a blue green pigment called biliverdin into the eggshell (Kennedy and Vevers 1976).
For more than a century, researchers have sought adaptive explanations for the evolution of blue green egg coloration (Kilner 2006). A number of hypotheses have been proposed, including aposematism (Swynnerton 1916; Cott 1948), thermoregulation (McAldowie 1886; Bakken et al. 1978; Lahti 2008), egg recognition (Victoria 1972; Jackson 1992; Soler and Møller 1996), and crypsis (Lack 1958). Despite a substantial amount of research devoted to this topic, the adaptive significance of blue green egg coloration remains a matter of debate, as these hypotheses either remain inconclusive (Underwood and Sealy 2002; Kilner 2006) or have been largely discredited (Lack 1958; Kilner 2006). Moreover, a recent comparative analysis failed to yield new insight into the adaptive significance of blue green egg coloration despite addressing multiple hypotheses using a comprehensive dataset spanning all of Aves (Kilner 2006).
Recently, Moreno and Osorno (2003) proposed a novel hypothesis for the evolution of blue green egg coloration. They suggested that blue green egg pigmentation acts as a sexually selected, condition-dependent signal of female quality. Moreno and Osorno (2003) reasoned that since biliverdin has been shown to have antioxidant properties (Kaur et al. 2003), females should balance the use of biliverdin for protection against free radicals and for deposition into eggshells. The sexual signaling hypothesis proposes that only high-quality females can afford the costs of depositing large amounts of biliverdin during the laying period, a time of high oxidative stress. Males should in turn respond to this signal by increasing their investment in clutches with more deeply pigmented blue green eggs (Moreno and Osorno 2003). The intraspecific assumptions and predictions arising from this hypothesis can be divided into four main categories. First, blue green egg pigmentation should be limiting and costly to deposit. Second, degree of blue green egg pigmentation should relate to female quality. Third, degree of blue green egg pigmentation should relate to offspring quality. Fourth, males should exert post-mating sexual selection on this trait by providing greater paternal investment to nests with more intensely pigmented blue green eggs.
The sexual signaling hypothesis has been investigated in a number of species, but support for the hypothesis has been mixed. For example, a positive association between male parental investment and blue green egg coloration was documented in some studies (Moreno et al. 2004, 2006b; Soler et al. 2008) but not in others (Krist and Grim 2007; Lopez-Rull et al. 2007). These findings, among others, suggest that more research needs to be undertaken to assess the general applicability of the sexual signaling hypothesis.
In this study, we investigated the sexual signaling hypothesis in ring-billed gulls (Larus delawarensis). This species is well suited for addressing the sexual signaling hypothesis because both males and females care for offspring, and these birds usually nest in large, densely packed colonies that provide ample opportunity for direct comparison of egg color across females (Ryder 1993). Additionally, females lay variably colored eggs, with some females laying particularly blue green eggs and others laying eggs that are brownish in color. This degree of variation could, in theory, facilitate assessments of relative mate quality based on egg color. Interestingly, all tests of the sexual signaling hypothesis to date have been conducted in cavity nesters or species that defend all-purpose nesting territories. In nest cavities, low light conditions may reduce visibility and make egg coloration more difficult to assess (Aviles et al. 2006). In species that defend all-purpose nesting territories, including some cavity-nesting species, territorial intrusions may make egg color assessments relatively costly, and the distance between nests prevents males from making direct comparisons of egg color between females.
We tested the following four assumptions and predictions of the sexual signaling hypothesis using a combination of correlational and experimental data. (1) If blue green pigmentation is limiting, we expected that blue green chroma would decrease with laying order. We expected this pattern because egg laying is particularly energetically demanding in gulls (Ricklefs 1974) and because the level of a potent antioxidant is known to decrease over the laying period in a congener (Monaghan et al. 1998). (2) If blue green pigmentation signals female quality, an important assumption of the hypothesis, we expected a positive relationship between female health and condition and the blue green chroma of her eggs. (3) If blue green pigmentation signals offspring quality, we predicted that chicks hatched from eggs with higher blue green chroma would be larger than chicks hatched from less chromatic eggs. (4) If males exert post-mating sexual selection based on blue green egg coloration, we predicted that males mated with females who laid more chromatic eggs would invest more in those clutches.
Materials and methods
Study species and study site
From 1 May to 14 July 2007, we studied ring-billed gulls near Windermere Basin in Hamilton, Ontario (43°15′49.30″ N, 79°46′54.83″ W). The ring-billed gull is a largely monogamous, colonial species. Males and females cooperate in building nests on the ground in low, open areas. Males and females share nearly equally in incubation, brooding, and feeding young (Ryder 1993). Clutches are generally complete in 3–5 days, and incubation lasts 25 days (Ryder 1993). In our study, most clutches were initiated on 4 May 2007 (mode) and hatched on 31 May 2007 (mode). Supernormal clutches are known to occur in this species (Conover et al. 1979), and these would complicate our study because these result from multiple females laying eggs into a single nest or a male pairing with two females at a single nest. Previous work has shown that 98% of two to three egg clutches are from male–female pairings (Conover 1989). As a conservative means of excluding supernormal clutches, we restricted our analysis to clutches with three or fewer eggs. Therefore, our average clutch size for clutches with colorimetric data was 2.9 ± 0.5 (n = 81).
