Abstract
The striking diversity of avian eggshell colour has long fascinated biologists. Recently, it has been proposed that the blue-green colour of some eggs may function as a post-mating sexually selected signal of female phenotypic quality to their mates to induce higher allocation of paternal care. It has been suggested that maternally deposited yolk carotenoids may be the specific aspect of reproductive quality that the female is signalling via eggshell colour. We use the known properties of the thrush visual system (Turdus sp.) to calculate photon capture for the four single cone photoreceptors, and the principal member of the double cone class for eggs in clutches of two introduced European thrush species (Turdus merula and Turdus philomelos) in New Zealand. We show that differences in the avian-perceived colours of individual eggs are not consistently correlated with different measures of maternal investment in the egg. Given the growing extent of the knowledge between maternal quality, parental investment and eggshell pigmentation across avian taxa, we encourage the use of avian perceptual modelling for testing alternative non-signalling explanations for the structural and physiological basis of these relationships.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The variation in eggshell appearance displayed by individual birds was traditionally viewed to function in concealing eggs from predators (Wallace 1889; Lack 1958). However, especially among arboreal nesting species, an antipredator role for the diversity in eggshell appearance has not been strongly supported by experimental research (e.g. Götmark 1992; Westmoreland and Kiltie 1996; Weidinger 2001), and a single hypothesis is unlikely to explain the wide variety of eggshell colours and patterns observed across avian lineages (Kilner 2006). For instance, while eggshell colour (between and within species) also co-varies with the pressures of intra- and interspecific brood parasitism on broad phylogenetic scales (Underwood and Sealy 2002; Lyon 2007), several lineages of birds with diverse eggshell colours are not impacted by brood parasites (Kilner 2006).
One of the most interesting and difficult aspects of eggshell colouration to explain is the striking blue-green pigmentation seen by humans in the eggs of some species, notably in the superfamily Muscicapoidea. This coloration has, in consequence, attracted considerable theoretical attention (Lack 1958; Oniki 1985; Underwood and Sealy 2002; Kilner 2006). Recently, it was hypothesised that the presence of certain pigments in blue-green coloured eggshells (in particular, the tetrapyrrole pigment biliverdin) might function as a post-mating sexually selected signal of female phenotypic quality to their mates to induce higher allocation of paternal care (Moreno and Osorno 2003). This novel hypothesis has quickly accumulated both observational and experimental data (Moreno et al. 2004, 2005; Morales et al. 2006; Moreno et al. 2006; Siefferman et al. 2006; Martinez-de la Puente et al. 2007) regarding a potential intraspecific signalling function of blue eggshell colour (but see Kilner 2006; Krist and Grim 2007). However, none of these studies have considered the visual perceptual properties of the birds themselves. This is particularly relevant because the use of portable reflectance spectrophotometers to measure eggshell spectra over the avian visible range (i.e. 300–700 nm) has considerably increased our ability to distinguish different eggshell colours (Cherry and Bennett 2001). Yet, physical reflectance measurements do not account for what the receivers’ (i.e. conspecific mates) visual sensory systems actually process (Endler and Mielke 2005; Cherry et al. 2007).
The sexually selected eggshell colour hypothesis (Moreno and Osorno 2003) postulates a relationship between eggshell colour and maternal investment in the egg (Moreno et al. 2004). Thus, it is inferred that female birds should be signalling an aspect of investment that males would, otherwise, not be able to perceive. Specifically, it has been suggested that maternally deposited yolk carotenoids may be the specific aspect of reproductive quality that the female is signalling via eggshell colour (Moreno et al. 2004). Carotenoids are a prominent component of avian egg yolk (Blount et al. 2000) and confer important antioxidant and immuno-stimulant roles to developing embryos and young birds (McGraw and Ardia 2003; Biard et al. 2005) and show extensive variation across species with diverse ecologies (Cassey et al. 2005; Hargitai et al. 2006). Here, we test for such a relationship using data for two related thrush species (the European blackbird Turdus merula and the song thrush Turdus philomelos) to assess whether there is evidence that eggshell colour is associated with metrics of female reproductive investment. These species are ideal for examining this relationship as they are both renowned for their blue-green eggshell colour (Lack 1958; Fig. 1a,b).
