Abstract
Bone morphogenetic proteins (BMPs) play important roles at multiple stages of chondrogenesis. This study was undertaken to investigate the potential role of bone morphogenetic protein-7 (BMP-7) in the differentiation of chondrocytes using tissue engineering techniques. The impact of BMP-7 on human amniotic epithelial cells (hAECs) was tested. The hAECs were treated either with recombinant human BMP-7 cDNA or with transforming growth factor beta 1 (TGF-β1) as a positive control for three weeks in vitro. Cartilaginous differentiation and proliferation were assayed by quantitative RT-PCR, histology, and in situ hybridization. Our results were such that hAECs treated with either BMP-7 or TGF-β1 expressed cartilage markers (aggrecan, Sox9, CEP-68, and type II and X collagens) within three weeks. Compared with a control vector, BMP-7 induced a decrease in type I collagen expression, while the transcription of the cartilage-specific type II collagen remained stable. In induction experiments, BMP-7 transgenic hAECs exhibited the largest amount of matrix synthesis. In conclusion, these data indicate that BMP-7 plays an important role in inducing the production of cartilage by hAECs in vitro. Cartilage differentiation and matrix maturation can be promoted by BMPs in a cartilage engineering paradigm. These properties make BMPs promising tools in the engineering of cartilaginous joint bio-prostheses and as candidate biological agents or genes for cartilage stabilisation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The epithelial cells derived from human amniotic tissue may be used to generate new tissues in vitro [1]. Human amniotic tissue-derived epithelial cells (hAECs) are homogeneous and differentiate reliably and reproducibly into many cell types, such as chondrocytes or neurocytes [2, 3]. Since the original description of the potential role of osteogenic protein-1 (bone morphogenetic proteins-7, BMP-7) in inducing cartilage at an ectopic site, it has taken more than three decades to bring BMPs to clinical treatment of cartilage lesions [4, 5]. BMP-7 (also known as osteogenic protein 1 or OP-1) stimulates the synthesis of chondrocyte matrix components such as proteoglycan and collagen in vitro [6]. BMP-7 was originally purified and identified in bone as proteins capable of inducing the formation of ectopic endochondral bone. However, it is now clear that they are expressed in a variety of tissues including adult articular cartilage [7], suggesting that a recombinant bone morphogenetic protein stimulates ingrowth of mesenchymal cells into the chondral defects which then transform into newly formed articular cartilage-like tissue. In addition, the regenerated cartilage contains a high content of proteoglycans and type II collagenas, as demonstrated in regeneration of articular cartilage chondral defects by OP-1/BMP-7 in sheep [8]. Taking advantage of these stimulatory properties, and of tissue-engineered 3-dimensional cartilage tissues, we used a combined approach to show that cartilage differentiation and matrix production are modulated by BMP-7 in artificially engineered cartilage tissues in vitro.
This study aimed to identify BMP-7 as a growth factor that induces chondrocyte differentiation in hAECs that can be used to engineer true hyaline cartilage in vitro and in vivo.
Materials and methods
Cell preparation
Human amniotic epithelial cells were derived from foetal membranes of the uterus harvested during caesarian sections performed in women delivering full-term infants.
The extracellular matrix was digested for 16 h at 37°C in RPMI with 10% foetal calf serum (FCS; Gibco BRL, Karlsruhe, Germany) supplemented with 2 mg/mL collagenase (Boehringer-Mannheim, Mannheim, Germany), 2 mg/ml type CLS II collagenase (Seromed, Berlin, Germany), and 0.1 mg/mL hyaluronidase (Sigma, Taufkirchen, Germany). Subsequently, the cell suspension was washed in Hank’s salt solution, and viability was determined by staining with trypan blue. hAECs were pooled and plated at a density of 75,000/cm2 and cultured in RPMI supplemented with 10% FCS at 37°C , 5% CO2, and 90% humidity. Half of the culture medium was replaced every other day.
Cultivation and expansion of hAECs in monolayer cultures
For expansion studies, hAECs were freshly isolated and seeded in monolayers at a density of 75,000 cells/cm2 (designated day 0) in RPMI with 10% FCS (n = 2). After reaching 80% confluence, cells were trypsinised and subcultured at a ratio of 1:4. Cells were fed every other day by replacement of 50% of the medium with fresh medium. The mononuclear cell layer formed by centrifugation was collected and resuspended in essential medium (Gibco; MEM) containing 20% FBS, antibiotics, and glutamine. Cells adhered and grew. Once the desired confluence was obtained (approximately 70%), 105 cells/well were plated in six-well plates.