We captured adult gulls in circular walk-in wire mesh traps placed on nests 9.69 ± 2.6 days prior to egg hatching (see Brown 1995). For each individual captured, we recorded tarsal length, bill length, length of head from tip of bill to base of skull, length of the exposed culmen, depth at the gonys, and wing chord (to the nearest millimeter) as well as mass (to the nearest gram). We obtained blood from adult birds by puncturing the brachial vein with a 26 1/2-gauge needle and drawing up a small amount of blood using a heparinized capillary tube. This blood was used to calculate heterophil to lymphocyte ratio in females (see below). We used a standard discriminant function (Ryder 1978) to determine sex upon first capture. This discriminant function is based on morphometric measurements and has a validated accuracy of 95.0%. Since the male is always larger than his female partner (Ryder 1993), we were able to confirm these classifications based on morphometric measurements when we caught both members of a mated pair. In addition, we confirmed these sex classifications based on visual size comparisons and behavioral observations. To facilitate visual identification of individual birds during behavioral observations, we applied unique combinations of colored leg bands as well as Nyanzol dye markings on the head or wings.
Egg color quantification
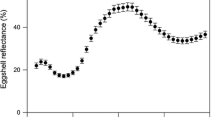
Female ring-billed gulls lay eggs that range from deep brown to deep olive green or paler blue green in ground coloration, with a variable amount of dark brown maculation (Fig. 1; Ryder 1993; Baicich and Harrison 1997). These eggs are visually similar in coloration to those of herring gulls (Larus argentatus) and black-headed gulls (Larus ridibundus), the ground coloration of which is known to result from a combination of protoporphyrin and biliverdin pigmentation (Kennedy and Vevers 1976). We quantified the coloration of ring-billed gull eggs using a USB 4000 spectrophotometer with a PX-2 pulsed xenon light source and a Spectralon white standard (Ocean Optics, Dunedin, FL, USA). For each egg, we measured reflectance on three different regions of the egg: the lower portion, the medial portion, and the upper portion. We took two measurements within each region, each of which comprised 30 readings averaged by the spectrophotometer operating software (OOIBase32), and used the mean of these readings in our analyses since colorimetric variables were highly repeatable within eggs (see below). Because maculation likely results entirely from protoporphyrin pigmentation (Kennedy and Vevers 1976), we only measured patches of ground coloration free of maculation, as blue green pigmentation was a focus of our study. Visual inspection of reflectance spectra revealed that as with other gulls (Kennedy and Vevers 1976), the ground coloration of ring-billed gull eggs is likely produced by a combination of biliverdin and porphyrin pigmentation. Most spectra had a series of long-wavelength peaks and troughs, as expected from the absorbance properties of protoporphyrin pigmentation (Scalise and Durantini 2004), and greenish eggs exhibited proportionally greater reflectance in the blue green portion of the spectrum, as expected from patterns of biliverdin absorbance (Fig. 1; Ding and Xu 2002; Falchuk et al. 2002).
Reflectance spectra of ring-billed gull eggs revealing extensive variation in egg color. Shown are the mean across all eggs sampled at Windermere Basin, Hamilton, Ontario, Canada in 2007 (n = 267; solid line), a blue green egg (dashed line), and a brownish egg (dotted line). The shapes of these spectra result from the combination of blue green biliverdin and brown porphyrin pigmentation (Ding and Xu 2002; Falchuk et al. 2002; Scalise and Durantini 2004)
We summarized variation in egg color using two colorimetric variables (Montgomerie 2006). We calculated blue green chroma as the proportion of reflectance in the blue green portion of the spectrum (450–550 nm). Similarly, we calculated red chroma as the proportion of reflectance in the red (600–700 nm) portion of the spectrum. We chose narrow ranges for these two variables to encompass the maximum reflectance generated by biliverdin (Ding and Xu 2002) and porphyrin (Scalise and Durantini 2004) pigmentation. Since pigment deposition has a subtractive influence on reflectance, it is unlikely to mask the independent effects of other pigments unless it absorbs strongly across all wavelengths. In addition, average clutch blue green and red chroma were not correlated (r = −0.12, n = 80, p = 0.28, CI0.95 = −0.33 to 0.10), suggesting that these two variables revealed different information about egg coloration. We did not include other colorimetric variables, such as hue, brightness, and other measures of chroma, as these tended to be correlated with either blue green or red chroma and were therefore redundant (all p < 0.0001 for either blue green or red chroma). Blue green and red chroma were highly repeatable across the different parts of each egg (blue green chroma: r = 0.84, p < 0.0001; red chroma: r = 0.71, p < 0.0001; Lessells and Boag 1987) and we therefore used an average value for each egg in our analyses. Based on a subset of 25 eggs measured at two different times, our measurements blue green and red chroma were very highly repeatable (0.97 and 0.94, respectively, both p < 0.0001; Lessells and Boag 1987).
In most of our analyses, we used the mean coloration of each female’s entire clutch. To ensure that averaging egg coloration within clutches was reasonable, we calculated the repeatability of egg coloration within clutches (Lessells and Boag 1987). If egg coloration reveals female quality, coloration should be repeatable within clutches (Moreno et al. 2004; Krist and Grim 2007). Red and blue green chroma were significantly repeatable within clutches (repeatabilities, 0.53 and 0.64 respectively, both p < 0.0001), indicating that egg coloration was more variable among than within clutches. This interclutch variation in egg coloration is striking to humans (personal observation) and is presumably detectable by the refined color discrimination abilities of birds (Cuthill 2006).