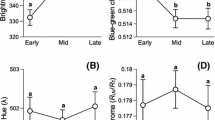
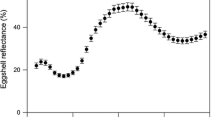
Three eggs from different clutches of a Turdus merula and b Turdus philomelos. Average reflectance spectra (±SD) for the six measurements from the central egg for c T. merula (black line) and T. philomelos (blue line). The dotted box indicates the region of the wavelength from which the proportion of reflected blue-green chroma (sensu Siefferman et al. 2006) is calculated. d Average relative photoreceptor cone capture frequencies for T. merula (solid bars) and T. philomelos (hollow bars) eggshell spectra. e Eggshell chromatic signals for T. merula (dark blue) and T. philomelos (cyan) plotted in a tetrachromatic 3D diagram using a receptor-noise-limited colour opponent model (Kelber et al. 2003), where colour loci are independent of the stimulus intensity, and Euclidean distances between points correspond to receptor-noise units (Kelber et al. 2003; Endler and Mielke 2005). Contours were constructed by a simple contamination process at every nanometre in the interval 300–700 nm. For each wavelength λ, the percentage reflectance was set at 100 while every other value was set at a fixed value p (p = 5, 10). This was repeated for all wavelengths (i.e. 401 times). The contour loci (X, Y, Z) were then generated for the 401 reference spectra following Eqs. 1–5 and joined by a simple fitted line. The black triangle at the centre of the figure represents the origin (or ‘grey point’ sensu Endler and Mielke 2005) and is where all four cones are stimulated equally and is perceived as colourless. The red triangles represent the loci of the multivariate affine equivariant median (Hettmansperger and Randles 2002; Nevalainen and Oja 2006) for each species
We use a previously described visual perception system for the blackbird (Hart et al. 2000) to calculate photon capture from eggshell colour signals for the four single cone photoreceptors and the principal member of the double cone class. Specifically, we assess whether variability in yolk carotenoid concentration and yolk mass (traits that cannot be directly discriminated) are more strongly associated with differences in eggshell colour than traits that can, otherwise, be physiologically perceived by these birds and, thus, judged directly by males (e.g. laying date, clutch size, egg size).
Materials and methods
Eggshell sampling
During the Austral breeding season (August–January) 2004–2005, we located 317 active (i.e. during incubation or brooding) blackbird (n = 136) and song thrush (n = 181) nests in the Central North Island, New Zealand. The median date for clutch initiation was 22 October and the majority of nests (53%) were located during the last 3 weeks of October and first week of November. Where possible, eggs were marked during the laying sequence, and clutch size was recorded after the female initiated incubation. We assumed that females laid a single egg daily during the laying sequence. In cases where laying sequence could be attributed to either early (first or second egg) or late (third or fourth egg), we randomly collected a single egg for analysis prior to the onset of incubation (blackbird n = 78 clutches; song thrush n = 102 clutches).
Both species are exotic horticultural pests in New Zealand, and all sampling was conducted on private land with the express permission of the landowners. The study sites, nest searching protocol and egg size measurements were previously described by Cassey et al. (2005, 2006a). Briefly, eggs were measured with precision vernier calipers (length × width to 0.1 mm), weighed with calibrated precision scales accurate to 0.001 g and, then, split equatorially; the yolk and albumin separated and frozen for analysis. The eggshell was washed in distilled water and, then, allowed to dry at room temperature before spectrophotometric analysis. Yolk carotenoid concentration was determined by high-performance liquid chromatography, and the methods—including carotenoid extraction and quantification, statistical analyses and repeatability estimates—are detailed in Cassey et al. (2005, 2006b).
Eggshell reflectance was measured in the laboratory using an Ocean Optics USB2000 Miniature Fiber Optic Spectrometer with illumination by a DT mini-lamp. A custom built light-proof cap was fitted over the probe to maintain a consistent angle (90°) between the eggshell and the measuring fibre optics. Spectra were recorded in ×0.4 nm steps and were expressed relative to a white Ocean Optics WS-1 diffuse reflectance standard. Three measurements were taken from the blue-green background shell colour in each hemisphere of the eggshell. To minimize measurement error, dark and white standard reflectance calibration measures were taken regularly during sampling.