Surface marker expression assays
FITC-CD14, CD29, CD33, CD34, CD44 and CD45 or HLA-ABC, DR, CD73 and CD105, CD133 and CD166 antibodies were used to label cells for analysis in a FACS Aria flow cytometer to detect cell activation according to current internationally recognised definitions.
In vitro induction and chondrogenic differentiation
A micromass technique modified from Ahrens et al. [9] was used. Briefly, cells from the second passage were harvested and resuspended in culture media at a concentration of 1 × 107 cells/ml. Culture droplets (10 μL) were placed in culture dishes and cells were allowed to adhere to each other and the substratum at 37°C for 90 min. After adhesion, chondrogenic media consisting of DMEM, 1% FBS, 1% penicillin/streptomycin, 37.5 mg/mL ascorbate-2-phosphate, an insulin/transferrin/selenium premix (BD Biosciences, Bedford, MA), and hAECs were treated with medium (MEM) containing 20% FBS with or without 1 ng/mL TGF-β1 as a positive control or 100 ng/mL recombinant BMP-7 (R&D Systems, Minneapolis, MN, USA) in duplicate [9]. We detected the effects of different concentrations of BMP-7 on hAEC survival with a colorimetric MTT test. By 24 hours, the cell aggregates had coalesced into spheres. The chondrogenic media was changed every three days. Micromasses were harvested for histological, protein, and RNA tests at various time points. These incubation time points were selected based on previous cell culture studies that have shown changes in cell number and shape [10] and an increase of chondrogenic markers [11] for up to three weeks after induction of differentiation. The cell medium was changed twice per week in all groups.
BMP-7 has previously been used at a concentration of 100 ng/mL in differentiation experiments. Among the different concentrations tested in our assay system, 100 ng/mL proved to be optimal for proliferation and was used for further analysis. Previous studies [12] showed that TGF-β1 exhibits its highest efficacy for proliferation and differentiation at a concentration of approximately 1 ng/mL.
Alcian blue staining
After a three-week incubation, cells were stained with Alcian blue (pH 2.5) for 30 min, followed by the addition of 3% acetic acid for 5 min. Then, the wells were washed with water for 1 min.
RT-PCR
Human chondrocyte-specific transcripts were detected by reverse transcriptase-polymerase chain reaction (RT-PCR). Briefly, 1 μg RNA from intact samples was reverse transcribed using the Omniscript Reverse Transcription Kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations. RT was carried out at 42°C for 50 min. The PCR primers (Biospring, Frankfurt, Germany) used were as follows: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense, 5′-GAAGGT-GAA-GGT-CGG-AGT C-3′, antisense, 5′-GGAAGC-CCA-TCA-CCA-TCT-TC-3′; aggrecan (AGC) sense, 5′-GGG-TCA-ACA-GTG-CCT-ATC-AG-3′, antisense, 5′-GGG-TGT-AGC-GTG-TAG-AGA-TG-3′; collagen type II (COL2A1) sense, 5′-TGG-CCTGAG-ACA-GCA-TGA C-3′; antisense, 5′-AGTGTT-GGG-AGC-CAG-ATT-GT-3′; collagen type X (COL10A1) sense, 5′-GCA-ACT-AAG-GGC-CTCAAT-GG-3′; antisense, 5′-GAG-CA-CTA-GGAATC-CTG-AG-3′; Sox9 sense, 5′-AGA-CCT-TTGGGC-TGC-CTT-AT-3′, antisense, 5′-AG-CCT-CCCTCA-CTC-CAA-GA-3′; and chondrocyte expressed protein-68 (CEP-68) as described [6]. After an initial denaturation at 95°C for ten minutes, the samples were subjected to 35 cycles of 95°C for 30 s, 56°C for 45 s, and 72°C for 50 seconds. The reaction products were resolved on a 2% agarose gel and visualised with ethidium bromide. β-actin was used as a standard control.
Cell ultrastructure observation
After induction with BMP-7, culture plates were washed with phosphate buffered saline (PBS) and cells were collected with a special rubber scraper. The cells were then transferred to a 15-mL centrifuge tube and centrifuged at 5,000 rpm for 15 min. The supernatant was applied to filter paper that was then suction dried, and then fixed with 25% glutaraldehyde at 4°C for more than two hours. The cells were then washed with PBS (pH = 7.2), post-fixed with 1% osmium tetroxide, dehydrated with an ethanol gradient, saturated with 100% acetone and embedded with epoxy resin. The embedded samples were sliced, transferred to copper grids, stained with lead citrate and uranyl acetate, and subjected to transmission electron microscopy observation (5000×).