Assessing laying order effects
To determine whether blue green egg pigmentation might be limiting to females, we compared egg coloration to position in the laying sequence while controlling for nest identification (ID). We monitored laying order by marking the blunt end of each egg with an indelible marker. In most cases, the egg was marked on the day it was laid with its number in the sequence. We used only eggs whose positions in the laying sequence were known in our analyses of laying order effects.
Assessing female and offspring quality
As a measure of female quality, we calculated the body condition of each female as size-adjusted body mass using the following equation: mass/(tarsus length + bill length; Kitaysky et al. 1999; Verboven et al. 2003; Buck et al. 2007). We used tarsus and bill length as measures of structural size since; unlike wing length, these remain constant over the breeding season (Kitaysky et al. 1999). Similar measures of female condition have been shown to relate to immunocompetence, reproductive success, and offspring quality in this (Boersma and Ryder 1983; Meathrel and Ryder 1987) and other gull species (Alonso-Alvarez and Tella 2001; Verboven et al. 2003). Additionally, we calculated heterophil to lymphocyte ratio as a measure of immune stress in females (Davis 2005). We stained blood smears created in the field using a Hema 3 staining kit (Fisher Scientific) and viewed these under oil immersion at 1,000× magnification. We counted the numbers of heterophils and lymphocytes were until approximately 10,000 red blood cells had been viewed to obtain a heterophil to lymphocyte ratio (H/L ratio). Heterophils are phagocytosing cells of the innate immune system, and lymphocytes consist primarily of T and B cells of the acquired immune system (Norris and Evans 2000). In birds, H/L ratio tends to increase in response to stressors such as disease, parasites, social stress, and starvation (Ots and Hõrak 1996) and represents an integrated measure of immune stress (Salvante 2006).
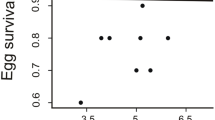
We calculated two related measures of offspring quality. First, we calculated the fresh egg mass of each egg using Hoyt’s (1979) formula (W = K w·LB 2). We measured the length (L) and breadth (B) of each egg on the day its color was measured and used the shape-dependent constant calculated by Hoyt (1979) for western gulls (Larus occidentalis livens); K w = 0.53, as the shape of their eggs, closely approximates that of ring-billed gull eggs. Egg size has been shown to relate to offspring quality and survival in many species (Grant 1991; Hipfner and Gaston 1999), including gulls (Parsons 1970; Lundberg and Väisänen 1979), even when controlling for parental quality (Bolton 1991). Second, we weighed chicks within several hours of hatching as an additional measure of offspring quality. Our sample size is more limited for this analysis as the risk of nest abandonment prohibited our obtaining more complete hatchling weight data. Chick mass has also been shown to relate to health and survival in a number of species (e.g., Moss et al. 1981; Grant 1991).
Assessing male investment and experimental manipulation
To investigate whether egg coloration influenced paternal care, we monitored male investment in relation to egg color at control nests and cross-fostered nests in the same colony. Our control nests consisted of 40 unmanipulated nests. However, any apparent influence of egg coloration on male investment in these control nests could result as a by-product of males responding to another female trait that is correlated with egg color or as a consequence of assortative mating between high-quality females that lay intensely colored blue green eggs and high-quality males that provide high levels of parental care. Therefore, in our experimental treatment, we conducted full clutch swaps for 15 pairs of nests on the day the third egg was laid. This ensured that any correlation between egg color and male care would be driven by the egg color per se. We chose this experimental design because we wanted to assess male responses to real eggs that exhibited natural variation in coloration. Although some studies have used artificial eggs or painted eggs, it is often difficult to mimic the appropriate spectral shape of egg pigments using these techniques, especially in the ultraviolet range. We assume that our experimental manipulation presented males with differently colored eggs because original egg color was not correlated with cross-fostered egg color for either blue green (r = −0.27, n = 11, p = 0.43, CI0.95 = −0.79 to 0.47) or red chroma (r = 0.25, n = 11, p = 0.45, CI0.95 = −0.49 to 0.78). Only 12 of the 30 fully swapped nests and 15 of the 40 controls survived to hatching, were visible for observation after hatching, or were not excluded as supernormal clutches. At one of the cross-fostered nests, the original eggs were depredated at their new location before we had the opportunity to measure their color.