Following previous studies (Morales et al. 2006; Siefferman et al. 2006), we calculated reflectance-based eggshell colour using an index of blue-green chroma as the proportion of total reflectance in the wavelength region (R400–575) across the total spectrum (R400–575/R300–700; see Fig. 1c). This is suggested to correspond to the region of least absorbance of the pigment biliverdin (Falchuk et al. 2002) and to be a useful metric of blue-green eggshell colouration (Siefferman et al. 2006). We note that both species reflect light maximally in this region (Fig. 1c) and that the distributions of the proportion of blue-green chroma were not significantly different from a normal distribution (blackbird n = 78, range = 0.42–0.51, Kolmogorov–Smirnov D = 0.06, p > 0.15; song thrush n = 102, range = 0.50–0.55, Kolmogorov–Smirnov D = 0.06, p > 0.15) and were, therefore, not transformed in subsequent analyses. We used the intraclass correlation coefficient (r) to investigate the proportion of variance in reflectance of blue-green chroma for each species that occurred within an egg rather than between different eggs (Lessells and Boag 1987).
A photoreceptor photon capture model
Birds have four spectral types of cone visual pigments that are located in two anatomically distinct cone types–single and double cones. Both types of cones are equipped with differently coloured oil droplets producing four spectrally distinct types of single cones and at least one spectral type of double cone (for review, see Hart 2001). Behavioural studies suggest that in birds, colour discrimination is mediated by single cones alone (Maier and Bowmaker 1993; Vorobyev and Osorio 1998). Consequently, it is widely believed that double cones are responsible for achromatic tasks (Maier and Bowmaker 1993; Hart et al. 1998; Osorio et al. 1999; Goldsmith and Butler 2005).
Because photoreceptors vary in the wavelengths of light they capture, it is necessary to calculate the light captured by each type of photoreceptor for different spectra under normal viewing conditions (Kelber et al. 2003; Endler and Mielke 2005). In order to compare the strength of eggshell colour signals as individual birds perceive them (Bennett and Théry 2007), we calculated the photon capture for each of the four avian single cone types (ultraviolet (UV)-wavelength sensitive, short-wavelength sensitive, medium-wavelength sensitive and long-wavelength sensitive) and for the principal member of the double cone class over the visible spectrum (300–700 nm in birds) when viewing eggs under a woodland shade (Endler 1993).
The flux of photons of wavelength, λ, reflected from the eggshell surface is the product of the number of incident photons at that wavelength, I(λ), and the surface reflectance, S(λ). For each eggshell colour signal, we calculated the quantum catch q k of each of the four single cone types (UV-wavelength sensitive, short-wavelength sensitive, medium-wavelength sensitive and long-wavelength sensitive; designated by k [1–4]) and for one type of double cone over the visible spectrum (λ = 300–700 nm in birds), as the integrated product of the spectral sensitivity of cone type k (R k ), the reflectance spectrum of an eggshell (S), and the ambient light spectrum illuminating the nest environment (I)
The stimulation output f k of photoreceptor k for the log-linear version of the receptor-noise model (Vorobyev et al. 1998, 2001) is then simply the natural logarithm of q k :
A 3D tetrachromatic colour space
The log-linear relationship of Eq. 2 relates the receptor signals to the quantum catches such that chromatic processing is insensitive to changes of stimulus intensity throughout colour space (Vorobyev et al. 1998, 2001). Consequently, we constructed a chromaticity diagram, where colour loci are independent of the stimulus intensity, and Euclidean distance between loci corresponds to the distance \(\sqrt {\Delta _e^2 } \) given by Eq. 5. The stimulation outputs f k are calculated using a visual model for the European blackbird (T. merula) and woodland ‘canopy-filtered green light’ irradience. For tetrachromatic vision, the following axes can be used to plot the colour loci (Kelber et al. 2003):
where:
and:
ω k is the Weber fraction of photoreceptor mechanism k. The Weber fractions for the four single cone mechanisms were obtained from the analysis of behavioural spectral sensitivity of a red-billed Leiothrix, Leiothrix lutea (Maier and Bowmaker 1993; Vorobyev et al. 1998). Values for ω k were: ω 1 = 0.1, ω 2 = 0.07, ω 3 = 0.07, ω 4 = 0.05 (Vorobyev 2003).