Statistical analysis
Results for PCR analysis, the expression of Col-2(I) and aggrecan were analysed by RT-PCR and compared using the unpaired t-test for analysis of variance. The expression of SOX9, proteoglycan and type II collagen was analysed by RT-PCR and compared using the SNK test for analysis of variance in SPSS 13.0. P values of less than 0.05 were considered to be statistically significant.
Results
Cell surface marker expression in hAECs
Flow cytometry revealed low expression of HLA-A, B, C, and DR antigens on hAEC membranes, but stronger expression of stem cell surface markers and gene products such as CD44, CD73, and CD105 (Fig. 1). The lack of HLA-A, B, C, and DR antigens suggests that hAECs do not express MHC-II on their surfaces.
Cells surface marker expression in hAECs. FITC-CD14, CD29, CD33, CD34, CD44 and CD45 or HLA-ABC, DR, CD73 and CD105, CD133 and CD166 were used to label cells for analysis in a FACS Aria flow cytometer to detect cell activation. Human amniotic epithelial cell membrane has a low expression of HLA-A, B, C, and DR antigens, but expresses at higher level of stem cell surface markers and gene products such as CD44, CD73, and CD105
In vitro chondrogenesis of hAECs
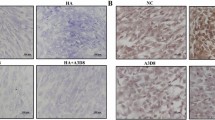
We used a special culture technique to induce chondrogenesis in hAECs [13]. To confirm that hAECs are able to progress along the chondrogenic program, we examined ECM proteoglycan production with Alcian blue staining and sGAG quantification, as well as collagen II production using immunofluorescence, at various time points. Differentiated micromasses demonstrated stronger Alcian blue and collagen II staining. On day three, the ECM production between the cells increased dramatically, micromasses condensed, and Alcian blue and collagen II staining became progressively stronger, all characteristics of natural cartilage. Mouse adipose tissue and femoral head cartilage were used as negative and positive controls, respectively, for the evaluation of chondrogenesis. Hematoxylin-eosin staining of adipose tissue revealed typical characteristics (Fig. 2a).
Chondrogenic differentiation of hAECs during cell culture expansion in vitro. a, b Alkaline phosphatase (AP) staining of hAECs during cell culture expansion. a Freshly isolated hAECs attached to the surface of the culture plate on day 3 were round and exhibited some expression of AP. b Further cultivation of cells yielded stretched fibroblasts that stained positive for AP. c Expression profile of differentiating hAECs during cell culture expansion. In mature cartilage (lanes 1–2), chondrocytes expressed Col-2(I) abundantly. The large cartilage proteoglycan aggrecan was expressed at moderate levels. The transcription of aggrecan was induced at an early phase of expansion and was upregulated again during hAEC differentiation. d Micrographs of Azan (collagen)- and Alcian blue (proteoglycan)-stained chondrocytes (BMP-7 transgenic or controls) were analysed by computer-assisted imaging software. Analysis of recombinant BMP-7 cells revealed a slight, but not significant (P = 0.3420, n = 6), induction of collagen matrix formation. The deposition of proteoglycan was significantly enhanced in BMP-7 transfected chondrocyte cultures compared to control cells (P = 0.1302, n = 6)
Freshly isolated cells were round, and a subset of these cells expressed AP. Within a few days, these cells attached to the plastic surface of the culture plate, stretched, took the shape of typical fibroblast-like cells, and progressively lost AP expression (Fig. 2a,b). At the molecular level, the expression of genes encoding the characteristic extracellular cartilaginous matrix products such as Col-2(I), and the large cartilage proteoglycan aggrecan (Fig. 2c) were observed. In mature articular cartilage, the expression of type I collagen could not be detected, while type II collagen was expressed abundantly.
Additionally, the expression of Col-2(I) and aggrecan was analysed by RT-PCR and compared using the unpaired t-test for analysis of variance. Mature cartilage cells showed a moderate expression of the cartilage-specific large cartilage proteoglycan aggrecan (Fig. 2d). Interestingly, the expression of the aggrecan gene was induced during the first few days of cell culture conditions.