To determine degree of paternal investment, two observers performed 30-min observation bouts on focal nests from an observation blind constructed in a central location within the colony. In addition to provisioning offspring, which represents direct investment in parental care, males may also invest in offspring indirectly. We therefore recorded nestling feeding visits, length of brooding bouts, threats towards neighbors (direct lunge at a neighbor), and long call rate as indicators of male parental investment. Long calling, which is characterized by a gull lowering its head and rapidly throwing it back to shoulder level while calling, is a known threat display and is also used in pair formation (Ryder 1993). We only included provisioning visits in our analyses if chicks ingested food. We standardized investment rates by the number of chicks in each nest. Parental feeding rate is known to decrease during the nestling period (Ryder 1993), and we therefore focused our observations on the first 11 days after hatching to minimize this effect (3.97 ± 1.56 observations per nest, range between 2 and 7). We also tested for relationships between nestling age and paternal investment within this age class. When controlling for nest ID, hatchling age was not predictive of male feeding rates (F27,75 = 1.22, R 2 = 0.30, p = 0.25; hatchling age: p = 0.41), brooding lengths (F27,75 = 1.03, R 2 = 0.27, p = 0.45; hatchling age: p = 0.03), or threatening rates (F27,75 = 3.52, R 2 = 0.56, p < 0.0001; hatchling age: p = 0.11). Male long call rate did significantly increase with nestling age (F27,75 = 4.12, R 2 = 0.60, p < 0.0001; hatchling age: p = 0.0002), and we therefore used the residuals of this regression in our analyses. We averaged these measures of investment recorded over multiple observations within each nest for our analyses. Since male effort may depend on the effort provided by his partner, we also considered proportional male investment. We found that proportional male care did not change with hatchling age for any investment variable (all p > 0.26), and we therefore averaged proportional effort recorded over multiple observations within each nest for our analyses. Our experimental manipulation did not appear to unduly affect male behavior, since there was no overall difference between control and cross-fostered nests in terms of male provisioning (F1,25 = 1.55, R 2 = 0.06, p = 0.22, d = 0.48, CI0.95 = −0.31 to 1.28), male threatening at the nest (F1,25 = 0.74, R 2 = 0.03, p = 0.40, d = −0.33, CI0.95 = −1.12 to 0.46), male long call rate (F1,25 = 3.17, R 2 = 0.11. p = 0.09, d = −.69. CI0.95 = −1.50 to 0.12), or male brooding length (F1,25 = 2.79, R 2 = 0.10, p = 0.11, d = −0.65, CI0.95 = −1.45 to 0.16).
Statistical analyses
We used transformations to normalize data where necessary. We used generalized linear models with nest ID as a random factor to assess the relationship between colorimetric variables and laying order or offspring quality. We used simple correlations to assess the relationship between our measures of female quality and average clutch coloration. Similarly, we used correlations to determine the association between chroma variables and paternal investment in control nests. For treatment nests, we used multiple regression analyses with original and cross-fostered chromas as predictor variables and measures of paternal investment as dependent variables. Some sample sizes vary because we were unable to obtain all measurements for all individuals or eggs included in this study.
We present standardized measures of effect size and the confidence intervals (CI) around those measures, where possible, to facilitate the interpretation of non-significant results in our study (Nakagawa and Cuthill 2007). Standardized effect sizes estimate the degree to which the null hypothesis is likely to be false (Cohen 1988; Nakagawa and Foster 2004). Presentation of confidence intervals around the effect size is particularly useful for the interpretation of non-significant results (Colegrave and Ruxton 2003; Nakagawa and Foster 2004). Small effect sizes with corresponding CIs that encompass zero provide support for the null hypothesis, indicating no real effect or a trivial effect if the null hypothesis is false. Standardized effect sizes can also be used to compare studies despite variation in sample sizes and are useful for meta-analyses (Nakagawa 2004; Nakagawa and Cuthill 2007). The presentation of standardized effect sizes and CIs is preferable to reporting retrospective power analyses which are biased, logically flawed (Nakagawa and Foster 2004), and provide no more information than p values (Colegrave and Ruxton 2003).
Results
Biliverdin as a limiting factor
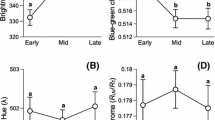
If blue green egg pigmentation is limiting in this species, we expected to see a decline in blue green egg chroma with laying order. When controlling for nest ID, we found a relationship between the level of blue green egg chroma and position in the laying order: The second egg had higher blue green chroma than the other eggs (Fig. 2; whole model: F 62,84 = 5.62, R 2 = 0.81, p < 0.0001; nest: p < 0.0001; laying order: p = 0.003). In a similar model, laying order did not predict red chroma (laying order: p = 0.08).
Egg coloration as a signal of female quality
If blue green egg coloration evolved as a signal of female quality, an important assumption of the sexual signaling hypothesis, it should correlate with female quality. We used female body condition index as a measure of female quality and H/L ratio as a measure of female immune stress. We found no association between female body condition index and either colorimetric variable (blue green chroma: r = −0.19, n = 24, p = 0.38, CI0.95 = −0.55 to 0.23; red chroma: r = 0.04, n = 24, p = 0.84, CI0.95 = −0.37 to 0.44). Similarly, we found no relationship between female H/L ratio and either colorimetric variable (blue green chroma: r = 0.03, n = 22, p = 0.88, CI0.95 = −0.39 to 0.44; red chroma: r = 0.05, n = 22, p = 0.82, CI0.95 = −0.36 to 0.45).
Egg coloration as a signal of offspring quality
According to our third prediction, blue green egg coloration should indirectly signal offspring quality, as investing in offspring of higher quality is the presumed benefit of increased male investment in more chromatic clutches. Neither blue green chroma nor red chroma were significant predictors of fresh egg mass when controlling for nest ID (Table 1). In a similar model, red chroma, but not blue green chroma, was a significant predictor of initial chick mass, such that larger chicks hatched from eggs that had higher red chroma (Table 1).