The distance between any two eggshell signals in the colour space can then be expressed as:
where X, Y and Z are given by Eq. 3.
Thrush spectral sensitivities were kindly provided by Nathan Hart following the analytical expressions for blackbird eyes used in Hart et al. (2000). Given their close phylogenetic and ecological relatedness (del Hoyo et al. 2006), we assumed that the spectral sensitivities provided for the blackbird were an adequate avian model for both Turdus species (Hart 2001). Thus, any variation in colour signals is entirely attributable to the different appearance of the eggs themselves.
Statistical analyses
Photoreceptor colour threshold models and analyses were conducted in SAS v 8.02. We used an Information Theoretic approach (Burnham and Anderson 2002; Whittingham et al. 2006) to investigate whether differences in eggshell colour were associated with variability in yolk carotenoid concentration, yolk mass, laying date, clutch size, egg volume and fresh egg mass across clutches for both species separately. We fitted generalised linear mixed models and compared them using a likelihood-based measure of model fit (Akaike information criterion (AIC), Akaike 1974). In many species, maternal constituents of the egg are known to vary with laying sequence across the clutch, including both eggshell colour (Moreno et al. 2005) and yolk carotenoid deposition (Saino et al. 2002). For both Turdus species, Cassey et al. (2005) previously reported that maternally deposited yolk carotenoids declined linearly (and significantly) with laying sequence. Relative laying order (early or late) was, therefore, included as a single random effect in all analyses. Model averaged estimates (and variances) were calculated for each parameter and all possible single order model subsets, using a model selection and multi-model inference spreadsheet (www.uvm.edu/~bmitchel/software.html).
We used multivariate Principal Component Analysis (PCA) to estimate the variance explained by the first major axis in eggshell colour across clutches in the three dimensions of tetrachromatic colour space (Fig. 1e). Note that we did not use PCA to describe differences among reflectance spectra, as it is well known that the assumptions of statistical tests to discriminate between the spectra are violated, and the tests will be invalid (Endler and Mielke 2005).
Results
Both thrush species lay eggs that appear bluish-green to the human eye (Fig. 1a,b). We obtained six reflectance spectra from each eggshell and calculated the proportion of variance in structural blue-green chroma reflectance for each species that occurred within an egg rather than between different eggs. The intraclass correlation coefficients (r) for both species were positive and large (blackbird r = 0.81; song thrush r = 0.70). This means that the variability between reflectance measurements within an egg was substantially less than the variability between different eggs from different clutches of the same species. In the following analyses, we used an average of the six reflectance spectra for each egg.
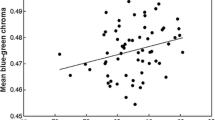
The majority of the variability in eggshell colour among clutches in both species is orientated along single tetrachromatic axes (Fig. 1e). Principal Component Analysis revealed the first major axis explained 60% of the variability in colour space for blackbirds (n = 78) and 65% in song thrush (n = 102). For consistency with previous studies, we calculated the variation in instrumental reflectance spectra as the proportion of blue-green chroma in the region of the wavelength relating to the absorbance of the bile pigment biliverdin (Siefferman 2006). Although the variability in this measure may not be specifically informative with regard to how birds are assumed to perceive colour signals, it was strongly correlated to the component scores for the principal major axis in tetrachromatic colour space in both species (Fig. 2).