RT-PCR
After three weeks of treatment with either BMP-7 or TGF-β, the distinct mRNA expression of the cartilage-specific markers Sox9 and collagen type II was observed, whereas control cells did not express these mRNAs. Weak expression of aggrecan was also observed in transfected cells (Fig. 3). These findings were consistent among hAEC populations from all donors.
RT-PCR shows particularly distinct changes in the expression of the cartilage-specific genes Sox9 and collagen type II. The positive control was mRNA isolated from normal chondrocytes. hAECs were treated with TGF-β or BMP-7 as described in Methods. RNA was extracted from cultured hAECs and analysed for the presence of transcripts of the indicated cartilage markers. a Sox9. Differentiation was induced in culture for three days in BMP-7 group. Differentiation was induced in culture for three days in TGF-β group. Differentiation was induced in culture for 21 days in BMP-7 group. Differentiation was induced in culture for 21 days in TGF-β group. b Col(Collagen)2A. Differentiation was induced in culture for three days in BMP-7 group. Differentiation was induced in culture for three days in TGF-β group. Differentiation was induced in culture for 21 days in BMP-7 group. Differentiation was induced in culture for 21 days in TGF-β group
Differentiation was induced in culture for three days or 21 days in each group. The expression of SOX9, proteoglycan and type II collagen was analysed by RT-PCR and compared using the SNK test for analysis of variance in SPSS 13.0. P values of less than 0.05 were considered to be statistically significant.
At 21 days after the induction of differentiation, the expression of SOX9 and type II collagen was significantly higher in the BMP-7 group than the TGF-β group (P < 0.05). The expression of proteoglycan was not significantly different (P > 0.05) in the BMP-7 and TGF-β groups. The expression of SOX9, proteoglycan and type II collagen in the control group was negative, as shown in Fig. 4. In the BMP-7 group, the expression of type II collagen, SOX9 and proteoglycan were significantly higher at 21 days after the induction of differentiation than at three days (Fig. 4).
The expression of Sox9 (a), Col(Collagen)2A1 (b) and proteoglycan (c) mRNAs, relative to GAPDH expression (%). Standard control of reference materials is β-actin. Differentiation was induced in culture for three days or 21 days in each group. Between BMP-7 and TGF-β groups: expression of SOX9 and type II collagen, P < 0.05; expression of proteoglycan, P > 0.05
Ultrastructural analysis
TEM observation (Fig. 5) of hAECs after induction in differentiation medium showed that cells exhibited features characteristic of cartilage cells, including a round or polygonal shape, membrane integrity, fan-clam-like protrusions, uniform cytoplasm with vacuoles and lysosomes of varying sizes, cytoplasm free of mitochondria, and nuclear integrity.
Epithelial morphology of a representative limbal clone on HAEC or 3T3 feeder layers at a low magnification (×5000). After the induction, cells were round or polygonal with cell membrane integrity, fan-clam-like protrusions, uniform cytoplasm with vacuoles and lysosomes of varying sizes and few mitochondria
Discussion
The purpose of this study was to assess the role of OP-1/BMP-7 in promoting chondrogenesis in hAECs and future application of OP-1/BMP-7 in the engineering of cartilage.
Current research in cartilage tissue engineering has used chondrocyte-seeded cells, embryo-derived cells, mesenchymal stem cells, periosteal cells, synovial cells, etc. [14]. Seed cells are receiving increased attention in the United States. Recently, some researchers have obtained embryonic stem cell-derived precursors from the umbilical cord or placenta and examined their morphology, surface marker antibodies, and differentiation capabilities; the clinical application of these tissues provides a new seed cell source for tissue engineering experiments [15].
hAECs derived from the membrane after cesarean section do not require the disruption of a normal embryo or the early termination of embryonic development, and will thus not spark medical ethics controversies. Most hAECs express only very low levels of HLA-A, B, C or DR antigens, suggesting that they will not stimulate post-transplant immune rejection, an important criterion for seed cells to be used in cartilage tissue engineering [16]. Currently, we are able to amplify cells’ proliferation ability after culture and grow stable, adherent cell lines that can differentiate into a variety of tissue cells [17]. Here, transfection with BMP-7 induced hAEC differentiation into chondrocytes. In our experiment, we found that hAECs become normal cartilage cells with an extracellular matrix rich in collagen and glucosamine GAG (glycosaminoglycans, GAGs). Immunohistochemical analysis with fan staining and 1% toluidine blue staining showed that while uninduced hAECs are weakly positive for amino-polysaccharides, induced cells are strongly positive; this indicates that the extracellular matrix of induced cells is rich in cartilage glycoprotein and probably contains type II collagen. Our transmission electron microscopy observations indicate that amniotic epithelial cells in human cartilage differentiation medium exhibited round or polygonal morphologies with cell membrane integrity, fan-clam-like protrusions, uniform cytoplasm with vacuoles and lysosomes of varying sizes but few mitochondria, and nuclear integrity. These features are all characteristic of cartilage cells.