Paternal investment
According to our fourth prediction, males should invest more in clutches with more chromatic blue green eggs. We addressed this prediction using both correlational and experimental data. In a group of unmanipulated (control) nests, we found that neither blue green nor red chroma were correlated with male investment in long call rate, feeding rate, neighbor threatening rate, and brooding length (all p > 0.58 and 0.36, respectively). However, in control nests, male response to egg coloration could be confounded by other variables (see “Materials and methods”). Therefore, we used an experimental manipulation to assess male parental care in relation to cross-fostered eggs. In generalized linear models and regression analyses, neither original nor cross-fostered egg color significantly predicted measures of paternal investment (Table 2; Fig. 3). We also assessed proportional male investment, relative to the total investment provided by both parents, in relation to egg coloration in control and cross-fostered nests. We found that in control nests, only proportional male feeding rate was significantly correlated with blue green egg chroma (r = 0.56, n = 15, CI0.95 = 0.01 to 0.85, p = 0.03; all other variables p > 0.14 for blue green chroma and all p > 0.60 for red chroma). Using similar models, we found no measure of proportional male investment related to either original or cross-fostered blue green or red chroma (all p > 0.13 and p > 0.33, respectively) in cross-fostered nests.
The relationship between egg coloration and male feeding rates in ring-billed gulls. Data show male feeding rates in relation to the original blue green (a) and red chroma (c) laid by his mate and the blue green (b) and red chroma (d) we subsequently cross-fostered into his nest. Univariate data are shown; see Table 2 for multivariate analyses
Discussion
In this study, we evaluated whether the sexual signaling hypothesis might explain egg color variation in ring-billed gulls. We tested four assumptions and predictions of this hypothesis: that blue green egg chroma would decrease over the laying period, that female health and condition would be positively correlated with the blue green chroma of her eggs, that more chromatic blue green eggs would be larger and would produce larger chicks, and that males would preferentially invest in clutches with more chromatic blue green eggs. We found little support for these predictions and therefore conclude that the sexual signaling hypothesis is unlikely to explain variation in blue green egg coloration in ring-billed gulls.
A key assumption of the sexual signaling hypothesis is that blue green egg pigmentation honestly reveals female quality and should therefore be limiting to females, such that only high-quality females can afford the cost of biliverdin deposition (Moreno and Osorno 2003). If biliverdin is limiting, we expected a negative relationship between blue green egg chroma and position in the laying order. Position in the laying order did influence blue green chroma; however, the direction of the effect was rather ambiguous, with the second egg being more chromatic. We expected a negative relationship because of the comparatively high oxidative and energetic costs of egg laying in gulls (Ricklefs 1974; Monaghan et al. 1998) and because levels of a potent antioxidant decrease across the laying period in a congener (Royle et al. 2001). Under these stressful conditions, antioxidant limitation could be manifested as a decreased ability to deposit the pigment as the laying sequence progresses (Moreno and Osorno 2003). Alternatively, one could argue that the sexual signaling hypothesis should favor homogeneous pigment deposition across the clutch. Although egg coloration was more similar within clutches than between clutches, our analysis shows that pigment deposition was not homogeneous across laying order. Three other studies have documented laying order effects on blue green egg coloration, including an increase in blue green chroma (Siefferman et al. 2006), a decrease in egg brightness (Moreno et al. 2005), and a nonlinear decrease in blue green egg chroma (Krist and Grim 2007). Taken together, these studies suggest that there is no generalized relationship between laying order and blue green egg pigmentation across species. Interestingly, one recent study found little difference between biliverdin levels in serum and excreta for hens laying blue-shelled and brown-shelled eggs; however, biliverdin levels differed significantly in the shell gland for these same females, suggesting that the biliverdin used in eggshell pigmentation is synthesized directly in the shell gland and that it may be largely independent of circulating levels of biliverdin (Zhao et al. 2006). Physiological studies assessing whether biliverdin is limiting to female birds during egg laying would provide a stronger test of this prediction.
Another assumption of the sexual signaling hypothesis is that blue green egg chroma signals female quality and, more specifically, female antioxidant capacity (Moreno and Osorno 2003). In this study, there was no significant association between the blue green chroma of a female’s eggs and her body condition index. We also found no association between female H/L ratio and average clutch blue green eggshell coloration. It would be prudent to consider other measures of quality before ruling out a link between female condition and egg color in ring-billed gulls. Several studies have supported an association between female quality and egg color. For example, blue green egg coloration was found to correlate with age, condition, or immunocompetence in a number of species (Moreno et al. 2005; Morales et al. 2006; Siefferman et al. 2006; Krist and Grim 2007), and two experimental studies have shown that manipulating female condition affects egg color (Moreno et al. 2006a; Soler et al. 2008). Despite negative results presented here and elsewhere (Moreno et al. 2004, 2005; Cassey et al. 2008), this is currently the most well-supported assumption of the sexual signaling hypothesis. Nevertheless, experimental manipulations of female antioxidant capacity or oxidative stress, and its resulting effect on egg pigmentation, would present stronger direct tests of this assumption. Moreover, it is important to recognize that other proposed functions of egg color could yield positive associations between female quality and color even if the color does not function as a signal directed at males (Bakken et al. 1978; Gosler et al. 2005; Higham and Gosler 2006; Martinez-de la Puente et al. 2007).