Bivariate scatterplot of the relationship between the proportion of reflected blue-green chroma (sensu Siefferman et al. 2006) and the first principal component (PC1) from the 3D tetrachromatic space in Fig. 1e for T. merula (solid points) and T. philomelos (hollow points). Ordinary least square regression lines and R 2 values are also given
We tested whether different measures of eggshell colour were independently related to yolk carotenoid concentration, yolk mass, laying date, clutch size, egg volume and fresh egg mass for each species separately (Table 1). In the blackbird, later laying dates were positively associated with ultraviolet-wavelength sensitive photon capture and negatively associated with long-wavelength sensitive photon capture. In all other cases, for both species, model averaged estimates had 95% confidence intervals that encompassed 0 (Table 1), indicating no consistent statistically significant relationships between eggshell colour signals and maternal investment traits.
In the blackbird, the best model for any of the colour metrics was most likely to contain laying date (w i = 0.28 to 0.97), fresh egg mass (w i = 0.07 to 0.42) and yolk carotenoid concentration (w i = 0.02 to 0.37). In the song thrush, the best model for any of the colour metrics was most likely to contain egg volume (w i = 0.15 to 0.38) and yolk carotenoid concentration (w i = 0.11 to 0.46). In both species, the best model for any of the colour metrics was least likely to contain clutch size (Table 1).
Discussion
The strikingly coloured eggs of thrushes and their relatives have long stimulated interest and research into the evolution and function of egg colour variation in birds (Lack 1958; Underwood and Sealy 2002), including the recent hypothesis that blue-green colouration acts as a sexually selected signal of female quality (Moreno and Osorno 2003). We found that the variation between eggshell colour and different measures of maternal investment in two Turdus thrush species represented a very weak (and information poor) correlation that is unlikely to support a visually perceived signaling function of maternal investment in eggs.
Blackbird and song thrush eggs differ in how strongly they stimulate different photoreceptors (Fig. 1d), and so their position in 3-D tetrachromatic space concomitantly varies (Fig. 1e). However, neither variation in instrumental reflectance spectra (i.e. blue-green chroma) nor photoreceptor photon capture of eggshell colour were consistently or strongly associated with any of our measures of maternal investment in the eggs across either species. Instead, we found that the strongest correlations with any measure of maternal investment were between the ultraviolet- and long-wavelength sensitive photon capture and laying date in blackbirds (Table 1). It seems unlikely that birds would be using eggshell colouration as a signal of laying date, as external environmental cues would provide a substantially more reliable method of tracking time through the breeding season.
Maternally transferred carotenoids play an important role in maintaining redox homeostasis during embryonic development and the first days after hatching (Surai 2002; McGraw et al. 2005). Yolk carotenoid concentration has been suggested to be the specific aspect of reproductive maternal quality that the female may be signalling via eggshell colour (Moreno et al. 2004), yet we found carotenoid concentration to be no more important in the models than predictors of egg size (egg volume or fresh egg mass), a variable trait that birds are able to assess directly (including Turdus species; Rothstein 1982). Egg size was consistently as likely to be contained in the best model as yolk carotenoid concentration. In addition, these predictors had model averaged estimates with 95% confidence intervals that included 0. Interestingly, Krist and Grim (2007) found that even though maternal quality was positively correlated with the structural intensity of blue-green egg colour, male brood provisioning was not associated with eggshell colour in cross-fostered eggs of the collared flycatcher Ficedula albicollis. Given that bird eggshell colours may depend on yearly fluctuations of environmental conditions, like rainfall and temperature (Aviles et al. 2007), it is less likely that they would be useful as a signal of yolk carotenoid concentrations, which is in agreement with our results.
Previous experimental studies have failed to provide evidence that the blue egg colour of song thrushes (T. philomelos) is an adaptation against predation risk (Götmark 1992; Weidinger 2001). Whereas, experimental work on the antiparasitic function of egg colours showed consistent rejections of non-mimetic ‘parasite’ eggs from Turdus nests (Grim and Honza 2001; Moskát et al. 2003; Honza et al. 2005). Recently, Honza et al. (2007) showed that the level of eggshell colour mimicry in the ultraviolet- and medium-wavelength parts of the colour spectrum significantly influenced egg rejection in the song thrush. We suggest that it seems more probable that intraspecific signalling is not related to yolk carotenoid concentration. We note, however, that even a strong existing correlation between female phenotypic quality and egg traits does not imply that there may be any signaling function of the latter factor (eggshell colour, maculation etc.)—female pigment investment may benefit developing embryos directly and without involving signaling to the mate or conspecifics in general. In fact, non-signalling functions, such as the regulation of eggshell strengthening and water loss (Gosler et al. 2005; Higham and Gosler 2006) or a parasol role (Bakken et al. 1978), are more likely to produce the statistical association between maternal quality, egg constituents and eggshell appearance (including eggshell patterning; Martinez-de la Puente et al. 2007) that have previously been observed.