BMP-7 was able to redirect in vitro expanded primary chondrocytes to stably express type II collagen, decrease type I collagen expression, and stabilise the artificial cartilage construction in vivo by protecting it from nonspecific infiltration and destruction. In our investigation using tissue engineering techniques, articular chondrocytes were isolated by enzymatic digestion of the extracellular matrix. Vital cartilage cells were cultured and expanded under conventional culture conditions. During these phases of culture and expansion, cartilage cells dedifferentiated and chondrocytes lost their characteristic phenotype, as evidenced by the down-regulation of type II collagen and the up-regulation of type I collagen gene expression. This typical switch in collagen synthesis is considered to be the main attribute of dedifferentiating chondrocytes, and may be reversed through the use of 3-dimensional culture conditions and treatment with distinct growth and differentiation factors [6].
Differentiation induced by these morphogens, especially by members of the BMP family, has been suggested for cartilage-derived cells or precursors [18]. Promotion and maintenance of the chondrocyte phenotype were documented for BMP-7 expressing chondrocytes, which expressed type II collagen rather than type I collagen during prolonged cultivation in vitro.
As suggested recently, specific BMPs may interfere with chondrocyte differentiation during distinct stages of chondrocyte development in time- and dose-dependent manners [19]. Additionally, BMPs 2, 5, and 6 have been shown to maintain and promote later stages of chondrocyte differentiation but not initiation of maturation [20], while BMP-7 promoted chondrocyte proliferation and inhibited terminal differentiation [21]. As has been shown in other studies, the addition of BMP-7 protein to short-term cultures of articular chondrocytes may promote redifferentiation in vitro by stimulating proteoglycan expression and the synthesis of type II collagen [22]. Thus, this initial screening for appropriate BMPs supporting chondrocytic differentiation revealed BMP-7 as a promising candidate gene for further analysis in vitro and in vivo. [23].
The selection of cells that express recombinant BMP is accompanied by prolonged cell expansion and senescence, which probably suppresses the responsiveness of articular chondrocytes to certain growth factors. Thus, to minimise expansion and senescence of cells and to allow maximum response to BMP, parental low-passage and BMP-7 transgenic chondrocytes were mixed at a 4:1 ratio and cultured under non-selective conditions [24]. The expression of recombinant BMP-7 was stable in mixed cartilage tissues during culture in vitro. Histochemical analysis revealed an increase of cartilage matrix production in BMP-7 transgenic cartilage tissue compared with control tissue. In addition, the expression of BMP-7 in a subset of chondrocytes was sufficient to convert adjacent dedifferentiated chondrocytes into mature differentiated cartilage cells, as shown by the switching of collagen synthesis from type I to type II collagen [25].
Therefore, BMP-7 acts as a paracrine acting growth factor and may not only stimulate matrix synthesis, but also improve the matrix quality of tissue-engineered cartilage tissue in vitro. Thus hAECs, which demonstrate the characteristics of embryonic stem cells under certain conditions in terms of morphology, proliferation, and immunophenotype, can be transformed into cartilage cells and provide a valuable experimental resource for future applications.