Under the sexual signaling hypothesis, males should invest more in clutches laid by females of higher quality, as revealed by their egg coloration, because higher quality females should produce higher quality offspring (Moreno and Osorno 2003). We did not find evidence that blue green chroma significantly predicted egg mass or nestling mass in ring-billed gulls. Nevertheless, an association between offspring quality and eggshell coloration in itself is not sufficient to broadly support the sexual signaling hypothesis, since these pigments may directly benefit the developing embryo without necessarily serving as a signal (Cassey et al. 2008). Studies of the relationship between offspring quality and blue green egg color in other species have yielded mixed results, and it is difficult to draw general conclusions since different authors tend to use different quality and egg color measures. For example, Krist and Grim (2007) found a relationship between blue green egg chroma and nestling tarsus length, but not mass or T-cell-mediated immunity. Moreno et al. (2005) found that nestlings had higher immunoglobulin levels, controlling for ectoparasites, when they hatched from eggs that were shifted away from blue green coloration. Morales et al. (2006) found that blue green egg chroma was positively associated with egg immunoglobulin levels. Most recently, Soler et al. (2008) found that nestlings supplemented with food showed a negative relationship between T-cell-mediated immunity and blue green egg chroma, whereas unsupplemented nestlings exhibited a positive relationship between the same two variables. Neither Siefferman et al. (2006) nor Lopez-Rull et al. (2007) found a relationship between egg coloration and egg characteristics. These findings suggest that this prediction of the sexual signaling hypothesis might also benefit from further experimental testing.
Our study also tested the prediction that males should provide a disproportionate amount of care to clutches with more chromatic blue green coloration. We tested this key prediction using correlational data and a cross-fostering experiment. Males did not provide greater parental investment to clutches with more chromatic blue green eggs in control clutches or experimentally cross-fostered clutches. In addition, male investment did not correlate with original egg coloration. When assessing proportional male investment, we found a positive relationship between blue green chroma and male feeding rate in control clutches, but not in experimental clutches. No other proportional male investment variables were correlated with either color variable. Our data suggest that male ring-billed gulls did not preferentially invest in more chromatic blue green clutches. In pied flycatchers, Ficedula hypoleuca, males provided more provisioning to clutches with greater average blue green clutch coloration (Moreno et al. 2004). A subsequent cross-fostering experiment in this species revealed that males did not adjust provisioning rate in response to average clutch color but rather adjusted proportional provisioning rate in response to the standard deviation of egg chroma and maximum egg chroma within a clutch (Moreno et al. 2006b). In the only study where egg coloration was experimentally manipulated, male spotless starlings, Sturnus unicolor, provided more care to artificial eggs painted a dark blue green than to artificial eggs painted pale blue green (Soler et al. 2008). By contrast, a recent experimental study found that males did not provide higher provisioning to more chromatic clutches in collared flycatchers, Ficedula albicolis (Krist and Grim 2007). Another spotless starling study did not support this prediction and showed that males instead used feather ornaments to assess female quality and provided less care to clutches with more chromatic blue green eggs (Lopez-Rull et al. 2007).
Using a combination of correlational and experimental data, we found that blue green egg coloration did not decrease with laying order, did not correlate with female or offspring quality, and did not influence parental investment by males. Taken together, our findings suggest that the sexual signaling hypothesis is unlikely to explain variation in blue green egg pigmentation in ring-billed gulls. Some of the analyses in our study were based on small sample sizes; however, most of the relationships did not suggest trends in the predicted direction and had low effect sizes with confidence intervals overlapping zero. Although further testing may be required before this hypothesis can be convincingly ruled out in ring-billed gulls, we suggest that other selective factors, such as egg recognition (Victoria 1972; Soler and Møller 1996; Lahti 2005) and crypsis (Lack 1958; Sánchez et al. 2004; Šálek and Cepáková 2006), are likely to play a more important role in explaining egg color variation in this species. Indeed, blue green egg coloration may have evolved in different avian lineages for different reasons (Kilner 2006). Since the sexual signaling hypothesis continues to receive mixed support in various species, future studies should continue to consider multiple hypotheses for the evolution of egg coloration in birds.