In summary, we have shown that variation in some measures of maternal reproductive investment (including yolk carotenoid concentration) was not consistently related to differences in quantifiable eggshell colour (including photoreceptor photon capture), and we posit, therefore, that it is unlikely for it to have a signalling function. This does not mean that the previous reports of relationships between maternal quality and pigmentation are erroneous. The challenge is both to document the extent and strength of these relationships within and between species and to develop testable hypotheses as to why different measures of maternal quality and eggshell pigmentation are linked. In this regard, it is important that we collect better data regarding avian-perceived discrimination and the adaptive function of eggshell colour variation.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Contr 19:716–723
Aviles JM, Stokke BG, Moksnes A (2007) Environmental conditions influence egg color of reed warblers Acrocephalus scirpaceus and their parasite, the common cuckoo Cuculus canorus. Behav Ecol Sociobiol 61:475–485
Bakken GS, Vanderbilt VC, Buttemer WA, Dawson WR (1978) Avian eggs: thermoregulatory value of very high near-infrared reflectance. Science 200:321–323
Bennett ATD, Théry M (2007) Avian color vision and coloration: multidisciplinary evolutionary biology. Am Nat 169:S1–S6
Biard C, Surai PF, Moller AP (2005) Effects of carotenoid availability during laying on reproduction in the blue tit. Oecologia 144:32–44
Blount JD, Houston DC, Moller AP (2000) Why egg yolk is yellow. Trends Ecol Evol 15:47–49
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practice information-theoretic approach. Springer, Berlin Heidelberg New York
Cassey P, Ewen JG, Boulton RL, Blackburn TM, Moller AP, Biard C, Olson V, Karadas F (2005) Egg carotenoids in passerine birds introduced to New Zealand: relations to ecological factors, integument coloration and phylogeny. Funct Ecol 19:719–726
Cassey P, Blackburn TM, Evans KL (2006a) Changes in egg size of exotic passerines introduced to New Zealand. Notornis 52:243–246
Cassey P, Ewen JG, Karadas F, Hauber ME (2006b) Repeatability of laboratory measurements for maternally derived yolk carotenoid concentrations in bird eggs. Aust J Zool 54:381–384
Cherry MI, Bennett ATD (2001) Egg colour matching in an African cuckoo, as revealed by ultraviolet-visible reflectance spectrophotometry. Proc R Soc Lond B 268:565–571
Cherry MI, Bennett ATD, Moskát C (2007) Host intra-clutch variation, cuckoo egg matching and egg rejection by great reed warblers. Naturwissenschaften 94:441–447
del Hoyo J, Elliott A, Christie DA (2006) Handbook of the birds of the world: cuckoo-shrikes to thrushes, vol. 10. Lynx, Barcelona
Endler JA (1993) The color of light in forests and its implications. Ecol Appl 63:1–27
Endler JA, Mielke PW (2005) Comparing entire colour patterns as birds see them. Biol J Linn Soc 86:405–431
Falchuk KH, Contin JM, Dziedzic TS, Feng ZL, French TC, Heffron GJ, Montorzi M (2002) A role for biliverdin IX alpha in dorsal axis development of Xenopus laevis embryos. PNAS 99:251–256
Goldsmith TH, Butler BK (2005) Color vision of the bugerigar (Melopsittacus undulatus): hue matches, tetrachromacy, and intensity discrimination. J Comp Physiol A 191:933–951
Gosler AG, Higham JP, Reynolds SJ (2005) Why are birds’ eggs speckled. Ecol Lett 8:1105–1113
Götmark F (1992) Blue eggs do not reduce nest predation in the song thrush, Turdus philomelos. Behav Ecol Sociobiol 30:245–252
Grim T, Honza M (2001) Differences in behaviour of closely related thrushes (Turdus philomelos and T. merula) to experimental parasitism by the common cuckoo Cuculus canorus. Biologia 56:549–556