References
Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U (2007) Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod 77:577–588
In't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH (2004) Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22:1338–1345
Liu T, Wu J, Huang Q, Hou Y, Jiang Z, Zang S, Guo L (2008) Human amniotic epithelial cells ameliorate behavioral dysfunction and reduce infarct size in the rat middle cerebral artery occlusion model. Shock 29:603–611
Pecina M, Vukicevic S (2007) Biological aspects of bone, cartilage and tendon regeneration. Int Orthop 31:719–720
Chubinskaya S, Hurtig M, Rueger DC (2007) Op-1/bmp-7 in cartilage repair. Int Orthop 31:773–781
Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A (2002) Interaction of fgf, ihh/pthlh, and bmp signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell 3:439–449
Pecina M, Jelic M, Martinovic S, Haspl M, Vukicevic S (2002) Articular cartilage repair: the role of bone morphogenetic proteins. Int Orthop 26:131–136
Jelic M, Pecina M, Haspl M, Kos J, Taylor K, Maticic D, McCartney J, Yin S, Rueger D, Vukicevic S (2001) Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth Factors 19:101–113
Sailor LZ, Hewick RM, Morris EA (1996) Recombinant human bone morphogenetic protein-2 maintains the articular chondrocyte phenotype in long-term culture. J Orthop Res 14:937–945
Bondeson J, Foxwell B, Brennan F, Feldmann M (1999) Defining therapeutic targets by using adenovirus: blocking nf-kappab inhibits both inflammatory and destructive mechanisms in rheumatoid synovium but spares anti-inflammatory mediators. Proc Natl Acad Sci USA 96:5668–5673
Hunziker EB, Rosenberg LC (1996) Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am 78:721–733
Poleni PE, Bianchi A, Etienne S, Koufany M, Sebillaud S, Netter P, Terlain B, Jouzeau JY (2007) Agonists of peroxisome proliferators-activated receptors (ppar) alpha, beta/delta or gamma reduce transforming growth factor (tgf)-beta-induced proteoglycans' production in chondrocytes. Osteoarthritis Cartilage 15:493–505
Perides G, Safran RM, Downing LA, Charness ME (1994) Regulation of neural cell adhesion molecule and l1 by the transforming growth factor-beta superfamily. Selective effects of the bone morphogenetic proteins. J Biol Chem 269:765–770
Nakamura Y, Wang X, Xu C, Asakura A, Yoshiyama M, From AH, Zhang J (2007) Xenotransplantation of long-term-cultured swine bone marrow-derived mesenchymal stem cells. Stem Cells 25:612–620
Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukun A, Uckan D, Can A (2007) Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells 25:319–331
Sudo K, Kanno M, Miharada K, Ogawa S, Hiroyama T, Saijo K, Nakamura Y (2007) Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells 25:1610–1617
Narukawa M, Suzuki N, Takayama T, Shoji T, Otsuka K, Ito K (2007) Enamel matrix derivative stimulates chondrogenic differentiation of atdc5 cells. J Periodontal Res 42:131–137
Hata K, Nishimura R, Muramatsu S, Matsuda A, Matsubara T, Amano K, Ikeda F, Harley VR, Yoneda T (2008) Paraspeckle protein p54nrb links sox9-mediated transcription with rna processing during chondrogenesis in mice. J Clin Invest 118:3098–3108
Pizette S, Niswander L (2000) Bmps are required at two steps of limb chondrogenesis: formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev Biol 219:237–249
Kameda T, Koike C, Saitoh K, Kuroiwa A, Iba H (2000) Analysis of cartilage maturation using micromass cultures of primary chondrocytes. Dev Growth Differ 42:229–236
Haaijman A, Burger EH, Goei SW, Nelles L, ten Dijke P, Huylebroeck D, Bronckers AL (2000) Correlation between alk-6 (bmpr-ib) distribution and responsiveness to osteogenic protein-1 (bmp-7) in embryonic mouse bone rudiments. Growth Factors 17:177–192
Chao PH, Grayson W, Vunjak-Novakovic G (2007) Engineering cartilage and bone using human mesenchymal stem cells. J Orthop Sci 12:398–404
Karlsson C, Brantsing C, Svensson T, Brisby H, Asp J, Tallheden T, Lindahl A (2007) Differentiation of human mesenchymal stem cells and articular chondrocytes: analysis of chondrogenic potential and expression pattern of differentiation-related transcription factors. J Orthop Res 25:152–163
Eyrich D, Wiese H, Maier G, Skodacek D, Appel B, Sarhan H, Tessmar J, Staudenmaier R, Wenzel MM, Goepferich A, Blunk T (2007) In vitro and in vivo cartilage engineering using a combination of chondrocyte-seeded long-term stable fibrin gels and polycaprolactone-based polyurethane scaffolds. Tissue Eng 13:2207–2218
Hwang NS, Varghese S, Puleo C, Zhang Z, Elisseeff J (2007) Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol 212:281–284
Acknowledgement
The authors wish to thank Michael Gross and Lian Fu Deng for their excellent technical assistance.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, J., Yu, G., Cao, C. et al. Bone morphogenetic protein-7 promotes chondrogenesis in human amniotic epithelial cells. International Orthopaedics (SICOT) 35, 941–948 (2011). https://doi.org/10.1007/s00264-010-1116-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-010-1116-3