References
Alonso-Alvarez C, Tella JL (2001) Effects of experimental food restriction and body-mass changes on the avian T-cell-mediated immune response. Can J Zool 79:101–105
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, NJ
Aviles JM, Soler JJ, Perez-Contreras T (2006) Dark nests and egg colour in birds: a possible functional role of ultraviolet reflectance in egg detectability. Proc R Soc Lond B 273:2821–2829
Baicich PJ, Harrison CJO (1997) A guide to the nests, eggs, and nestlings of North American birds, 2ndnd edn. Academic, San Diego
Bakken GS, Vanderbilt VC, Buttermer WA, Dawson WR (1978) Avian eggs: thermoregulatory value of very high near-infrared reflectance. Science 200:321–323
Boersma D, Ryder JP (1983) Reproductive performance and body condition of earlier and later nesting ring-billed gulls. J Field Ornith 54:374–380
Bolton M (1991) Determinants of chick survival in the lesser black-backed gull—relative contributions of egg size and parental quality. J Anim Ecol 60:949–960
Brown KM (1995) Does blood-sampling ring-billed gulls increase parental desertion and chick mortality. Colon Waterbird 18:102–104
Buck CL, O'Reilly KA, Kildaw SD (2007) Interannual variability of black-legged kittiwake productivity is reflected in baseline plasma corticosterone. Gen Comp Endocrinol 150:430–436
Cassey P, Ewen JG, Blackburn TM, Hauber ME, Vorobyev M, Marshall NJ (2008) Eggshell colour does not predict measures of maternal investment in eggs of Turdus thrushes. Naturwissenschaften 95:713–721
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Erlbaum, New York
Colegrave N, Ruxton GD (2003) Confidence intervals are a more useful complement to nonsignificant tests than are power calculations. Behav Ecol 14:446–450
Conover MR (1989) Parental care by male–female and female–female pairs of ring-billed gulls. Colon Waterbird 2:148–151
Conover MR, Miller DE, Hunt GL (1979) Female–female pairs and other unusual reproductive associations in ring-billed and California gulls. Auk 96:6–9
Cott HB (1948) Edibility of the eggs of birds. Nature 161:8–11
Cuthill IC (2006) Color perception. In: Hill GE, McGraw KJ (eds) Bird coloration, vol I. Harvard University Press, Cambridge, MA, pp 3–40
Darwin C (1871) The descent of man, and selection in relation to sex. Murray, London
Davis AK (2005) Effect of handling time and repeated sampling on avian white blood cell counts. J Field Ornith 76:334–338
Ding ZK, Xu YQ (2002) Purification and characterization of biliverdin IXα from Atlantic salmon (Salmo salar) bile. Biochemistry (Moscow) 67:927–932
Falchuk KH, Contin JM, Dziedzic TS, Feng Z, French TC, Heffron GJ, Montorzi M (2002) A role for biliverdin IXα in dorsal axis development of Xenopus laevis embryos. PNAS 99:251–256
Gosler AG, Higham JP, Reynolds SJ (2005) Why are birds’ eggs speckled? Ecol Lett 8:1105–1113
Grant MC (1991) Relationships between egg size, chick size at hatching, and chick survival in the whimbrel Numenius phaeopus. Ibis 133:127–133
Higham JP, Gosler AG (2006) Speckled eggs: water-loss and incubation behaviour in the great tit Parus major. Oecologia 149:561–570
Hipfner JM, Gaston AJ (1999) The relationship between egg size and posthatching development in the thick-billed murre. Ecology 80:1289–1297
Hoyt DF (1979) Practical methods of estimating volume and fresh weight of bird eggs. Auk 96:73–77
Jackson WM (1992) Estimating conspecific nest parasitism in the northern masked weaver based on within-female variability in egg appearance. Auk 109:435–443
Kaur H, Hughes MN, Green CJ, Naughton P, Foresti R, Motterlini R (2003) Interaction of bilirubin and biliverdin with reactive nitrogen species. FEBS Lett 543:113–119
Kennedy GY, Vevers HG (1976) A survey of avian eggshell pigments. Comp Biochem Physiol B 55B:117–123
Kilner RM (2006) The evolution of egg colour and patterning in birds. Biol Rev Camb Philos Soc 81:383–406
Kitaysky AS, Wingfield JC, Piatt JF (1999) Dynamics of food availability, body condition and physiological stress response in breeding black-legged kittiwakes. Funct Ecol 13:577–584
Krist M, Grim T (2007) Are blue eggs a sexually selected signal of female collared flycatchers? A cross-fostering experiment. Behav Ecol Sociobiol 61:863–876
Lack D (1958) The significance of the colour of Turdine eggs. Ibis 100:145–166
Lahti DC (2005) Evolution of bird eggs in the absence of cuckoo parasitism. Proc Natl Acad Sci U S A 102:18057–18062
Lahti DC (2008) Population differentiation and rapid evolution of egg color in accordance with solar radiation. Auk 125:796–802
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities—a common mistake. Auk 104:116–121
Lopez-Rull I, Celis P, Gil D (2007) Egg colour covaries with female expression of a male ornament in the spotless starling (Sturnus unicolor). Ethology 113:926–933
Lundberg CA, Väisänen RA (1979) Selective correlation of egg size with chick mortality in the black-headed gull (Larus ridibundus). Condor 81:146–156
Martinez-de la Puente J, Merino S, Moreno J, Tomas G, Morales J, Lobato E, Garcia-Fraile S, Martinez J (2007) Are eggshell spottiness and colour indicators of health and condition in blue tits Cyanistes caeruleus? J Avian Biol 38:377–384
McAldowie AM (1886) Observations on the development and the decay of the pigment layer on birds’ eggs. J Anat Physiol 20:225–237
Meathrel CE, Ryder JP (1987) Intraclutch variation in the size, mass and composition of ring-billed gull eggs. Condor 89:364–368
Monaghan P, Nager RG, Houston DC (1998) The price of eggs: increased investment in egg production reduces the offspring rearing capacity of parents. Proc R Soc Lond B 265:1731–1735