Hargitai R, Matus Z, Hegyi G, Michl G, Toth G, Torok J (2006) Amtioxidants in the egg yolk of a wild passerine: differences between breeding seasons. Comp Biochem Physiol, B Biochem Mol Biol 143:145–152
Hart NS (2001) The visual ecology of avian photoreceptors. Prog Retin Eye Res 20:675–703
Hart NS, Partridge JC, Cuthill IC (1998) Visual pigments, oil droplets and cone photoreceptor distribution in the European starling (Sturnus vulgaris). J Exp Biol 201:1433–1446
Hart NS, Partridge JC, Cuthill IC, Bennett ATD (2000) Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.). J Comp Physiol, A 186:375–387
Hettmansperger TP, Randles RH (2002) A practical affine equivariant multivariate median. Biometrika 89:851–860
Higham JP, Gosler AG (2006) Speckled eggs: water-loss and incubation behaviour in the great tit Parus major. Oecologia 149:561–570
Honza M, Kuiper SM, Cherry MI (2005) Behaviour of African turdid host towards experimental parasitism with artificial red-chested cuckoo Cuculus solitarius eggs. J Avian Biol 36:517–522
Honza M, Polaciková L, Procházka P (2007) Ultraviolet and green parts of the colour spectrum affect egg rejection in the song thrush (Turdus philomelos). Biol J Linn Soc 92:000–000
Kelber A, Vorobyev M, Osorio D (2003) Animal colour vision—behavioural tests and physiological concepts. Biol Rev 78:81–118
Kilner RM (2006) The evolution of egg colour and patterning in birds. Biol Rev 81:383–406
Krist M, Grim T (2007) Are blue eggs a sexually selected signal of female collared flycatchers? A cross-fostering experiment. Behav Ecol Sociobiol 61:863–876
Lack D (1958) The significance of the colour of turdinae eggs. Ibis 100:145–166
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lyon BE (2007) Mechanism of egg recognition in defenses against conspecific brood parasitism: American coots (Fulica americana) know their own eggs. Behav Ecol Sociob 61:455–463
Maier EJ, Bowmaker JK (1993) Colour vision in the passeriform bird, Leiothrix lutea: correlation of visual pigment absorbency and oil droplet transmission with spectral sensitivity. J Comp Physiol, A Sens Neural Behav Physiol 172:295–301
Martinez-de la Puente J, Merino S, Moreno J, Tomas G, Morales J, Lobato E, Garcia-Fraile S, Martinez J (2007) Are eggshell spottiness and colour indicators of health and condition in blue tits Cyanistes caeruleus. J Avian Biol 38:377–384
McGraw KJ, Ardia DR (2003) Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am Nat 162:704–712
McGraw KJ, Adkins-Regan E, Parker RS (2005) Maternally derived carotenoid pigments affect offspring survival, sex ratio, and sexual attractiveness in a colorful songbird. Naturwissenschaften 92:375–380
Morales J, Sanz JJ, Moreno J (2006) Egg colour reflects the amount of yolk maternal antibodies and fledging success in a songbird. Biol Lett 2:334–336
Moreno J, Osorno JL (2003) Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality. Ecol Lett 6:803–806
Moreno J, Osorno JL, Morales J, Merino S, Tomas G (2004) Egg colouration and male parental effort in the pied flycatcher Ficedula hypoleuca. J Avian Biol 35:300–304
Moreno J, Morales J, Lobato E, Merino S, Tomas G, Martinez-de la Puente J (2005) Evidence for the signaling function of egg color in the pied flycatcher Ficedula hypoleuca. Behav Ecol 16:931–937
Moreno J, Lobato E, Morales J, Merino S, Tomas G, Martinez-de la Puente J, Sanz JJ, Mateo R, Soler JJ (2006) Experimental evidence that egg color indicates female condition at laying in a songbird. Behav Ecol 17:651–655
Moskát C, Karcza Z, Csörgo T (2003) Egg rejection in European Blackbirds (Turdus merula): the effect of mimicry. Ornis Fenn 2003:86–91
Nevalainen J, Oja H (2006) SAS/IML macros for a multivariate analysis of variance based on spatial signs. Journal of Statistical Software 16:1–17
Oniki Y (1985) Why robin eggs are blue and birds build nests: statistical tests for Amazonian birds. Ornithological Monographs 36:536–545
Osorio D, Miklosi A, Gonda Z (1999) Visual ecology and perception of coloration patterns by domestic chicks. Evol Ecol 13:673–689
Rothstein SI (1982) Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species. Behav Ecol Sociobiol 11:229–239
Saino N, Bertacche V, Ferrari RP, Martinelli R, Moller AP, Stradi R (2002) Carotenoid concentration in barn swallow eggs is influenced by laying order, maternal infection and paternal ornamentation. Proc R Soc Lond, B Biol Sci 269:1729–1733
Siefferman L (2006) Egg coloration and recognition of conspecific brood parasitism in eastern bluebirds. Ethology 112:833–838
Siefferman L, Navara KJ, Hill GE (2006) Egg coloration is correlated with female condition in eastern bluebirds (Sialia sialis). Behav Ecol Sociobiol 59:651–656
Surai PF (2002) Natural Antioxidants in Avian Nutrition and Reproduction. Nottingham University Press, Nottingham
Underwood TJ, Sealy SG (2002) Adaptive significance of egg coloration. In: Deeming DC (ed) Avian Incubation: Behaviour, Environment and Evolution. Oxford University Press, Oxford, pp 280–289
Vorobyev M (2003) Coloured oil droplets enhance colour discrimination. Proc R Soc Lond, B Biol Sci 270:1255–1261
Vorobyev M, Osorio D (1998) Receptor noise as a determinant of colour thresholds. Proc R Soc Lond B 265:351–358
Vorobyev M, Osorio D, Bennett ATD, Marshal NJ, Cuthill IC (1998) Tetrachromacy, oil droplets and bird plumage colours. J Comp Physiol, A Sens Neural Behav Physiol 183:621–633
Vorobyev M, Brandt R, Peitsch D, Laughlin SB, Menzel R (2001) Colour thresholds and receptor noise: behaviour and physiology compared. Vis Res 41:639–653
Wallace AR (1889) Darwinism: An exposition of the theory of natural selection with some of its applications. Macmillan, London
Weidinger K (2001) Does egg colour affect predation rate on open passerine nests. Behav Ecol Sociobiol 49:456–464
Westmoreland D, Kiltie RA (1996) Egg crypsis and clutch survival in three species of blackbirds (Icteridae). Biol J Linn Soc 58:159–172
Whittingham MJ, Stephens PA, Bradbury RB, Freckleton RP (2006) Why do we still use stepwise modelling in ecology and behaviour. J Anim Ecol 75:1182–1189
Acknowledgements
We are extremely grateful to R. Peacock, B. and G. Cassey, K. Mathews, G. and S. Cassey, M. Thompson and D. Armstrong for accommodation and access to private land. Y. Richard, N. MacArthur and R. Boulton assisted in locating naturally occurring nests, and this study would not have been completed without their input. Spectral sensitivities were kindly provided by N. Hart, J. Lockwood, and three anonymous referees provided comments that greatly improved our draft manuscript. Financial support was provided, in part, by the University of Auckland Research Council (to PC and MEH), the Universitas 21 Birmingham Travel Fund (to PC), a Human Frontier Science Program award (to PC and MEH), the New Zealand Marsden Fund (to MEH) and a Royal Society Grant (to JGE).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cassey, P., Ewen, J.G., Blackburn, T.M. et al. Eggshell colour does not predict measures of maternal investment in eggs of Turdus thrushes. Naturwissenschaften 95, 713–721 (2008). https://doi.org/10.1007/s00114-008-0376-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-008-0376-x