Montgomerie R (2006) Analyzing colors. In: Hill GE, McGraw KJ (eds) Bird coloration, vol I. Harvard University Press, Cambridge, MA, pp 90–148
Morales J, Sanz JJ, Moreno J (2006) Egg colour reflects the amount of yolk maternal antibodies and fledging success in a songbird. Biol Lett 2:334–336
Moreno J, Osorno JL (2003) Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecol Lett 6:803–806
Moreno J, Osorno JL, Morales J, Merino S, Tomas G (2004) Egg colouration and male parental effort in the pied flycatcher Ficedula hypoleuca. J Avian Biol 35:300–304
Moreno J, Morales J, Lobato E, Merino S, Tomas G, Martinez-de la Puente J (2005) Evidence for the signaling function of egg color in the pied flycatcher Ficedula hypoleuca. Behav Ecol 16:931–937
Moreno J, Lobato E, Morales J, Merino S, Tomás G, de la Puente JM, Sanz JJ, Mateo R, Soler JJ (2006a) Experimental evidence that egg color indicates female condition at laying in a songbird. Behav Ecol 17:651–655
Moreno J, Morales J, Lobato E, Tomás G, de la Puente JM (2006b) More colorful eggs induce a higher relative paternal investment in the pied flycatcher Ficedula hypoleuca: a cross-fostering experiment. J Avian Biol 37:555–560
Moss R, Watson A, Rothery P, Glennie WW (1981) Clutch size, egg size, hatch weight and laying date in relation to early mortality in red grouse Lagopus lagopus scoticus chicks. Ibis 123:450–462
Nakagawa S (2004) A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol 15:1044–1045
Nakagawa S, Foster MT (2004) The case against retrospective statistical power analyses with an introduction to power analysis. Acta Ethol 7:103–108
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605
Norris K, Evans MR (2000) Ecological immunology: life history trade-offs and immune defense in birds. Behav Ecol 11:19–26
Ots I, Hõrak P (1996) Great tits Parus major trade health for reproduction. Proc R Soc Lond B 263:1443–1447
Parsons J (1970) Relationship between egg size and post-hatching chick mortality in herring gull (Larus argentatus). Nature 228:1221–1222
Ricklefs RE (1974) Energetics of reproduction. In: Paynter RA (ed) Avian energetics, vol 15. Nuttall Ornithological Club, Cambridge, pp 152–297
Royle NJ, Surai PF, Hartley IR (2001) Maternally derived androgens and antioxidants in bird eggs: complementary but opposing effects? Behav Ecol 12:381–385
Ryder JP (1978) Sexing ring-billed gulls externally. Bird-Banding 49:218–222
Ryder JP (1993) Ring-billed gull. In: Poole A, Stettenheim P, Gill F (eds) The birds of North America. The National Academy of Sciences, Philadelphia
Šálek M, Cepáková E (2006) Do northern lapwings Vanellus vanellus and little ringed plovers Charadrius dubius rely on egg crypsis during incubation? Folia Zool 55:43–51
Salvante KG (2006) Techniques for studying integrated immune function in birds. Auk 123:575–586
Sánchez JM, Corbacho C, del Viejo AM, Parejo D (2004) Colony-site tenacity and egg color crypsis in the gull-billed tern. Waterbirds 27:21–30
Scalise I, Durantini EN (2004) Photodynamic effect of metallo 5-(4-carboxyphenyl)-10,15,20-tris(4-methylphenyl) porphyrins in biomimetic AOT reverse micelles containing urease. J Photochem Photobiol A 162:105–113
Siefferman L, Navara KJ, Hill GE (2006) Egg coloration is correlated with female condition in eastern bluebirds (Sialia sialis). Behav Ecol Sociobiol 59:651–656
Soler JJ, Møller AP (1996) A comparative analysis of the evolution of variation in appearance of eggs of European passerines in relation to brood parasitism. Behav Ecol 7:89–94
Soler JJ, Navarro C, Contreras T, Aviles J, Cuervo J (2008) Sexually selected egg coloration in spotless starlings. Am Nat 171:183–194
Swynnerton CFM (1916) On the coloration of the mouths and eggs of birds. II. On the coloration of eggs. Ibis 4:529–606
Underwood TJ, Sealy SG (2002) Adaptive significance of egg coloration. In: Deeming DC (ed) Avian incubation: behaviour, environment, and evolution. Oxford University Press, New York, pp 280–298
Verboven N, Monaghan P, Evans DM, Schwabl H, Evans N, Whitelaw C, Nager RG (2003) Maternal condition, yolk androgens and offspring performance: a supplemental feeding experiment in the lesser black-backed gull (Larus fuscus). Proc R Soc Lond B 270:2223–2232
Victoria JK (1972) Clutch characteristics and egg discriminative ability of African village weaverbird Ploceus cucullatus. Ibis 114:367–376
Wallace AR (1889) Darwinism: an exposition of the theory of natural selection with some its applications. Macmillan, London
Zhao R, Xu GY, Liu ZZ, Li JY, Yang N (2006) A study on eggshell pigmentation: biliverdin in blue-shelled chickens. Poult Sci 85:546–549
Acknowledgments
We are very grateful to Jim Quinn for the advice, guidance, and logistical support he provided throughout this project. We thank M. Vujacic, S. Mai, and especially A. Mistakidis for their efforts in the field. The Doucet Lab provided insightful comments on the manuscript. We also thank anonymous reviewers and the editorial staff for their helpful suggestions. Our study was funded by an Explorer’s Club grant to D.H. and by research and equipment grants from the Natural Sciences and Engineering Research Council of Canada and the University of Windsor to S.M.D. This study was conducted under compliance of regulations provided by the Canadian Council on Animal Care.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. McGraw
Rights and permissions
About this article
Cite this article
Hanley, D., Doucet, S.M. Egg coloration in ring-billed gulls (Larus delawarensis): a test of the sexual signaling hypothesis. Behav Ecol Sociobiol 63, 719–729 (2009). https://doi.org/10.1007/s00265-008-0705-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-008-0705-2